Keywords
Partial coverage restorations, posterior teeth, ceramic, Zirconia reinforced lithium silicate, lithium disilicate, CAD/CAM
This article is included in the All trials matter collection.
Partial coverage restorations, posterior teeth, ceramic, Zirconia reinforced lithium silicate, lithium disilicate, CAD/CAM
High survival rates, fracture resistance and proper marginal integrity of CAD/CAM partial coverage restorations (PCRs) were reported in studies simulating 5-year clinical service1–4.
However, clinical behavior of PCRs utilizing morphology driven preparation design was never assessed in randomized clinical trials5–11.
Furthermore, long-term clinical studies have shown that bulk fracture and marginal deterioration of PCRs has a direct correlation to the use of brittle ceramic materials, such as feldspathic and leucite-based ceramics12–15, which encouraged researchers to use higher strength lithium disilicate glass ceramic in such restorations16–21. Although some clinical studies tested the performance of lithium disilicate PCRs, no randomized clinical trial tried to compare between lithium disilicate ceramic material and the newly introduced zirconia-reinforced lithium silicate ceramic material in posterior partial coverage22–24.
The aim of this randomized controlled split-mouth clinical study was to evaluate the clinical outcomes of zirconia-reinforced lithium silicate (Vita Suprinity) and lithium disilicate (IPS e.max CAD) partial coverage restorations. The null hypothesis was that there would be no difference between the two ceramic materials over 12 months.
This study was approved by Ethics Committee of Faculty of Oral and Dental Medicine in October 2016 (Approval number: 03102016).
Written informed consent for all the study procedural steps and publication of their clinical results and images were obtained from the patients.
This trial was registered with ClinicalTrials.gov under trial number NCT02861729 on the 04/08/2016.
This study was a double blinded, split-mouth randomized controlled clinical trial, with an allocation ratio of 1:1.
This article was written in concordance with the CONSORT checklist 2010 (see Reporting guidelines).
All patients were recruited from the outpatient clinic of the Department of Fixed Prosthodontics, Faculty of Oral and Dental Medicine, Cairo University, Cairo, Egypt. Between May 2017 and June 2017, a total of 14 adult patients (8 females and 6 males) were included in this study after fulfilling all inclusion criteria. A total of 46 premolars and molars (20 maxillary and 26 mandibular) were restored in this study, according to split-mouth design; at least two restorations (one of each ceramic material) were placed in each patient.
Inclusion criteria:
Adult patient aged 18–50 years old. Patient with good oral hygiene (papillary bleeding index (PBI < 35%).
Teeth: vital, with large carious lesions/defective restorations and teeth in occlusion.
Exclusion criteria:
Patient with severe systemic disorder, smokers, xerostomia or buxism
Teeth: non-vital, endodontically treated, mobile or periodontally affected teeth.
Based on the previous paper by Guess et al. 200915, the probability of surface roughness among interventions is 0.48. If the true probability among controls is 0.11, it was estimated that a total of 46 samples (n= 23 of each ceramic material group) would be required to reject the null hypothesis that the exposure rates for case and controls are equal with probability (power) 0.8. The Type I error probability associated with this test of this null hypothesis is 0.05. Sample size was calculated using G* Power program, version 3.0.10.
A random sequence was generated by computer software (http://www.randomizer.org/) in the Center of Evidence Based Dentistry, Cairo University. The table was kept with the assistant supervisor (CHH). Participants received numbered papers each contains a number from 1 to 2 representing ceramic material and a letter R or L representing the side where PCR will be placed on folded paper placed in sealed opaque envelops. The patient selected the ceramic material for the first tooth randomly, and then the following tooth received the alternate ceramic material according to the split-mouth design.
A new cavity preparation design; morphology driven preparation (MDP) design was selected for this study. In this design, preparations were guided by the anatomical and structural morphology of the teeth25–27.
Interior walls were prepared with 6–10°divergence, well-defined margins and rounded inside angles. Inter-proximal box was prepared with 1–1.2mm butt-joint, all obtained with medium-grit 80μm diamond truncated conical bur (4137-856-025, Microdont, USA). (Figure 1)
Occlusal reduction of 1.5–2mm was performed with egg-shaped football bur (3118-368-023, Microdont, USA), and verified with silicon index. The outer axial walls with inclined planes were prepared with hollow chamfer margin obtained with round bur (1014-801-014, Microdont, USA). (Figure 2)
All preparation was finished with fine finishing bur (4137F-856-025, Microdont,USA).
All undercuts were blocked with Herculite™ ultra-flow composite (Kerr-Germany, Catalogue no.: 2201-35392).
Full arch Vinylpolysiloxane (EliteHD+. Zermack-Germany, Catalogue no.: F121007 - 2016-05) impression and interocclusal records (Occlufast. Zermack-Germany, Catalogue no.: F121009 - 2016-05) were taken. Provisional restorations were fabricated with Structure-2 bis-composite (Voco-Germany, Catalogue no.: VC 84 001479 GB 0918 V) and cemented with temporary cement (Dentotemp-Itena. France, Catalogue no.: K03330 9).
Master models were poured with type IV dental stone (FujiRock-EP, GC-Belgium, Catalogue no.: 890366), then scanned with an extraoral scanner (Identica-blue. Medit, England). Final PCRs were designed using CAD/CAM software (Exocad-DentalCAD, Exocad GmbH-Slovenia) and milled with 5-axis machine (CAM5-S1impression.Vhf, Ammerbuch-Germany) of Suprinity (VITA-ZahnfabrikH. Rauter-GmbH-Germany, Catalogue no.: 2002E – 0114 (X.) S Version (02)) and e.maxCAD (Ivoclar-Vivadent, Schaan-Liechtenstein, Catalogue no.: 721198/e/2018-11) blocks. After staining, PCRs crystallization was done in ceramic furnace (Programat-P310) according to manufacturer instructions.
Definitive PCR try-in was performed to confirm the restoration proper seating, marginal integrity, shade matching and proper occlusal and proximal contacts.
All PCRs bonding steps were performed under rubber-dam isolation.
The internal surfaces of PCRs were etched for 20 second with 9.5% hydrofluoric acid (BISCO-USA, Catalogue no.: E-5702EP), rinsed, air dried, then Bis-silane (BISCO-USA, Catalogue no.: B-2221P) was applied, left for 60 seconds and air dried.
Enamel margins of the preparations were etched with 37% phosphoric acid (BISCO-USA) for 30 seconds, rinsed and air dried. All-bond universal adhesive was applied, air thinned, and cured for 20 seconds (Elipar™, 3MESPE, USA, Catalogue no.: 70-2013-0430-3-B). Duolink adhesive resin cement (BISCO-USA, Catalogue no: A-19010P), was applied to fitting surface; restoration was seated with gentle pressure, glycerin barrier was applied to margins (Deox.Ultradent-USA, Catalogue no: 238), then light curing was performed for 40 seconds (Elipar™, 3MESPE, USA, Catalogue no.: 70-2013-0430-3-B).
Residual cement was removed and occlusion was carefully checked.
The PCRs were assessed for clinical outcomes by an independent outcome assessors according to the modified United States Public Health Service (USPHS) criteria28–30; at baseline, 6 and 12 months post-treatment. PCRs were visually inspected with mirror, probe and dental floss; all changes were recorded and photographed31.
Primary outcome: survival rate
For survival rate, only Alpha ratings were considered success.
Absolute failure was defined by loss of retention, fracture, crack development which required a replacement of the entire restoration, secondary caries or endodontic complications32–35 (Table 1).
| Kaplan-Meier estimate | |
|---|---|
| Median survival time (Vita Suprinity) | > 50% survival |
| Median survival time (IPS e.max CAD) | > 50% survival |
| Confidence interval | 0.4045 - 2.0774 |
| p-value | 0.8254 |
Secondary outcomes: marginal adaptation and marginal discoloration
Alpha and Bravo scores were considered success, while PCRs rated Charlie or Delta were considered failure15,16,32,35.
This study was a double-blinded study; both patient and outcomes’ assessors were blinded to the assigned PCR material for each tooth throughout all preparation and clinical evaluation steps. However, the operator wasn’t blinded for purpose of lab communication and ceramic material construction steps.
The blinded assessors were asked to fill a chart for each outcome with the number corresponding to each patient without knowing the PCR material allocated to each side of the mouth for each participant. The template for clinical assessment chart can be found with the trial protocol (Extended data36).
The results were analyzed using IBM SPSS, version 21 (SPSS, Chicago, IL, USA). Chi square test was performed for categorical data, a value of P < 0.05 was considered statistically significant. Sample size (n=23/group) was large enough to detect significant effects and perform pair-wise comparisons with a satisfactory level of power set at 80% and a 95% confidence level.
All 14 patients (8 females and 6 males) attended 6 and 12 month follow-up. A total of 46 PCRs were fabricated in this study. A patient flow diagram is available as part of the Reporting guidelines section.
Survival rates on Kaplan Meier survival curve are provided in (Table 1) and (Figure 3). After 12 months, all PCRs of both groups remained in situ, with a survival-rate of 100% (P=0.8254).
For criteria marginal adaptation, e-max CAD group showed a statistically non-significant decrease in Alpha ratings to 95.65% (p=0.1560), while Vita Suprinity group maintained 100% Alpha scores after 12 months (Table 2, Figure 4 and Underlying data36).
For marginal discoloration, e-max CAD group showed a non-significant decrease in Alpha ratings to 95.65 % (p = 0.1560) after 6 months and 87 % ( p=0.6078) after 12 months (Table 3 and Figure 5).
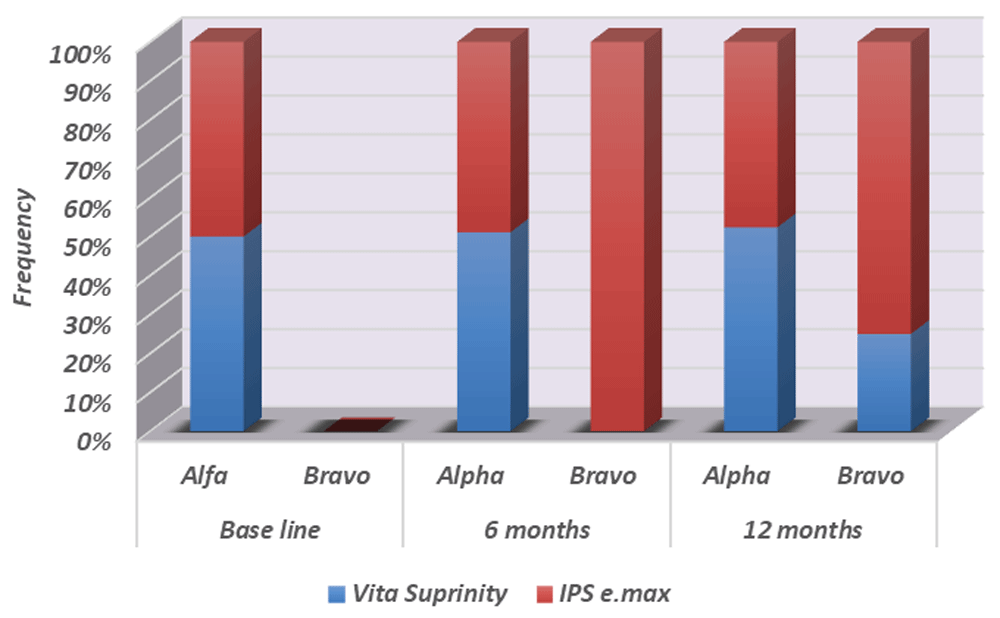
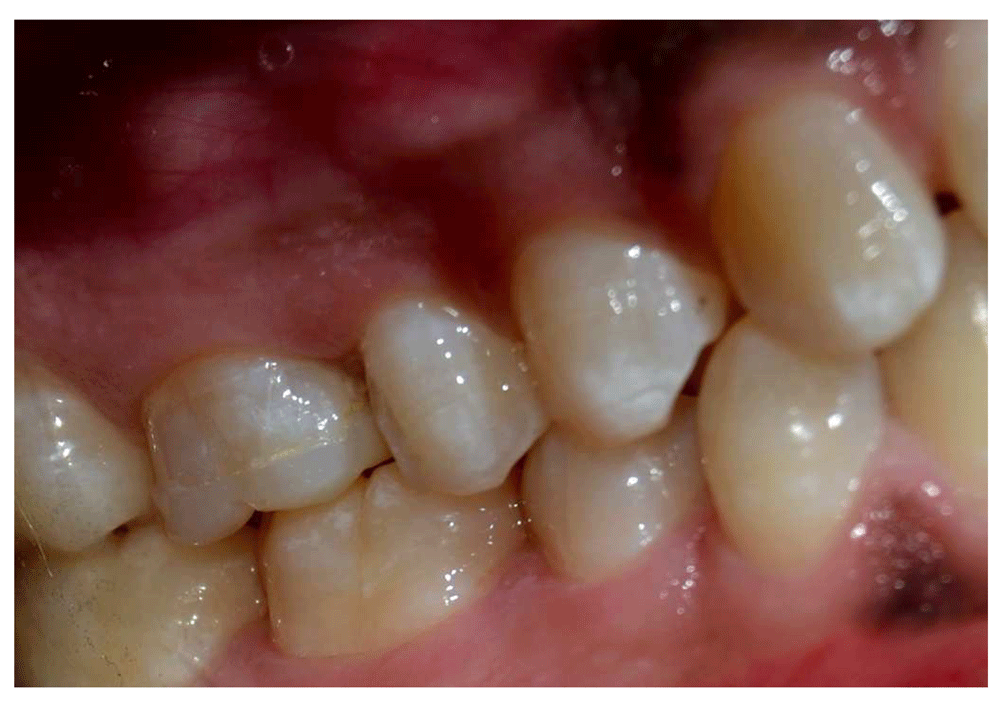
Bravo ratings of 13% for discoloration and palpable marginal ditching were recorded in e-max CAD group after 12 month (Figures 6–Figure 8), while 4.35% Bravo rating were recorded in the Vita Suprinity group after 12 months.
This study aimed to compare the clinical performance of Vita Suprinity and e-max CAD partial coverage restorations in a prospective double-blinded split-mouth design. Selection biases can be avoided in split-mouth studies as the patient acts as their own control, in this way direct comparison of two ceramic materials can be performed37–39.
Compared to full coverage restorations, posterior partial coverage restoration utilizing more tooth structure conservation concept has the potential to reinforce and protect tooth structure, preserve enamel, and safeguard pulp vitality while achieving the desired aesthetic results5,6. Overtime, various ceramic materials have been developed for restoring posterior teeth7–9. The excellent combination of high mechanical strength and optical properties of lithium disilicate glass ceramic material made it the gold standard for comparison of new monolithic ceramic materials9,11,12. In our study CAD/CAM lithium disilicate ceramic material (IPS e.max CAD) was selected as the control to compare the clinical outcomes of the newly introduced zirconia-reinforced lithium silicate ceramic material (ZLS) (VITA Suprinity). Reinforced with about 10% zirconium dioxide, ZLS belongs to a new generation of CAD/CAM ceramic that combines positive mechanical characteristics of zirconia with glass-ceramic aesthetic appearance22. Still all findings regarding this new material are either laboratory or initial clinical experience findings22–24. Moreover, the indication for this material should be chosen with strict observation of the material-specific processing instructions regarding the necessary minimum wall thickness and required adhesive luting22,23. All of these findings make it crucial to conduct randomized clinical studies to verify the clinical performance of this new material.
In this study, a novel tooth preparation design; MDPT was selected. According to Venezian M25, this preparation design aims to minimize the loss of healthy tooth tissue and reduce the areas of dentin exposure. A hollow chamfer margin was created on the outer surface of the preparation to optimize the cutting of enamel prisms, thereby bonding and color blending at the transitional zone between tooth and restoration are enhanced25–27(Figure 1), (Figure 2).
Regarding our results, Kaplan Meier analysis was used for survival assessment during the observational period of 12 months32–35; both Suprinity and e.max-CAD had a survival rate of 100%. All restorations remained in situ and in good function.
Comparison of our results to similar clinical studies regarding Suprinity is limited due to the novelty of the material22,23.
Clinical studies on e.max PCRs reported 98.99–100% survival rates over periods of 1–5 years15,17,40–43. In a long-term evaluation study, Guess et al.16 reported 100% survival rates for e.max PCRs with only evidences of relative failures as small repairable chipping after 8 years, but none of PCRs were fractured or de-bonded.
In our study, none of PCRs were de-bonded after 12 months. Other studies reported de-bonding of PCRs as one of the common causes of failure13,14. In those studies, de-bonding mainly was associated with endodontically treated teeth which were among the exclusion criteria in this study.
For marginal adaptation, all PCRs were rated Alpha at base-line and after 6 months. However; after 12 months, palpable margin ditching resulted in Bravo ratings for one of the e.max-CAD PCRs (4.35%). Marginal deterioration might be attributed to degradation of cement due to fatigue in the oral cavity15,16.
Suprinity PCRs sustained Alpha rating after 12 months, which can be attributed to the higher marginal quality and fatigue resistance of zirconia lithium silicate over lithium disilicate as reported by Preis et al.23,43.
Nevertheless, results by Elsaka and Elnaghy24 were in disagreement with our results as they reported lower brittleness index of e.max CAD compared to Vita Suprinity, and consequently according to the parameters determined by Boccaccini44 and Chaysuwan et al.45; e.max CAD might show lower marginal chipping rates than Suprinity24.
Marginal discoloration of e.max PCRs has been reported by Guess et al.15,16 and Santos et al.17 as the most common clinical finding occurring in 37.5% of PCRs after 7 years.
For marginal discoloration; three of e.max CAD (13%) and one Vita Suprinity (4.35%) restorations showed yellowish marginal staining (Bravo) after 12 months. Still both materials showed clinically acceptable margins.
The null hypothesis for this study was accepted as there was no statistically significant difference in clinical outcomes of the two tested ceramic materials.
This study is randomized clinical trial conducted on relatively big sample size patients, in real clinical settings and was conducted efficiently. This is the first study to compare the clinical performance of e.max CAD and Vita Suprinity partial coverage restorations utilizing a novel preparation design (MDP). Our present study proposes a more conservative and efficient alternative to full coverage restorations for treatment of decayed, vital posterior teeth with high survival rates and excellent marginal quality.
The following limitations should be considered: The morphology driven preparation technique is a new design that wasn’t tested in previous randomized clinical trials before, reliability of the new design irrespective of the ceramic material used needs to be investigated in further clinical trials.
The short follow-up period was one of our study limitations, although no significant differences were found between the two materials, there was notable differences regarding marginal discoloration, thus longer term clinical trials are required to investigate the clinical performance of these ceramic materials.
Both Vita-Suprinity and e.max CAD partial coverage restorations are considered reliable treatment options for restoring larger defects in posterior dentition.
Open Science Framework: Clinical Outcomes of Zirconia-reinforced Lithium Silicate Partial Coverage Crowns Compared to Lithium Disilicate Partial Coverage Crowns. A Randomized Controlled Split-mouth Clinical Study. https://doi.org/10.17605/OSF.IO/UNGCJ36
This project contains the following underlying data:
Open Science Framework: Clinical Outcomes of Zirconia-reinforced Lithium Silicate Partial Coverage Crowns Compared to Lithium Disilicate Partial Coverage Crowns. A Randomized Controlled Split-mouth Clinical Study. https://doi.org/10.17605/OSF.IO/UNGCJ36
This project contains the following extended data:
Open Science Framework: CONSORT checklist and flow diagram for ‘Clinical outcomes of zirconia-reinforced lithium silicate partial coverage crowns compared to lithium disilicate partial coverage crowns. A randomized controlled split-mouth clinical study’ https://doi.org/10.17605/OSF.IO/UNGCJ36
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Written informed consent for publication of their clinical details was obtained from the patients.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Guess PC, Selz CF, Steinhart YN, Stampf S, et al.: Prospective clinical split-mouth study of pressed and CAD/CAM all-ceramic partial-coverage restorations: 7-year results.Int J Prosthodont. 26 (1): 21-5 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Prosthetic dentistry, Dental Biomaterial, Clinical Trials.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Prosthetic dentistry, clinical studies
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 19 Mar 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)