Keywords
Pre-eclampsia, eclampsia, pregnancy, Magnesium Sulphate
Pre-eclampsia, eclampsia, pregnancy, Magnesium Sulphate
Preeclampsia/eclampsia is a pregnancy-specific disease with multisystem physiological disorder, which may lead to increased risk of maternal and perinatal mortality and morbidity1. Regarding etiology, no clear cause has been defined, but some reports indicate that oxidative stress and placental defects during early pregnancy may represent the main causes2. Preeclampsia is defined as pregnancy-related systemic disorder, which is associated with incidence new onset of hypertension as well as proteinuria3. Eclampsia is described as the beginning of grand mal seizure symptoms in preeclamptic women4. Epidemiologically, around 50,000–60,000 pregnant women die due to preeclampsia/eclampsia each year, which represents around one-quarter of maternal deaths in Latin America, and approximately one-tenth of maternal deaths in Africa and Asia5,6.
Magnesium sulphate (MgSO4) is one of the main interventions used to decrease the morbidity and mortality from preeclampsia/eclampsia. In 2011, MgSO4 was been strongly suggested by the World Health Organization (WHO) to be the drug of choice for both treatment and prevention of eclampsia7. Currently, the WHO and other international organizations recommend two MgSO4 prophylactic protocols for eclampsia. The first one is the Pritchard regimen, in which MgSO4 is administered intramuscularly, while it is injected intravenously in the second protocol named as Zuspan regimen8. MgSO4 might be toxic in some conditions, and the toxicity depends on the serum magnesium levels, for example: loss of the knee jerk may develop at 3.5–5 mmol/l; respiratory paralysis may occur at 5–6.5 mmol/l, cardiac conduction is altered at higher than 7.5 mmol/l9. Meanwhile, it can lead to death if the serum magnesium exceeds 12.5 mmol due to cardiac arrest9.
In Sudan, preeclampsia/eclampsia is considered as one of the main factors leading to an increase in morbidity and mortality among pregnant women2. There is not enough assessment data reported about MgSO4 efficacy, benefits and risks in pre-eclamptic and eclamptic women in this setting. Thus, the present study was carried out to assess MgSO4 usage in preeclamptic and eclamptic women in Khartoum, Sudan.
A cross-sectional pilot hospital-based study was conducted between January and April 2017 at Omdurman Maternity Hospital (Khartoum, Sudan) using a checklist, which was completed by the researcher. Data was collected from patient medical records.
Eligibility criterion was as follows: women who were pregnant and had preeclampsia/eclampsia. The total number of preeclamptic/eclamptic patients admitted during the time period was 130 patients. Therefore, the data records of 130 preeclamptic/eclamptic pregnant women were included in this study.
The checklist (Extended data10) collected the following data variables from records: age; blood pressure; protein urea appearance; admission diagnosis (eclampsia/pre-eclampsia); reason of using MgSo4 (prophylactic in pre-eclampsia or treatment in eclampsia); MgSo4 administration policy (loading dose and maintenance dose); outcomes after MgSo4 therapy; monitoring of MgSo4 side effects (BP, pulse rate, respiratory rate, pulse oximeter, urine output, deep tendon patellar reflexes); Mgso4 toxicity recording.
Misdiagnosis of admitted patients might be a source of bias. This was addressed as follows.
As per hospital policy, any patient who was admitted with blood BP≥ 140/90 and protein urea the following criteria were measured and only patients who have one or more of the following criteria were diagnosed and admitted as pre-eclamptic patients: refractory oliguria (<500 cc over 24 h), renal function compromise (minimal criterion would be a rise in serum creatinine of 1 mg/dl above baseline), persistent right upper quadrant or epigastric pain or both, persistent headache, scotomata or blurred vision, shortness of breath with reduced oxygen saturation or pulmonary edema, thrombocytopenia (platelets <100,000/cu.mm), hemolysis (based on peripheral smear analysis or increased bilirubin), impaired liver function of unclear etiology, and estimated fetal weight below 5th percentile for gestational age are important parameters to be noted.
Patients who were admitted with blood BP≥ 140/90, protein urea and grand mal seizures were diagnosed as eclamptic patients.
After data collection, IBM SPSS Statistics software (version 21) was used to descriptively analyze the data.
Ethical clearance (FPEC-06-2018) was obtained from the Ethical Committee of the University of Khartoum, Faculty of Pharmacy. Official agreement from the general manager and the medical directors of the hospital preceded the conduction of this study. After records were as being eligible for the study, the patients were contacted for their consent to use the records in this study. Since the majority of the patients were illiterate, oral informed consent was preferred to written informed consent.
As shown in Figure 1A, 62 participants were 25–34 years (~48%). About 34% of participants were 15–24 years and only 18% were 35–45 years old. Regarding admitting diagnosis, the majority (78%) were diagnosed with preeclampsia, while 22% were diagnosed with eclampsia (Figure 1B).
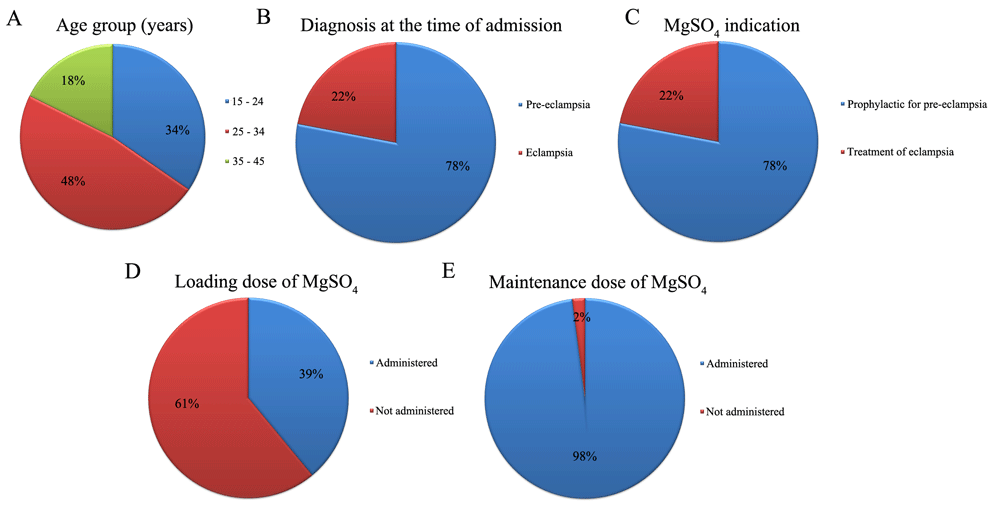
Graphs showing the distribution of the study population according to (A) age; (B) admitting diagnosis; (C) MgSO4 indication; (D) administration of loading dose of MgSO4; (E) administration of maintenance dose of MgSO4
Every patient recruited to the study was followed up while they received MgSo4 therapy. During follow-up some of the preeclamptic women developed eclampsia; therefore, the ratio of preeclampsia:eclampsia became 88:42 instead of 101:29.
MgSO4 was used as a prophylactic agent in 88 preeclamptic women and as treatment for 42 eclamptic women, as shown in Figure 1C. Moreover, magnesium sulphate was administered as a loading dose in 51 (39.2%) patients, while most treated patients (98.5%) were administered a maintenance dose of MgSO4 (Figure 1D and E).
To avoid practitioner-related errors, patients’ vital signs and parameters must be monitored frequently during MgSO4 administration11. In this study, only vital signs (Pulse Rate, Blood Pressure, Respiratory Rate, and Pulse Oximeter) were checked ten minutes after starting loading dose (LD). At the end of LD, 4 hourly checks were performed during maintenance dose (Figure 2A). Other important parameters, such as urine output and deep tendon patellar reflexes, were checked in 42% and 25% of patients, respectively.
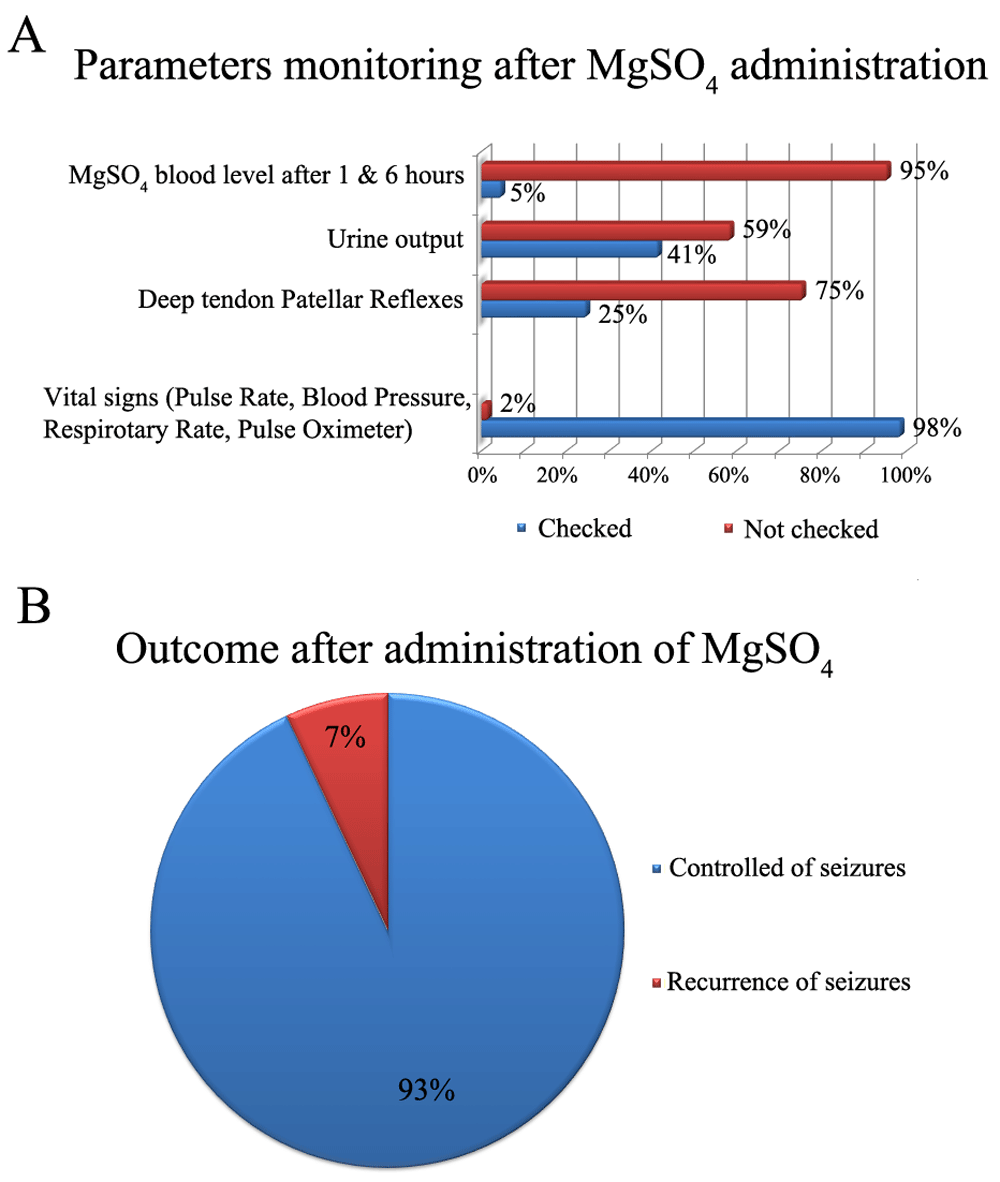
(A) Parameters monitored after MgSO4 administration; (B) distribution of study population according to seizure outcome after administration of MgSO4.
Regarding outcomes after administration of MgSO4, the majority of patients (121; 93.1%) had controlled seizures (Figure 2B). Only one patient developed MgSO4 toxicity (respiratory paralysis).
Being pregnant at age 35 or more is one of the highest risks of developing preeclampsia/eclampsia12, however, in the present study the 25–34 year age group was the largest age group of women with preeclampsia/eclampsia. MgSO4 is considered as the golden choice for treatment and prevention of eclampsia, as endorsed by the WHO7. Therefore, it is no wonder that MgSO4 has been used to control every admitted eclamptic/preeclamptic case included in the present study.
Regarding the administration protocol, MgSO4 must be given by either Pritchard or Zuspan regimen. In Pritchard regimen, a loading dose of 4 g is injected intravenously, then a maintenance dose will be started immediately by intramuscular injections of 10 g followed by 5 g 6 times/day. In Zuspan regimen, 4 g dose is given a loading dose, then maintenance dose by using 1 to 2 g/h controlled infusion pump13. In present study, poor practice is observed as 61% of admitted patients didn’t receive loading doses and two patients didn’t receive maintenance dose.
Some reports indicate that the rate of eclamptic seizures was recorded as being lower than 35% of all recruited cases after using MgSO4, while in present study the rate of uncontrolled-recurrent seizures was only 7% after using MgSO414. In this study, no patient died as a result of using MgSO4 whereas some literature indicates that “if MgSO4 was universally prescribed for all pre-eclamptic pregnant women, the death from magnesium toxicity may be more than that from seizures”14. On the other hand, the prevalence of MgSO4 toxicity is very mild and only one toxicity case had been reported worldwide to date15.
Although practitioners in the present study did not properly adhere to MgSo4 protocol, the successful output of its usage as a treatment for eclampsia and prophylaxis for pre-eclampsia was shown by the majority of patients (93.1%) only having controlled seizures, with only one patient developing toxicity.
Practitioners’ adherence to MgSo4 protocol should be assured in the future to increase the percentage of the well-controlled patients.
Open Science Framework: Assessment of Magnesium Sulphate Usage in Pre-eclampsic and Eclampsic Women in Omdurman Maternity Hospital, 2017, https://doi.org/10.17605/OSF.IO/YV76K10.
Checklist used in the present study: https://doi.org/10.17605/OSF.IO/YV76K10.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Obstetrics and Gynecology; High-risk pregnancy, Hypertension in Pregnancy, Evidence-based Medicine, Prenatal, Chilbirth and Postpartum Care, Maternal Mortality, Abortion, Reprodutive Rights
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Preeclampsia–Eclampsia. Official Journal of the federation of Obstetric and gynaecological Societies of India. 2014; 64 (1).Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Maternal medicine and high-risk obstetrics, infertility and laparoscopic surgery
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 11 Apr 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)