Keywords
Arabidopsis, meiosis, gene expression, promoter, meiocytes, zinc finger, fluorescence reporter, negative selection marker
Arabidopsis, meiosis, gene expression, promoter, meiocytes, zinc finger, fluorescence reporter, negative selection marker
The cell division process of meiosis generates haploid gametes for fertilization. Cells that are primed for meiosis (meiocytes) undergo two consecutive rounds of chromosome segregation. The first division (meiosis I) results in separation of the homologous chromosomes1, which is facilitated by the formation of physical connections (chiasmata) at double stranded break sites generated by transesterases, such as SPO11-12,3 and SPO11-24 in the model plant species Arabidopsis thaliana. Resolution of these junctions can lead to the reciprocal exchange of parts of chromosome arms, a process known as meiotic crossing over5. The second round of division (meiosis II), results in separation of the sister chromatids1, finally yielding four haploid daughter cells from a diploid meiocyte. In Arabidopsis, male and female meiosis are separated within the flower and take place in the anthers and carpels, respectively. Male meiocytes are organized as column-like structures (referred to in this study as male meiocyte columns)6,7, and upon completion of meiosis give rise to four haploid pollen cells that are physically attached to each other as structures known as tetrads. Maturation of tetrads eventually leads to the release of individual pollen cells.
The process of meiosis is of great interest to plant breeders for different reasons. Most importantly, crossover events are the driving force behind genetic variation in plants5,8,9. Research on crossover frequency has thus received a considerable amount of attention in literature (e.g. 10–12). To facilitate the engineering of crossover-related events in meiocytes without affecting non-meiotic tissue, there is interest in identifying gene promoters that allow for effector activity during meiosis. Although there is a vast amount of literature available on the genetics and cytogenetics of plant meiosis1, it has been challenging to identify DNA sequences that can be used to express proteins of interest preferably in meiotic cells.
Relatively recent studies on the male meiocyte transcriptome6,13 have provided cues for the identification of genes that are more or less specifically expressed during male meiosis. The upstream regions of these genes (generally representing the gene control region14) are mostly referred to as ‘promoters’. Such promoters can be regarded as portable elements for the assembly of gene expression cassettes that can mediate meiotic effector protein expression. Accurately quantifying meiotic transgene expression, however, is subject to a number of practical limitations. Firstly, relative expression levels do not necessarily correlate with promoter activity, which is determined by endogenous RNA metabolism as well (e.g. 15). Secondly, the isolation of meiocytes for quantitative gene expression analysis is technically very challenging and laborious, and could easily result in contamination of RT-qPCR or transcriptome data with non-meiocyte transcripts. Qualitative assessment of promoter activity using fluorescence microscopy is an attractive possibility. However, the dynamic cytoplasmic and nuclear states of meiocytes make it difficult to determine the exact localization of fluorescence, and to distinguish it from autofluorescence.
In this study, we have investigated two novel strategies to screen for portable promoter elements to express effector proteins specifically or at high levels in meiocytes. The first strategy was based on expressing a previously constructed GFP-tagged zinc finger protein (1803F-GFP) to visualize pericentromeric fluorescence foci in meiocytes. Here, we show that 1803F-GFP expression driven by the promoter of the Arabidopsis RPS5A gene (pRPS5A) which is active in vegetative16–18 and meristematic tissue18, resulted in clear GFP foci in male meiocytes. We also selected and cloned 14 promoter sequences that could be of interest for expression of transgenes in meiocytes based on published transcriptome data, and assessed their activities in meiocytes using the 1803F-GFP reporter system. As a second strategy, we assembled constructs encoding the highly cytotoxic Agrobacterium tumefaciens virulence protein VirD5, which was recently shown to disturb chromosome segregation in plant cells by interacting with the kinetochores of chromosomes19. We reasoned that, due its mode of cytotoxicity, VirD5 could be used as a novel negative selection marker to assess effector protein expression in meiocytes. Here, we show that the VirD5 reporter system can be used to obtain in planta cues about promoter strength and specificity. Altogether, our data show that both strategies can provide valuable in vivo information regarding promoter activity in meiocytes.
To investigate the activity and specificity of promoters of interest to drive effector protein expression in meiocytes, we first cloned the promoters of a selection of genes which are involved in meiosis or highly expressed in meiocytes. In total, we cloned the promoter sequences of 14 genes based on the two available studies on the male meiocyte transcriptome6,13. An overview of these 14 genes is provided in Table 1. In brief, we firstly selected five genes with important roles in meiosis (ZYP1A, SPO11-2, NBS1, SPO11-1, MUS81) that are expressed in male meiocytes6,13, and rather specifically so based on meiocyte/anther and meiocyte/seedling expression ratios6 (Table 1). In addition, we selected four genes (ASK1, DMC1, SMC1 and MS5) which are involved in meiosis and are expressed in male meiocytes6,13, but not specifically so (Table 1). Secondly, we selected four genes (At1g27710, At5g25980, At5g26622, At5g42530) which are the most highly expressed genes in male meiocytes (13 and personal communications with the authors). Finally, we selected the promoter of the gene At4g40020, which has been described to be expressed specifically during meiosis I7.
The genes are listed in locus ID order.
Previously, we have described a GFP-based reporter to monitor chromosomes in vivo in vegetative tissue of Arabidopsis plants20. This construct encoded a fusion between the fluorescent protein GFP and an array of three zinc fingers (3F) targeting a cognate 9 bp DNA sequence14 in the highly conserved pericentromeric 178 bp repeats which make up a large portion of the pericentromeric DNA in Arabidopsis21. Expression of this fusion protein, which is referred to here as ‘1803F-GFP’, can result in distinctive GFP signals that visualize the pericentromeric regions of chromosomes or the cytological chromocenters20,22. These signals are particularly easy to observe in non-green tissues, such as roots22. An important benefit of the formation of foci is that the GFP signal is strongly concentrated, making discrimination from autofluorescence much easier compared to the detection of diffuse GFP signal. In addition, as the 178 bp repeat regions are strongly conserved and present in the pericentromeric regions of all five Arabidopsis chromosomes, the 1803F-GFP reporter system also allows for ploidy determination. For these reasons, we considered the 1803F-GFP system a suitable read-out for meiotic protein expression. An overview of the 1803F-GFP strategy is provided in Figure 1.

A promoter of interest (promoter of interest) is cloned into the binary vector pRF 1803F:(GGGGS)3:GFP Kana (e.g. as a PCR-amplified SalI-NotI fragment). The promoter drives expression of a fusion protein consisting of three zinc fingers (ZF1, ZF2 and ZF3) together recognizing 9 bp of DNA which are unique to a major fraction of the 178 bp pericentromeric repeats, fused to the open reading frame of GFP (GFP), spaced by a flexible linker peptide (Linker), and with an N-terminal nuclear localization signal (NLS) and FLAG tag (FLAG). Transcription termination is under control of the NOS terminator sequence (tNOS). The construct is introduced into Arabidopsis plants as a T-DNA with left and right border sequences (LB and RB, respectively), through Agrobacterium tumefaciens-mediated transformation. Primary transformants are selected for by kanamycin resistance due to NPTII expression. Expression of 1803F-GFP under control of the promoter of interest can subsequently be visualized as nuclear GFP foci by microscopy.
Previously, we have used the 1803F-GFP reporter system20,22 under control of the promoter of the Arabidopsis RPS5A gene (pRPS5A) in the binary vector pRF 1803F:GFP Kana. For this study, we slightly modified the backbone of pRF 1803F:GFP Kana to accommodate the introduction of other promoter fragments. In addition, we introduced a new, flexible hydrophilic (GGGGS)3 linker23,24 to possibly enhance the read-out of the 1803F-GFP system. This newly generated binary vector was named pRF 1803F:(GGGGS)3:GFP Kana. More details about the construction of pRF 1803F:(GGGGS)3:GFP Kana are provided in the Methods section. An overview of the DNA sequence and the key components of pRF 1803F:(GGGS)3:GFP Kana is provided in Figure 2, and the amino acid sequence encoded by the 1803F:(GGGGS)3:GFP open reading frame is provided in Figure 3.
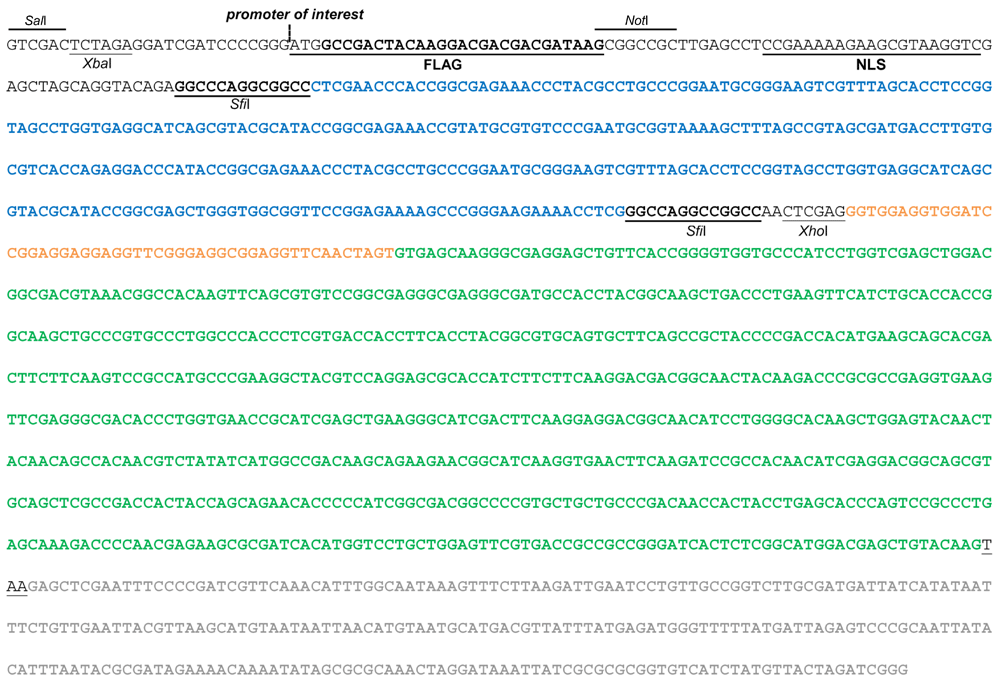
The fusion construct consists of the 1803F encoding sequence (bold blue font) flanked by SfiI sites, fused to the eGFP encoding sequence (bold green font) through a flexible linker (bold orange font), tagged with a FLAG tag and a nuclear localization signal (NLS) at the 5´ end. Other relevant unique restriction sites are also indicated. Expression of the fusion construct is under control of a promoter of interest, which can be inserted at the indicated position, and of the NOS terminator sequence (bold grey font).

The fusion protein consists of 1803F (consisting of three ZFs; ZF1 in blue, ZF2 in red and ZF3 in purple font, respectively) fused to GFP (bold green font) through a flexible linker peptide consisting of a trimer of the aa sequence GGGGS, and tagged with a FLAG tag and a nuclear localization signal (NLS) at the N-terminus (398 amino acids in total).
Promoters of interest can easily be introduced into the pRF 1803F:(GGGGS)3:GFP Kana backbone, for instance by ligating SalI-NotI fragments of PCR-amplified promoter sequences into SalI-NotI digested pRF 1803F-GFP Kana, thereby directly placing the 1803F-GFP open reading frame under their control. The binary vector is also compatible with other cloning strategies, e.g. as described in this study for pSPO11 (further details described in the Methods section).
To investigate the 1803F-GFP system as a novel tool to visualize protein expression in vivo in meiocytes, we firstly verified whether the nuclear GFP foci distinct for 1803F-GFP binding to pericentromeric regions (Figure 1) could be observed in root tip cells (diploid; 2n=10) of transgenic plants harboring the pRPS5A::1803F:(GGGGS)3:GFP construct. Nuclei of root tip cells could easily be visualized as expected, and in most cases contained ten distinct GFP foci in a full Z-stack of images, corresponding to the diploid (2n=10) number of chromosomes (Figure 4A). The foci could be examined directly by zooming in on confocal microscopy images and did not require further image analysis.

(A) Confocal fluorescence microscopy images of a root tip (T2 generation) expressing 1803F-GFP under control of pRPS5A. Highlighted is a nucleus in which 10 distinct GFP foci corresponding to the diploid number of chromosomes (2n=10) are visible. Scale bar represents 10 μM. A contrasted black and white (‘Binary’) version of the GFP image is included to clearly mark GFP-fluorescent chromocenters with red arrows. (B) Confocal fluorescence microscopy images of male meiocytes of wild-type Col-0 plants, primary transformants (T1 generation) expressing 1803F-GFP under control of the RPS5A promoter (pRPS5A::1803F-GFP), and primary transformants expressing 1803F-GFP under control of the GAL4-based two-step expression system (p2S-RPS5A::1803F-GFP). Images taken with the RFP channel are presented as negative controls for autofluorescence. Scale bars represent 20 μM. (C) False color images of nuclei of meiocytes harboring p2S-RPS5A::1803F-GFP (n=8 presented). Below every nucleus the number of counted GFP foci is presented. A diploid somatic cell (2n=10) is provided as a control. (D) DAPI-stained spread of wild-type Col-0 flower bud tissue. Highlighted are a diploid cell (2n=10) and a haploid cell (n=5). Scale bar represents 10 μM.
To assess the 1803F-GFP system in meiocytes, we subsequently isolated male meiocyte columns from wild-type plants and from transgenic plants harboring pRPS5A::1803F:(GGGGS)3:GFP, and examined them by confocal fluorescence microscopy. Male meiocytes from transgenic plants harboring pRPS5A::1803F:(GGGGS)3:GFP clearly displayed GFP foci corresponding to 1803F-GFP fusions binding to pericentromeres (Figure 4B), while meiocytes isolated from wild-type Col-0 plants did not (Figure 4B). These observations demonstrated that the 1803F-GFP reporter system provides for a clear read-out for meiotic effector protein expression, and that pRPS5A is active during meiosis, which is a novel finding. To corroborate these observations, we constructed a GAL4-based two-step (2S) variant of the pRPS5A expression cassette, in which pRPS5A drives the expression of GAL4-VP16, which in turn can transactivate a flanking 4xUAS-minimal35S::1803F:(GGGGS)3:GFP reporter gene (p2S-RPS5A::1803F:(GGGGS)3:GFP in short). This 2S construction, which was based on an earlier published enhancer trap approach25,26, was expected to provide for an essentially similar expression pattern as in pRPS5A:: 1803F:(GGGGS)3:GFP reporter lines, but, due to the fact that GAL4-VP16 is a potent activator of multiple UAS-driven gene expression, would have higher overall strength. We subsequently isolated male meiocyte columns from transgenic plants harboring the two-step p2S-RPS5A:: 1803F:(GGGGS)3:GFP variant, and examined them by confocal microscopy. Nuclei of meiocytes harboring p2S-RPS5A::1803F:(GGGGS)3:GFP indeed displayed clear GFP foci which now had a slightly higher signal intensity (Figure 4B), and displayed less background GFP fluorescence than meiocytes from transgenic plants harboring pRPS5A:: 1803F:(GGGGS)3:GFP. These data showed that the 2S expression cassette is also a suitable reporter for meiotic activity of promoters of interest.
We further investigated the resolution of the 1803F-GFP system by assessing the number of visible chromosomes in the nuclei of meiocytes in male meiocyte columns (Figure 4C). When examining 1803F-GFP foci in the surrounding somatic tissue, the number of chromosomes per cell was always ten (2n=10). This dropped to approximately five GFP foci in meiocytes of transgenic plants harboring p2S-RPS5A::1803F:(GGGGS)3:GFP (Figure 4C), confirming that these cells were either haploid, or that chromocenters were in close proximity. In this case, it also has to be noted that the raw images did not require any further processing besides zooming in on nuclei. Hence, the 1803F-GFP reporter system combined with confocal microscopy had sufficient resolution to visualize chromosomes in vivo in meiocytes using the 2S expression cassette. To further corroborate this, we compared images taken from meiocytes expressing 1803F-GFP to confocal images of chromosome spreads which were prepared from flower bud tissue (Figure 4D). We found that images of DAPI-stained spreads of flower bud cells (Figure 4D) had a resolution which in terms of visibility of chromocenters was very similar to images obtained through squashing of 1803F-GFP expressing flower buds (Figure 4C), although the latter were captured in vivo without requiring any tissue fixation and staining. These observations indicated that the 1803F-GFP system had sufficient resolution to match the standard of the best available cytological images.
To further investigate the 1803F-GFP system as a read-out for meiotic effector protein expression, the cloned promoter sequences of meiotic genes (Table 2) were each ligated into binary vector pRF 1803F-GFP Kana, thereby replacing pRPS5A. Arabidopsis Col-0 plants were transformed with these fusion constructs using the floral dip method. Male meiocyte columns were then isolated from young flower buds of kanamycin-resistant primary transformants and examined by confocal fluorescence microscopy, as was done for pRPS5A. As controls, we also examined tetrads and pollen, and other tissues of flower buds which were released during the isolation of meiocytes. An overview of the results of the confocal fluorescence microscopy is provided in Table 2. Confocal microscopy images of tissues in which fluorescence foci were observed, are provided in Figure 5.
Flower buds from on average five independent primary transformants (n=5) were examined. The promoters are listed in locus ID order. Positive fluorescence signals are indicated in grey.
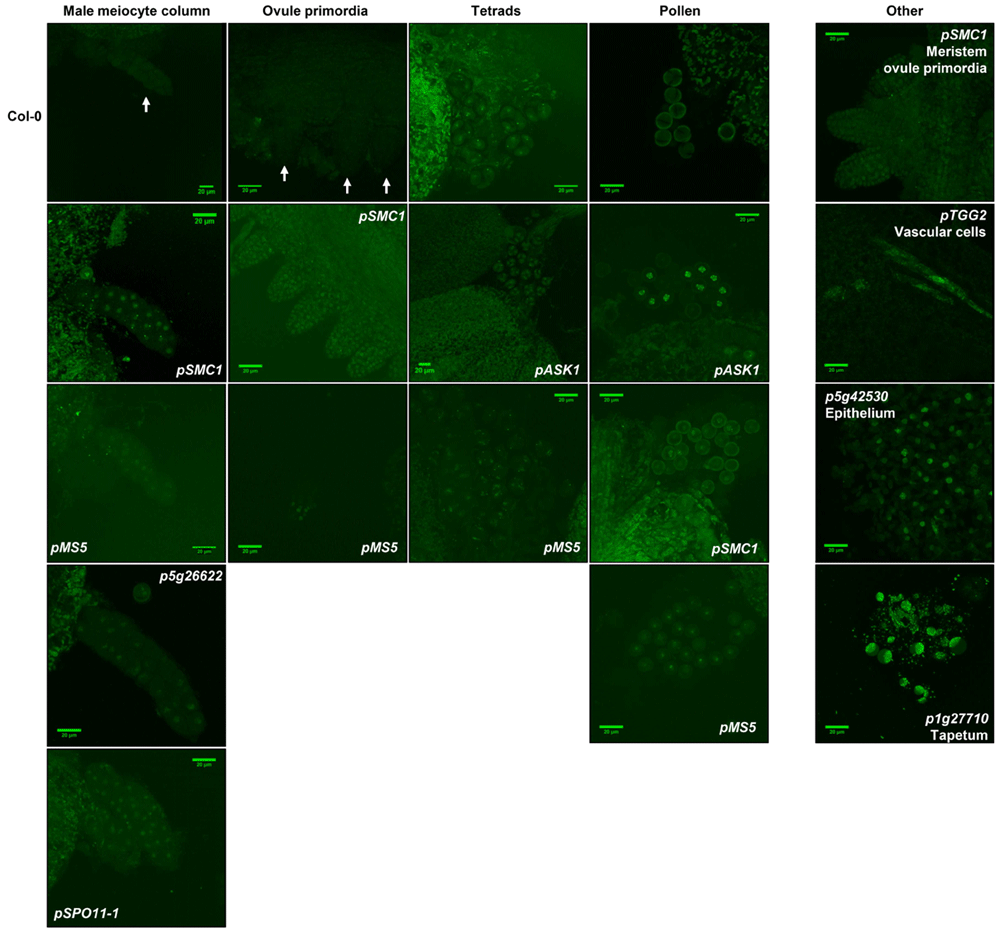
Confocal images of male and female meiocytes (male meiocyte columns and ovule primordia, respectively) are presented, along with tetrads and pollen. For the sake of clarity of the figure only the cases where GFP signals could be observed are presented. Images of all four tissue types in Col-0 are presented as a negative control, with white arrows indicating the location in case of low background fluorescence. Scale bars represent 20 μM.
Using the 1803F-GFP system, we could show that the activity of the promoters of SPO11-1, SMC1, MS5 and p5g26622 indeed resulted in GFP foci in male meiocytes (Table 2 and Figure 5). For pSPO11-1 and p5g26622 we did not observe foci in any other tissues of the flower bud (Table 2), indicating that these promoters are indeed specifically active during meiosis. In the cases of seven other promoters (pZYP1A, pSPO11-2, pNBS1, pDMC1, pMUS81, p4g40020, and p5g42530) we could not detect GFP foci in any tissue type (Table 2), while transcriptome data for five of these genes (ZYP1A, SPO11-2, NBS1, DMC1, and MUS81) are indicative of rather specific expression during meiosis (Table 2)6. These observations suggested that the expression levels of these genes in meiocytes are too low to be detected using the 1803F-GFP system as a reporter, which is in accordance with their low expression values in meiocytes (Table 2)6,13. For three promoters (p1g27710, pTGG2, and p5g42530) we did not observe expression in meiocytes, tetrads, or pollen, but noted GFP signals in other tissues in the flower bud, such as the tapetum, epithelium, and vascular cells (Table 2; Figure 5), suggesting that these promoters have very low activities in meiocytes and are not at all meiosis specific.
An overview of promoter activities described in this study which either confirm findings described in literature or are completely novel is presented in Figure 6.
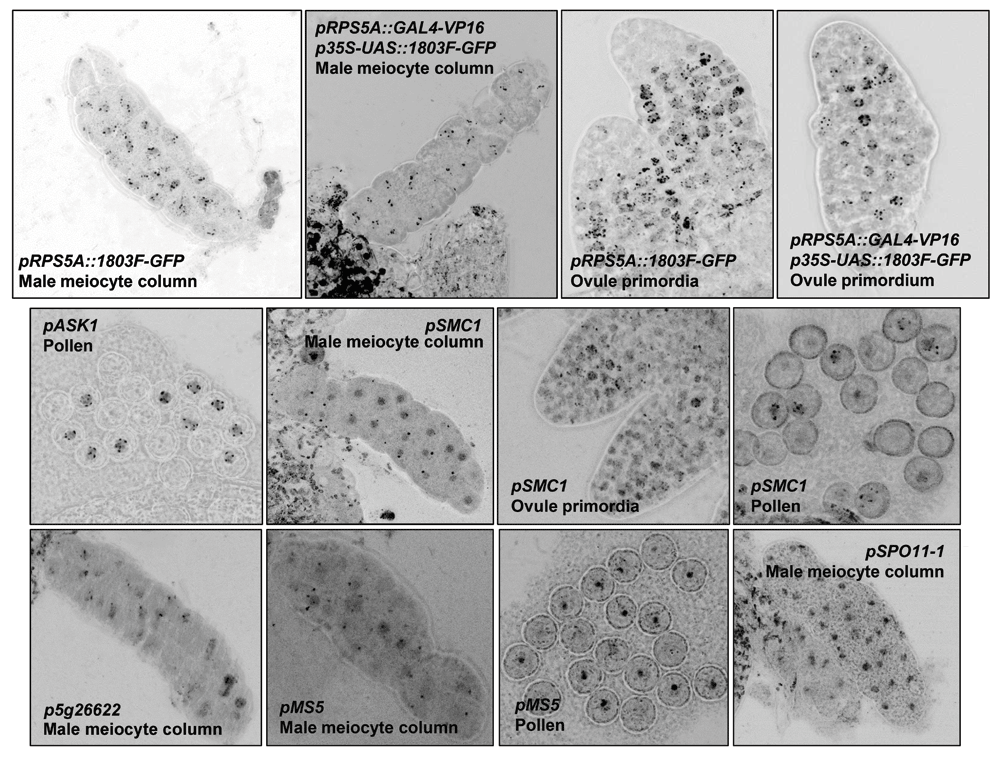
Presented are the most clear examples of 1803F-GFP expression in meiocytes and pollen under control pRPS5A, the GAL4-based two-step expression system (p2S-RPS5A::1803F-GFP) and of the meiotically active promoters verified in this study. Fluorescence intensity and size of the confocal images was optimized to qualitatively show the nuclear foci representing 1803F-GFP fusions binding to pericentromeric regions. Therefore, no scale bars are shown.
As a second strategy to assess the strength and specificity of meiotic promoter activity, we assembled reporter constructs with the Agrobacterium tumefaciens virulence gene virD5. Recently, the virulence protein VirD5 was described as being cytotoxic to plant cells by binding to kinetochores through an interaction with the protein Spt4, thereby disturbing mitotic cell division and leading to cell death and aneuploidy19. Transgenic plants expressing VirD5 under control of an inducible promoter could be obtained, but these died upon induction of expression19. In the present study, we hypothesized that VirD5 could also be cytotoxic to meiotic cells, and that plants expressing VirD5 under control of a meiotic promoter would grow normally, but would show partial or full sterility due to abortion of meiosis. In that manner, VirD5 expression might act as a dominant negative indicator of meiotic protein expression. We chose for VirD5 over other dominant negative selection marker systems because it specifically interferes with chromosome segregation and is thus likely to be particularly toxic to actively dividing cells, such as meiocytes. A schematic overview of this approach is presented in Figure 7. To establish a VirD5 based reporter system we cloned the virD5 open reading frame (ORF) from Agrobacterium tumefaciens strain LBA110027 and generated fusion constructs for a selection of nine different cloned meiotic promoters of genes for which both expression data and specificity data were available6,13 (Table 1).

A promoter of interest (Promoter of interest) is introduced into the binary vector pVirD5 Kana (e.g. as a PCR-amplified SalI-NotI fragment). The promoter then drives expression of the virD5 open reading frame with an N-terminal FLAG-tag (FLAG) and nuclear localization signal (NLS), under control of the NOS terminator sequence (tNOS). The construct is introduced into Arabidopsis plants as a T-DNA with left and right border sequences (LB and RB, respectively), through Agrobacterium tumefaciens-mediated floral dip transformation into wild-type Col-0 plants. Primary transformants (T1 generation) are selected as kanamycin-resistant individuals due to expression of the NPTII gene. Depending on the strength and tissue specificity of the activity of the promoter of interest, the viability, growth and fertility of the primary transformants can be affected. Possible examples of these effects are illustrated. In case of meiosis-specific activity, vegetative and floral development are unaffected, but the plants are sterile.
Wild-type Col-0 plants were transformed with VirD5 expression constructs under control of the nine cloned promoters using the floral dip method. Viable primary transformants could be obtained at normal transformation frequencies with all of the promoters except for pASK1 and pSMC1 (Table 3), which did not yield any viable primary transformants (Table 3), indicating that these promoters are not at all meiosis-specific and already active in embryos and seedlings. In case of pASK1 this is in accordance with already observed vegetative ASK1 expression patterns (Table 1), but was unexpected for pSMC1, which is, more or less, a meiosis-specific promoter based on available gene expression data (Table 1). Viable, fully developed primary transformants could be obtained at high frequencies for the other seven promoters, indicating that none of them were very active in vegetative tissue.
The promoters are listed in locus ID order.
| Locus ID | Promoter name | Meiosis specificity (meiocyte/seedling expression ratio) Based on Chen et al. (2010) | ::virD5 | |||
|---|---|---|---|---|---|---|
| Transformation efficiency (%) | Number of primary transformants obtained (from X floral dip seeds) | Viable primary transformants (%) | Primary transformants with reduced fertility (%) | |||
| At1g22260 | pZYP1A | Specific (17.0) | 0.31 | 62 (20,000) | 98 | 21.2 |
| At1g63990 | pSP011-2 | Rather specific (2.0) | 0.15 | 30 (20,000) | 93 | 14.3 |
| At1g75950 | pASK1 | Not specific (1.0) | 0.02* | 3* (20,000) | 0* | N.A. |
| At3g02680 | pNBS1 | Rather specific (1.6) | 0.16 | 32 (20,000) | 94 | 6.7 |
| At3g13170 | pSPO11-1 | Rather specific (2.9) | 0.32 | 63 (20,000) | 95 | 34.1 |
| At3g22880 | pDMC1 | Specific (6.0) | 0.18 | 36 (20,000) | 100 | 5.6 |
| At3g54670 | pSMC1 | Rather specific (1.8) | 0.01* | 2* (20,000) | 0* | N.A. |
| At4g20900 | pMS5 | Not specific** (N.A.) | 0.16 | 32 (20,000) | 100 | 0.0 |
| At4g30870 | pMUS81 | Rather specific (4.0) | 0.20 | 39 (20,000) | 97 | 2.6 |
| pRPS5A::1803F-GFP (negative control) | 0.54 | 54 (10,000) | 100 | 7.4 | ||
We further noted that VirD5 expression under control of pZYP1A, pSPO11-1 and pSPO11-2 did not impact vegetative development and flowering, but did lead to strongly reduced fertility (Table 3). Phenotyping VirD5 expressing primary transformants thus provided experimental evidence that these promoters are largely inactive during vegetative development and therefore might indeed be meiosis specific. In the case of pZYP1A this would be in accordance with a meiocyte/seedling expression ratio of 17.0 (Table 1 and Table 3), indicating meiosis-specific expression. Furthermore, these data indicated that both pSPO11-1 and pSPO11-2 could be more meiosis-specific than might be expected based on the meiocyte/seedling expression ratios (Table 1). We decided to further analyze the effects of pZYP1A and pSPO11-1 in more detail, because these primary transformants were the most viable and at the same time displayed the most reduced fertility, indicating meiosis specificity.
To further assess meiosis specificity of pZYP1A and pSPO11-1 we stained siliques of representative primary transformants with cottonblue-lactophenol to examine the quality of seed set and the viability of the embryos. Whereas siliques of control plants expressing 1803F-GFP under control of pRPS5A exhibited normal silique development with all positions in the siliques producing viable seeds (Figure 8A), siliques of transformants expressing VirD5 under control of pZYP1A and pSPO11 were mostly much shorter and also contained many fewer viable seeds (Figure 8A). As the flowers did not show any obvious defects, these observations indicated that VirD5 is indeed cytotoxic to meiocytes or to their precursor cells, leading to substantially reduced fertility. In the case of pSPO11-1, loss of fertility was accompanied by substantial heterogeneity in seed size among the T2 seed pool (Figure 8B). Remarkably, most small seeds still germinated and finally produced normal plants. It has to be noted, however, that even though expression of VirD5 under control of pSPO11-1 severely affected fertility, it never completely abolished seed set, indicating that pSPO11-1, even though meiosis-specific, is not a very strong promoter. As an alternative possibility, it might be that interference with meiotic chromosome segregation by VirD5 could still allow for the generation of a small fraction of balanced gametes with 5 chromosomes, just as observed for spo11-1 mutants28. To further address this, we constructed a 2S expression construct (placing expression of GAL4-VP16 under control of pSPO11-1, and transactivating a flanking 4xUAS-minimal35S::virD5 reporter construct) to artificially enhance the activity of pSPO11-1, as we observed for the p2S-RPS5A construct (Figure 8B). Primary transformants expressing VirD5 under control of this 2S construct were only slightly hampered in vegetative rosette development but were fully sterile compared to control plants (Figure 8C). This again corroborated that pSPO11-1 is likely to be a very meiosis-specific promoter with at most low activity in other tissues. To further investigate the cause of the reduced fertility observed in plants harboring pSPO11-1 and pZYP1A, we analyzed pollen production and pollen viability in five different plant lines for each construct. Staining of stage 12 flower buds29,30 showed that pollen production as well as viability were indistinguishable from wild-type plants. Hence, using the promoters mentioned, VirD5 expression did not distort the development of cells prior to the formation of pollen, neither of pollen development itself. It might have been that female gametogenesis was partially distorted, but further experimental exploration of this possibility has not yet been investigated.

(A) Cotton blue staining of siliques of primary transformants expressing VirD5 under control of pZYP1A and pSPO11. Two stained and unstained siliques, respectively, are presented for three individual primary transformants. Siliques of primary transformants harboring the construct pRPS5A::1803F-GFP are presented as a negative control. Scale bars represent 2 mm. (B) Photos of seeds of three independent primary transformants expressing VirD5 under control of pSPO11. Seeds of a representative primary transformant harboring pRPS5A::1803F-GFP are presented as a negative control. The seeds were spread on grid paper, with each square representing 1 mm2. (C) Photos of primary transformants expressing VirD5 under control of the GAL4-based two-step expression system (p2S-RPS5A::1803F-GFP). Primary transformants harboring the construct pRPS5A::1803F-GFP are presented as a control. Scale bar represents 10 mm.
Here, we have described two novel strategies to assess the suitability of portable promoter elements to drive effector protein expression in meiocytes. The first strategy, based on expression of 1803F-GFP fusions under control of promoters of interest, has proven to be a suitable method to investigate such activity in meiocytes in vivo without the requirement of tissue lysis, fixation or staining. The second strategy, based on expression of the cytotoxic Agrobacterium tumefaciens virulence protein VirD5, proved to be an additional and partly complementary tool to gather in planta cues regarding promoter strength and specificity, especially when combined with available gene expression data sets.
The 1803F-GFP strategy allows for the relatively simple introduction of PCR-amplified promoter fragments into the binary vector pRF 1803F-GFP Kana. Visualization of GFP signal in meiocytes can then be performed directly in the T1 generation after floral dip transformation. In addition, an important advantage of this approach over other available approaches is that promoter activity can be studied in vivo without the requirement of disruptive procedures such as lysis, fixation or staining. Finally, the formation of GFP foci localized to the pericentromeric regions of all five chromosomes allows for the discrimination of true GFP signals from autofluorescence as well as for ploidy determination. The 1803F-GFP system could also be combined with other techniques (e.g. chromosome spreading) to draw further conclusions with respect to expression levels or cytology. Of course, just as for any other reporter of gene expression, claims regarding promoter specificity should be interpreted with care. GFP signals in meiocytes could in theory also be due to inheritance of 1803F-GFP transcripts or proteins from progenitor cells.
In the present study we could not detect GFP foci when 1803F-GFP expression was driven by the promoters of ZYP1A, SPO11-2, NBS1, DMC1 and MUS81 (Table 2). These genes are by their function known to be expressed during early prophase I of meiosis I1, but their mRNA levels are relatively low in meiocytes (Table 1). It could therefore be that low and subphase-specific promoter activity is difficult to detect with the 1803F-GFP system. In addition, it is known that meiocytes progress through prophase I of meiosis I relatively quickly32, making the isolation of meiocytes columns which are in a specific subphase of prophase I a very challenging task. The usefulness of the 1803F-GFP reporter system is therefore to some extent limited by the ability to isolate meiocytes in the correct meiotic subphase of interest.
The VirD5 reporter system in principle allows for the screening of promoter elements with tissue-type specific activities without the requirement of any a priori knowledge. Valuable biological cues regarding promoter strength and specificity can already be gathered by phenotyping primary transformants expressing VirD5 under control of a promoter element. In addition, when gene expression data sets are available, the VirD5 reporter system can be used to further verify promoter activity in a tissue type of interest, or to confirm that expression patterns are indeed limited to a specific tissue type. It must be realized that expression of VirD5 in cells that support reproductive tissues could also lead to sterility. Further investigation of promoter activity might thus be required when VirD5 expression is observed to trigger sterility.
The strategy of using VirD5 expression as a dominant negative indicator for cell ablation offers an interesting alternative to the use of other negative selection markers, because it specifically interferes with chromosome segregation19. Its expression is therefore only detrimental to dividing cells, and less likely to be toxic to other types of cells when expressed at low levels. By comparison, the protein synthesis inhibiting diphtheria toxin from the pathogenic bacterium Corynebacterium diphteriae, which is widely used as a potent negative selection marker (e.g. 33–35), is highly toxic to all cells undergoing mRNA translation and therefore lethal to every cell type, also when expressed at low levels. The VirD5 strategy might therefore offer an interesting alternative by excluding lethality due to biologically insignificant levels of transgene expression.
Altogether the data presented here demonstrated that effector protein expression in meiocytes can be visualized in vivo using the 1803F-GFP reporter system, which facilitates the assessment of meiotic promoter activity. The promoters investigated in this study were selected primarily based on published studies on male meiosis6,13, but for future studies the activity and strength of any promoter could in principle be assessed in meiocytes (or other tissue types of interest) using the 1803F-GFP reporter system. In addition, we showed that expression of the Agrobacterium tumefaciens virulence protein VirD5 can be used as a novel negative selection marker and can also provide for experimental cues on tissue specificity and strength of cloned promoter fragments of interest. Combining the 1803F-GFP and VirD5 reporter systems with available gene expression data sets could greatly facilitate the identification of portable promoter elements with desired tissue specificity and/or strength.
All plants in this study were grown on soil in a climate-controlled growth chamber at a constant temperature of 20°C, 70% relative humidity, a light intensity of approximately 150 μmol m-2 s-1 of photosynthetically active radiation from SPAR tubes, and at a 16 h photoperiod. All transgenic plants were generated in the Col-0 background.
The selected promoter sequences (Table 1) were PCR-amplified from genomic DNA of Col-0 plants isolated with the CTAB protocol36, using nested PCR with a gene-specific forward primer (Table 4) and four overlapping reverse primers (three gene-specific and one universal reverse primer; Table 4). The PCR conditions are listed in Table 5. Where possible, PCR primers were designed to amplify the full genomic region between the ATG start codon of the gene of interest and the first or last exon of the adjacent gene (depending on the ORF of that gene lying on the positive or negative strand). Due to a lack of suitable primer target sites, the cloned intergenic regions were 300 bp shorter for At1g75950 (ASK1) and 170 bp shorter for At3g54670 (SMC1). For At1g22260 (ZYP1A), the cloned 2102 bp fragment reached up to At1g22275 (ZYP1B) and included the complete At1g22270 (SMO2) sequence. The DMC1 gene (At3g22880) is separated by 7280 bp from the neighboring At3g22886 (MIR167A) sequence. In this case, we amplified a 3123 bp upstream sequence which includes an 1881 bp LIMPET1 transposable element. The amplified sequence is 177 bp longer than the reportedly active Col-0 sequence used in a previous study7 but might be effectively shorter than the ~3200 bp upstream sequence from the Landsberg erecta ecotype which does not contain the LIMPET1 element37. The forward primer and the universal reverse primer were added to the PCR mix at the final concentration advised by the manufacturer of Phusion High-Fidelity DNA Polymerase (Thermo Fisher Scientific). Reverse primers 3, 2, and 1 were added to 1/5, 1/10, and 1/10 of the final concentration advised by the manufacturer, respectively. In this manner, relatively large PCR products of the promoter sequences fused to a FLAG tag and a nuclear localization signal (NLS) at the 3´ end, flanked by SalI and NotI restriction sites (5´ and 3´ ends, respectively, except for the promoter sequence of SPO11-1, which was flanked by XhoI and NotI sites) could be generated in a single PCR reaction. Although the FLAG tag did not serve a direct purpose in the present study, it can be useful for immunological purposes such as pulldown of meiocyte proteins interacting with FLAG-tagged proteins of interest. The PCR products were ligated into the pJET Blunt cloning vector using the CloneJet PCR Cloning Kit (Thermo Fisher Scientific) and their identity was confirmed by Sanger sequencing (Macrogen Europe).
The genes are listed in locus ID order.
| Step | Temperature (°C) | Time (min:s) | Cycles |
|---|---|---|---|
| Initial denaturation | 98 | 3:00 | 1x |
| Denaturation | 98 | 0:10 | 2-4x |
| Annealing | 68 | 0:20 | |
| Extension | 72 | 1:30 | |
| Denaturation | 98 | 0:10 | 39x |
| Extension | 72 | 1:30 | |
| Final extension | 72 | 5:00 | 1x |
For the construction of pRF 1803F:(GGGGS):GFP Kana, the previously published binary vector pRF EAR Kana16 was taken as a cloning scaffold. The oligo DNA fragments ‘Kpn EAR Sac FW’ and ‘Kpn EAR Sac RV’ (Table 6) were annealed and ligated into KpnI and SacI digested pRF EAR Kana, thereby removing the KpnI site and introducing (among others) XhoI and SpeI sites into the backbone. Subsequently, oligo DNA sequences encoding the (GGGGS)3 flexible linker (GGGGS FW and RV; Table 6) were annealed and ligated into the backbone between the XhoI and SpeI sites, yielding the plasmid pRF (GGGGS)3 Kana. The sequence encoding eGFP was PCR amplified from the plasmid pART738, and subsequently ligated between the former KpnI site and the SacI site, yielding the plasmid pRF (GGGGS)3:GFP Kana. The 1803F encoding sequence20,22 was ligated as a SfiI fragment into SfiI digested pRF (GGGGS)3:GFP Kana, finally yielding the binary vector pRF 1803F:(GGGGS)3:GFP Kana.
The cloned promoter sequences were digested from pJET with SalI and NotI (or XhoI and NotI in the case of pSPO11-1), and were ligated into SalI and NotI digested pRF 1803F:GFP Kana (containing pRPS5A)20. For the construction of a GAL4-based two-step (“2S”) expression system (pRPS5A or any other promoter of choice driving the expression of GAL4-VP16, which transactivates a flanking 4xUAS-minimal35S::1803F:GFP reporter construct), a 1471 bp DNA sequence with key elements essentially as described previously25,26, but directly compatible with our vector systems was synthesized (BaseClear, Leiden, The Netherlands; Figure 9), and subsequently ligated as an EagI fragment into pRF 1803F:GFP Kana predigested with NotI.
For the amplification of the virD5 ORF, genomic DNA of Agrobacterium tumefaciens strain LBA1100 harboring the disarmed octopine-type Ti plasmid pTiB627 was extracted using the DNeasy Blood & Tissue Kit (QIAGEN) according to the manufacturer’s instructions, except that lysis of the cells was performed in TES (10 mM Tris HCl pH 8.0, 50 mM EDTA, 50 mM NaCl) containing 400 μg/ml lysozyme. The virD5 ORF followed by its TGA stop codon and 54 bp of its downstream non-coding sequence was PCR amplified from the genomic DNA of LBA1100 using the forward and reverse primer combination VirD5 NotI FW (5´- ATTAGCGGCCGCTGACAGGAAAGTCGAAAGTTCAC-3´; NotI site underlined) and VirD5 SpeI RV (5´- TAATACTAGTTATCAACCAGCGATCGATGC-3´; SpeI site underlined). The resulting PCR product was ligated into the pJET Blunt cloning vector using the CloneJet PCR Cloning Kit (Thermo Fisher Scientific) and sequenced by Sanger sequencing (Macrogen Europe). Subsequently, the virD5 ORF was ligated as a NotI-SpeI fragment into pRF 1803F:GFP Kana derivatives described above harboring the cloned promoter sequences of MUS81, NBS1, SMC1, SPO11-1, SPO11-2 and ZYP1A, respectively. The relevant restriction sites are depicted in Figure 2. This procedure deleted the 1803F coding domain as well as the adjacent sequence encoding the flexible linker, replacing it by the VirD5 coding region with its own TGA stop codon. Due to the presence of a SpeI site in the cloned promoter sequences of ASK1 and MS5, a similar cloning strategy was followed, except that the virD5 ORF was PCR amplified using VirD5 NotI FW combined with the reverse primer VirD5 SalI RV (5´-TAATGTCGACTATCAACCAGCGATCGATGC-3´; SalI site underlined), and was ligated as a NotI-SalI fragment into NotI-XhoI digested binary vectors harboring the cloned promoter sequences of ASK1 and MS5, respectively. Additionally, in these cases, the virD5 ORF was followed by its TGA stop codon and 54 bp of its downstream non-coding sequence. As a small but functionally irrelevant difference with the other constructs, the short sequence encoding the flexible linker now remained downstream of the virD5 ORF (see position of restriction sites depicted in Figure 2).
Binary vectors were mobilized to the Agrobacterium tumefaciens strain AGL1 through triparental mating39. Col-0 plants were transformed with the constructs using the floral dip method40. The seed pools resulting from floral dip transformation were sterilized and stratified at 4°C for 3-4 days, after which they were plated on MA medium containing 35 μg/ml kanamycin. After one to two weeks of growth on selection medium, kanamycin-resistant seedlings were transferred to soil.
Young, unopened flower buds6 were harvested from the primary transformants harboring promoter::1803F-GFP constructs. For the isolation of male meiocyte columns, anthers were sectioned from the flower buds and placed in a drop of physiological buffer solution (0.9% w/v NaCl, Tris buffered to pH 7.0) on microscope slides. Male meiocyte columns were then released by gently applying slight pressure on the cover slide, thereby also releasing tetrads and pollen into the buffer solution. Chromosome spreads of Col-0 flower tissue were performed as described previously41,42, and subsequently stained with 4’,6-Diamidino-2-phenylindole (DAPI). Confocal fluorescence microscopy was performed using a Zeiss LSM5 exciter (Zeiss, München, Germany) with Illuminator HXP 120V and a standard photomultiplier tube (PMT) detector (maximal gain of 1250). Excitation of the tissue was performed at a wavelength of 488 nm. Red fluorescence was collected as a negative control with a 560 nm long-pass filter; GFP fluorescence was collected with a 505-530 nm band-pass filter, with a master gain of 678 ± 66 (mean ± standard deviation). Images were collected as Z-stacks with a pixel dwell time of 1.7 ± 1.4 s (average ± standard deviation). All images were collected with the same laser power.
The Z-stack confocal images (.lsm files) were transformed into 3D projections using the ‘3D project’ function of the program Fiji (Image J version 1.48f). Representative projections were compressed to 2D images, and brightness of the images was slightly enhanced using Adobe Photoshop CC 2018 (19.1.5 release) for the sake of clarity of the figures. This was done in the same way for all images. For the false color images presented in Figure 6 an overlay of the GFP channel and bright field channel was created in Adobe Photoshop, and opacity was adjusted to visualize the GFP signal inside of the tissue. The images were then merged, inverted and the intensity of black pixels was enhanced to qualitatively visualize foci of fluorescence.
Siliques of primary transformants harboring pRPS5A::1803F-GFP, pZYP1A::virD5 and pSPO11::virD5 constructs were harvested in Carnoy’s fixative (60% ethanol, 30% chloroform and 10% glacial acetic acid, all v/v) and stored at 4 °C until further use. Siliques were then stained with cottonblue-lactophenol/lactophenol as described previously43. The primary transformants were subsequently allowed to further set seeds (T2), which were collected. Seed quality and homogeneity were assessed by collecting images of T2 seeds on grid paper using a LeicaMZ16FA stereomicroscope (Leica, Wetzlar, Germany).
Figshare: Two novel strategies to assess in vivo meiotic protein expression in Arabidopsis thaliana. https://doi.org/10.6084/m9.figshare.780837831.
This project contains raw confocal microscopy images of meiocytes (and other flower bud tissues) isolated from transgenic plants expressing 1803F-GFP under control of the different promoter elements described in this study.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Plant materials and bacterial strains are available from the corresponding author upon reasonable request.
This research was supported by a partnership between Rijk Zwaan and the Dutch Technology Foundation STW (partnership project number 12427), which is the applied science division of NWO, and the Technology Programme of the Ministry of Economic Affairs, Agriculture and Innovation.
We would like to thank Dr. Yinxiang Wang and Prof. Dr. Hong Ma (State Key Laboratory of Genetic Engineering, Institute of Plant Biology, Fudan University, Shanghai, China) for personal communications about promoters of genes described in Yang et al., 201113. We would like to thank Prof. Dr. Hans de Jong (Wageningen UR) for helpful discussions. We would like to thank Shuai Shao for extracting genomic DNA from Agrobacterium strain LBA1100, and Dr. Reza Roushan for help with pollen analysis.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for developing the new method (or application) clearly explained?
Yes
Is the description of the method technically sound?
Partly
Are sufficient details provided to allow replication of the method development and its use by others?
Partly
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
Partly
References
1. Fransz P, De Jong JH, Lysak M, Castiglione MR, et al.: Interphase chromosomes in Arabidopsis are organized as well defined chromocenters from which euchromatin loops emanate.Proc Natl Acad Sci U S A. 2002; 99 (22): 14584-9 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Meiosis, Arabidopsis, cytogenetics, Homologous Recombination
Is the rationale for developing the new method (or application) clearly explained?
Partly
Is the description of the method technically sound?
Partly
Are sufficient details provided to allow replication of the method development and its use by others?
No
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
No
References
1. Armstrong SJ, Franklin FC, Jones GH: Nucleolus-associated telomere clustering and pairing precede meiotic chromosome synapsis in Arabidopsis thaliana.J Cell Sci. 2001; 114 (Pt 23): 4207-17 PubMed AbstractCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Meiosis, Recombination, molecular genetics
Is the rationale for developing the new method (or application) clearly explained?
Yes
Is the description of the method technically sound?
No
Are sufficient details provided to allow replication of the method development and its use by others?
Partly
If any results are presented, are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions about the method and its performance adequately supported by the findings presented in the article?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: meiosis, plant reproduction, cytogenetics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 24 Apr 19 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)