Keywords
Metabolic syndrome, lipotoxicity, obesity, type 2 diabetes, hypertriglyceridemia
Metabolic syndrome, lipotoxicity, obesity, type 2 diabetes, hypertriglyceridemia
The metabolic syndrome (MetS) concept gathers in a single entity a set of metabolic abnormalities that have in common a close relationship with ectopic deposit of lipids, insulin resistance, and chronic low-grade inflammation. In many cases, a chronic exposure to a positive caloric balance is the driving force for the appearance and progression of this condition. The main traits included in MetS diagnosis are arterial hypertension, central adiposity, hyperglycemia, and atherogenic dyslipidemia1. Several other conditions are related to similar metabolic derangements (that is, liver steatosis, polycystic ovary syndrome, and hyperuricemia) but are not part of the MetS diagnostic criteria. The main long-term complications of MetS are type 2 diabetes2, atherogenesis3, and cognitive impairment4.
Controversy has surrounded the definition of MetS5,6. Several definitions (composed of dissimilar traits and thresholds) were proposed for its identification over the past 20 years. This clinical construct has been used as a diagnosis, a risk factor, or even an outcome. Contrasting results have been informed when the added value of MetS is compared against the information derived from each one of its individual components for the prediction, prevention, and treatment of its long-term complications. Also, it has been considered a valuable teaching tool to help health professionals to understand and integrate the consequences of a chronic positive caloric balance and the adverse metabolic consequences of excess body weight7.
In this short review, we discuss recent advances in the diagnosis and clinical use of MetS. Also, new findings in its pathophysiology and treatment are highlighted in the final section of the article.
MetS definitions are composed of one or more clinical variables or biomarkers of the presence of the main traits (Table 1) included in the MetS concept (central adiposity, hypertension, high triglycerides, low high‐density lipoprotein cholesterol, and hyperglycemia)8–13. The selection of the variables and its thresholds has been a complex task because the main MetS traits could be assessed by several indicators that provide complementary information (for example, fasting plasma glucose, HbA1c, or even the 2-hour glucose concentration after an oral glucose load for the “hyperglycemia” trait). In addition, authors have applied diverging conceptual models to build their diagnostic frame. For example, the International Diabetes Federation and the National Cholesterol Education Program (NCEP) definitions are focused on central obesity; in contrast, the World Health Organization definition is focused on insulin resistance. As a result, these tools identify heterogeneous populations as having the same condition. The existence of several MetS definitions created inconsistent results and confusion. However, the practicality of the NCEP criteria9 (or its harmonized version8) has made this diagnostic tool the most accepted diagnostic criterion. Its use has been included in some obesity and cardiovascular risk guidelines14. However, it is questionable that the inclusion of MetS in the clinical assessment or the prognostic evaluation of at-risk cases for having non-communicable diseases provides more information than that obtained by the evaluation of each one of the MetS traits. As a consequence, several groups have proposed alternative approaches intended to be used in research. To avoid arbitrary decisions in the selection of the thresholds to define abnormal values of the MetS traits, our group proposed a score based on the population-based distribution of each one of the MetS components15. One point was given per decile for every component. The total number of points accumulated was used to classify subjects. Individuals in the upper quartile of the point scale (≥39 points) had an odds ratio to have incident diabetes significantly greater than that found with the NCEP criteria (12.71 versus 11.14). The advantage of this approach is the detection of subjects at the extreme of the range of diabetes risk and the ability to estimate the risk as a continuum. A similar approach was applied to develop the MetS-Z score, which is based on the weighted contribution of each component on a sex- and race-specific basis derived from the US National Health and Nutrition Examination Survey. This approach is capable of predicting incident type 2 diabetes and cardiovascular events16. In a recent report, MetS-Z score was applied to assess the response to a healthy lifestyle program in the Diabetes Prevention Program. The 1- to 5-year risk of incident diabetes was strongly associated with 1-year changes in MetS-Z score and waist circumference (hazard ratios for a 1–standard deviation increase = 1.80), whereas the risk of cardiovascular disease was associated with a 1-year change in MetS-Z score, glucose, and systolic blood pressure17. These observations suggest that the MetS definition is still a work in progress. The use of continuous scores may be an option to improve the identification of at-risk individuals and to assess their response to therapy. Some of the continuous definitions are highlighted in Table 217–21. However, its translation into clinical practice will require the demonstration of a clear advantage of its use over current conventional practice. As a consequence, MetS cannot be considered a treatment goal or an outcome22.
AACE, American Association of Clinical Endocrinologists; AHA/NHLBI, American Heart Association/National Heart, Lung, and Blood Institute; ATP, Adult Treatment Panel; BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; EGSIR, European Group for the Study of Insulin Resistance; HDL, high‐density lipoprotein; IDF, International Diabetes Federation; MetS, metabolic syndrome; SBP, systolic blood pressure; TG, triglycerides; WHO, World Health Organization
| Authors | Statistical approach | Clinical use | Reference |
|---|---|---|---|
| Aguilar-Salinas et al. | Population-based distribution of the main traits of metabolic syndrome | Prediction of incident diabetes | 15 |
| DeBoer et al. | Metabolic syndrome–Z score | Prediction of incident diabetes and cardiovascular events Assessment of response to a diabetes prevention program | 16 17 |
| Magnussen et al. | Principal component analyses Sum of standardized Z scores Sum of standardized residuals | Prediction of cardiometabolic outcomes | 18 |
| Wijndaele et al. | Principal component analyses | Assessment of the severity of the metabolic abnormalities | 19 |
| Janghorbani and Amini | Age and gender Z scores | Prediction of incident diabetes | 20 |
| Kang et al. | Sum of points derived from a Cox regression model | Prediction of cardiovascular outcomes | 21 |
Nearly 30% of obese individuals are free of metabolic co-morbidities23. This phenotype has been called “metabolically healthy obesity” (MHO). The MetS definitions have been adapted to identify this condition. However, more than five versions derived from the NCEP definition have been proposed. We should learn from the past24. The definition of a clinical construct should be based on evidence and a conceptual model. The MHO is a biological model with limited clinical implications.
The underlying mechanism by which the MetS components share aspects of their pathophysiology is a matter of intense research. The capacity of the adipose tissue to expand and store energy substrates plays a critical role in its pathophysiology. However, other metabolic pathways should be involved since the same metabolic abnormalities seen in MetS could happen in lean individuals25. Systems biology approaches and omics-based research have been critical to develop new holistic models26. This is the case for the recently published systems biology studies carried out in patients with non-alcoholic steatohepatitis27. Based on this approach, the involvement of several metabolic pathways (de novo lipogenesis, beta oxidation, pyruvate utilization, and serine and glutathione synthesis) was recognized. Some of them are activated to handle the excessive flux of energy precursors found in MetS and to prevent the accumulation of oxygen radicals. Furthermore, the critical role of pyruvate kinase liver red cell (PKLR) as a determinant of triglyceride accumulation in the liver was identified28. In summary, lipotoxicity, low-grade chronic inflammation, and insulin resistance are the prevailing complementary mechanisms that the majority of groups consider the core of MetS pathogenesis. Its description goes beyond the scope of this review. Interested readers will find the description of the state of the art of this field in outstanding reviews included in the References section29–33.
In the near future, the study of the contribution of new approaches (that is, metagenomics, impact of environmental factors on the epigenome, and expansion or differentiation of adipose tissue, among others) may become novel evidence to improve innovative targets for prevention or treatment34–37.
Treatment targets on MetS are the adoption of a healthy lifestyle, weight loss, and the control of co-morbidities (hyperglycemia, dyslipidemia, and arterial hypertension, among others)38 (Figure 1). The long-term goals are the prevention of type 2 diabetes, cardiovascular events, and other MetS-related outcomes (that is, dementia and chronic liver disease).
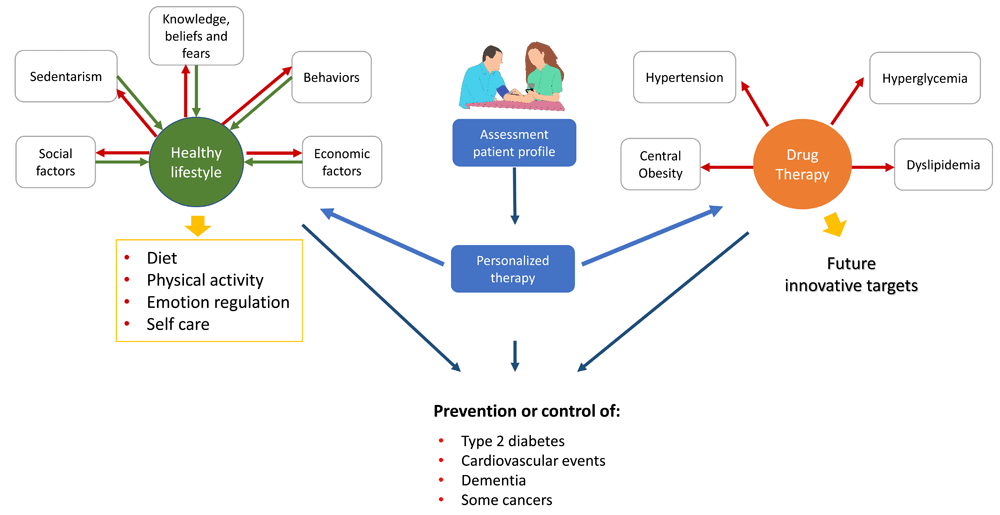
A personalized approach should be applied in the adoption of a healthy lifestyle and in the prescription of drug therapy. Adherence is a major challenge that could be overcome by identifying the patient profile, estimating the empowerment and addressing the main or most common barriers to include therapy in the daily routines.
The adoption of a healthy lifestyle is the cornerstone of MetS treatment. Diet, physical activity, sleep, emotion control, peer support, and avoidance of tobacco, alcohol, and other drugs/medications that alter satiety or body weight are key targets of any healthy lifestyle program. Each one requires a systematic assessment and a patient-centered intervention plan. Knowledge, beliefs, fears, barriers to achieve adherence to therapy, and motivation to change should be evaluated for each target39. Algorithms or intervention plans based on patient profiles are conventional strategies to intervene. In many health systems, innovative approaches (that is, app-based support systems and web-based interventions) have reinforced the intervention plans. However, many challenges remain unsolved. In a large proportion of cases, the effect of the intervention is short-lived. The intervention does not alter major environmental and sociodemographic factors that determine health-related conduct; as a result, unhealthy behaviors persist.
The MetS concept could be used as a teaching tool in healthy lifestyle programs. This approach was applied in the The Reversal Intervention for Metabolic Syndrome (TRIMS) study40. During a 55-minute session, participants discussed with health professionals the contributing factors for having MetS and the health risks that could be avoided. However, the impact of the session was not analyzed separately from the overall intervention. The same limitation is found in meta-analyses that have measured the effect of lifestyle programs on the prevention of type 2 diabetes and other MetS long-term risks41.
A prime objective is weight loss. Even a small weight loss (3% or more) results in an improvement in several MetS components. The most likely explanation for that is the high turnover rate of the intra-abdominal fat depots; as a result, a slight weight loss reduces the liver exposure to fatty acids and other pro-inflammatory mediators. A sustained weight loss greater than 10% could be enough to reverse glucose intolerance, arterial hypertension, and several of the lipoprotein abnormalities. The best source of evidence of the prominent role of weight loss in MetS treatment is bariatric surgery, in which a 30 to 40% weight loss is capable of reversing the majority of the MetS abnormalities42. This action is not exclusive to bariatric surgery43. In addition, complementary mechanisms of bariatric surgery beyond weight loss (that is, farnesoid X receptor [FXR] activation, changes in incretin secretion, or modification of microbiota) have been postulated44. Despite the above, weight loss is an unmet need for many MetS cases. Few cases attain ideal body weight or maintain weight loss over time.
The third treatment target is the control of co-morbidities. Effective drug therapies are available for treating hyperglycemia, arterial hypertension, and dyslipidemia. As a result, the accomplishment of this target is the most likely approach to be implemented in primary care centers to prevent MetS-related outcomes. Metformin, statins, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers are among the most frequent drugs used to treat co-morbidities. In the last few decades, new drugs derived from the identification of innovative treatment targets have come on the market. This is the case with the glucagon-like-peptide 1 (GLP-1) agonists, sodium-glucose co-transporter 2 (SGLT2) inhibitors, and dipeptidyl peptidase 4 (DPP4) inhibitors44. These drugs have positive effects on more than one MetS component (that is, hyperglycemia and weight control). Preliminary data suggest that GLP-1 agonists and SGLT2 inhibitors may reduce cardiovascular or renal outcomes45,46. Long-term studies are under development to demonstrate the effectiveness of such interventions. Cost-effectiveness analyses are needed to integrate these therapies in the majority of health systems. New potential treatment targets are under study. This is the case with FXR agonists47, new peroxisome proliferator-activated receptor (PPAR)-gamma or PPAR-alpha modulators48,49, GIP/GLP1 dual agonists50, dual SGLT1/SGLT2 inhibitors51, G protein–coupled receptor (GPCR/GPR) agonists52, apical sodium-dependent bile acid transporter (ASBT) inhibitors53, chemokine receptor 2 and 5 antagonists, fibroblast growth factor 19 agonists54, and modulators of microbiota or their products among many others MetS treatment is an area under intense investigation. However, owing to long-term safety issues, the introduction of new agents is not as fast as needed.
MetS is a topic in which many disciplines are interested. Besides which definition is used, the MetS concept is a valuable tool in medical education. It provides an overview of the complex mechanisms by which chronic exposure to a positive caloric balance or lipotoxicity (or both) causes long-term complications. Implementing cost-effective therapies and developing new therapeutic strategies for MetS should be considered high-priority areas.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 03 Apr 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)