Keywords
Origin of metastasis, Cancer alloustasis, Complementary hypothesis
Origin of metastasis, Cancer alloustasis, Complementary hypothesis
Since the days of the German pathologist Rudolf Virchow (1821–1902), the prevailing view for the origin of cancer metastasis has been the assumption that the anatomic progression of a malignant cell population is a stepwise movement of cells from the primary tumor to the regional lymph nodes and thence to more distant organs. Cancer metastases are believed to be the end result of a multistage process that includes local tissue invasion by primary tumor cells, intravasation into blood vessels or lymphatic system, survival during transit in circulation, arrest within a distant organ, extravasation, survival in the new tissue environment, and proliferation to produce metastatic colonization (Gupta & Massague, 2006).
In line of this hypothesis, Nowell proposed the clonal selection model, where only small sublines of cells within primary tumor acquire genetic permits grow as metastases under stepwise selection (Nowell, 1976). Whereas weiss emphasize on dynamic or compartmental heterogeneity, stating that the primary tumor is composed of a heterogeneous population of cells with metastatic capability, but metastatic phenotype is only a property of a given tumor cells due to epigenetic changes (Weiss, 1990). Other major models of metastatic growth include the clonal dominance model (Kerbel et al., 1988), metastasis gene transfer theory (Garcia-Olmo et al., 2004), the fusion model (Pawelek, 2005; Pawelek & Chakraborty, 2008) and the parallel progression theory (Klein, 2009). All these models share the common belief that metastasis emerge from primary tumor or primary lesion. I nominate these models for the origin of metastasis as the Primary Site Derived hypothesis (PSD hypothesis).
There are several complex biological phenomena that challenge this assumption of metastatic progression. One important clinical observation is of metastatic tumors from an unknown primary source. It is estimated that 2–6% of cancer patients (according to various literature reports) presenting to oncology units have cancer metastases in the absence of an identifiable primary tumor.
In these clinic cases, the patient is considered to have ‘unknown’ or ‘occult’ primary tumor and characterized as unknown primary origin (CUP) or unknown primary tumors (UPT) (Ettinger et al., 2011). The existence of CUP suggests that metastases may not always be derived from primary tumors (Greco, 2014; Oskarsson et al., 2014; Vanharanta & Massague, 2013).
Moreover, several studies show that the formation of metastatic tumors is not definitely rely on the formation of primary tumor. The morphologically normal breast cells from genetically engineered mice were injected into the tail veins of other female mice. The results showed that tumors were formed resulted in the lungs but not in the breast after oncogene induction (Podsypanina et al., 2008). In addition, the cultured metastatic cells may even be detected in the primary organs rather than secondary sites, for example, the kidneys and heart (Friberg & Nystrom, 2015). Another uncertainty is whether metastasis is really the result of cancer cell dissemination to a secondary organ through the bloodstream or lymphatic vessels as circulating tumor cells (CTCs). For example, a study has estimated that the probability of not detecting any CTCs in blood from metastatic breast cancer patients is 0.6 (Shahriyari, 2016). Although this finding may be due to technical issues to detect CTCs in all patients, it may also suggest that other mechanisms may operate to create tumors in other organs.
The above experimental or clinical observations generate a follow-up doubt: Do the tumors in secondary sites develop always from the cancer cells preexisting within a primary tumor or absolutely from premalignant cells originated from primary lesion? It is important to note that our current understanding of the biology of cancer metastasis is strongly influenced by experimental models including in vitro and ex vivo systems, mimic in vivo models developed on Drosophila Melanogaster, zebrafish, fertilized chicken eggs and mices (van Marion et al., 2016). These models contribute to study the tumor progression especially combined with whole body imaging techniques, which enables the early detection of small metastases and track longitudinally their fate in the same animal. However, the clinical observations is frequently not consistent with the metastatic process in the animal models.
In this regard, the ideal model about the origin of secondary tumors should be summarized solely from clinical observation. Clinically, most secondary tumors occur later when the primary tumor is larger. Nevertheless, secondary tumors can occasionally form early in tumor progression when the primary tumor is still small or even undetectable. The parallel progression model (Klein, 2009) explain this phenomena as tumor cells depart the primary lesion before the acquisition of fully malignant phenotypes to undergo somatic progression and metastatic growth at a distant site. This model well illustrates the characteristic biology of early dissemination (Hosseini et al., 2016; Husemann et al., 2008; Klein et al., 2002) while remaining questions are left open. If parallel progression hypothesis is applicable for all secondary tumors, we would expect that the ever-increasing ability to detect secondary tumors at earlier stages should certainly lead to a major reduction in mortality. In fact, despite earlier diagnosis and improved treatment modalities and supportive care, age-adjusted mortality rates have not appeared to decrease.
Cancer is a complex disease and our current understanding of systemic cancer is insufficient. Unfortunately, neither prevailing models about the origin of secondary tumors is supported by direct and incontrovertible evidence. Therefore, I address the possibility that some tumors formed de novo in secondary sites are not coming from the primary tumor or primary lesion. These particular tumors, in a sense, grow up like ‘primary tumors’ and according to their own principles.
I propose a model to describe the origin of cancer metastases in some patients and nominate this model as Own Site Derived hypothesis (OSD hypothesis). The major points that involved into the OSD hypothesis are:
Metastasis is a Greek word meaning ‘displacement’, from μετά, meta, ‘next’, and στάσις, stasis, ‘placement’. It was coined in 1829 by Jean Claude Recamier and is now defined as ‘the transfer of disease from one organ, or part, to another not directly connected to it’ (Fidler & Balch, 1987). Since cancer metastasis is commonly considered as the secondary tumors derived from primary tumor, we create a term ‘cancer alloustasis’ to define the tumors developed from particular cancer cells that are not coming from primary tumor. This idea is inspired by changing μετά, meta, ‘next’ to άλλού, allou, ‘elsewhere’. Accordingly, ‘cancer alloustasis’ means ‘elsewhere placement of tumor’. ‘Alloustasis’ is a singular noun. Its plural, adjective and transitive verb is denoted as alloustases, alloustatic and alloustasize, respectively.
Cancer is a systemic disease. The origin of cancer cells is the result of clonal selection and evolution. It is possible that there are transformed cells arising from both primary and secondary organs before the occurrence of primary tumor. The fate of these progenitors of cancer is determined by their adaptability for the host environment. The tumor that acquires sustainable growth capability will continue to grow as a malignant lesion. Otherwise the transformed cells will maintain their premalignant state or be eliminated by their own microenvironment (Figure 1). According to the OSD hypothesis, the first malignant lesions are defined as primary tumors and the organ where they emerge from is described as primary organ. The transformed cells located into primary organ are distinct with those transformed cells located into secondary sites. Even after the outgrowth of primary tumor(s), the transformed cells in secondary sites do not emerge from a subpopulation of cells present within a primary tumor but rather selected out by their own microenvironment.
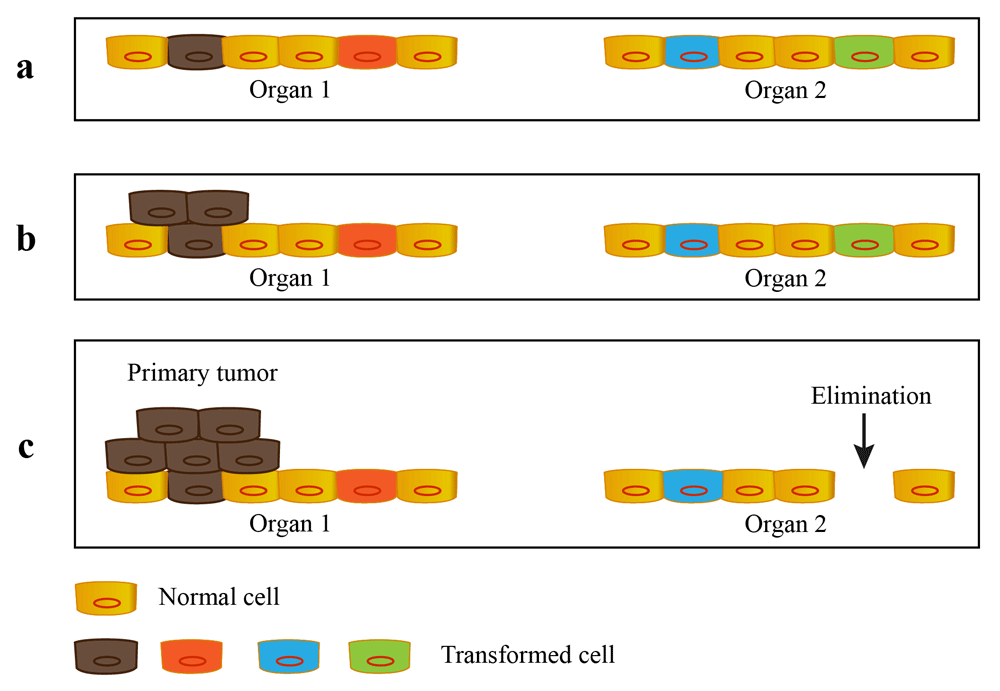
The premalignant cells arise from both primary and secondary organs before the outgrowth of primary tumor. The fate of these progenitors is determined by their adaptability for the host environment and their own microenvironment. a. The transformed cells arise from multiple organs of patient. b. The special transformed cell acquires priority selection. c. The selected transformed cell continues to grow as primary tumor and other transformed cells maintain their premalignant state or are eliminated by their own microenvironment.
The host environment of the patient is dynamic. The successful growth of the primary tumor may remodel the host environment of the patient’s whole body by excreting humoral factors (such as hormones or cytokines) or by an immune response against those tumor cells. It follows that primary tumors change the microenvironment of secondary site and induce the outgrowth of alloustasis which, in the same manner, produces secondary alloustases (so-called ‘alloustasis of alloustases’) (Figure 2). Thus, in a short time, a small primary tumor may produce a cascade of alloustases. This mechanism reveals why cancer exponentially increases the clinical impact to the host and causes severe diseases, for example paraneoplastic syndrome (Finora, 2003; Torrielli et al., 1971) and cachexia (Fearon & Moses, 2002).
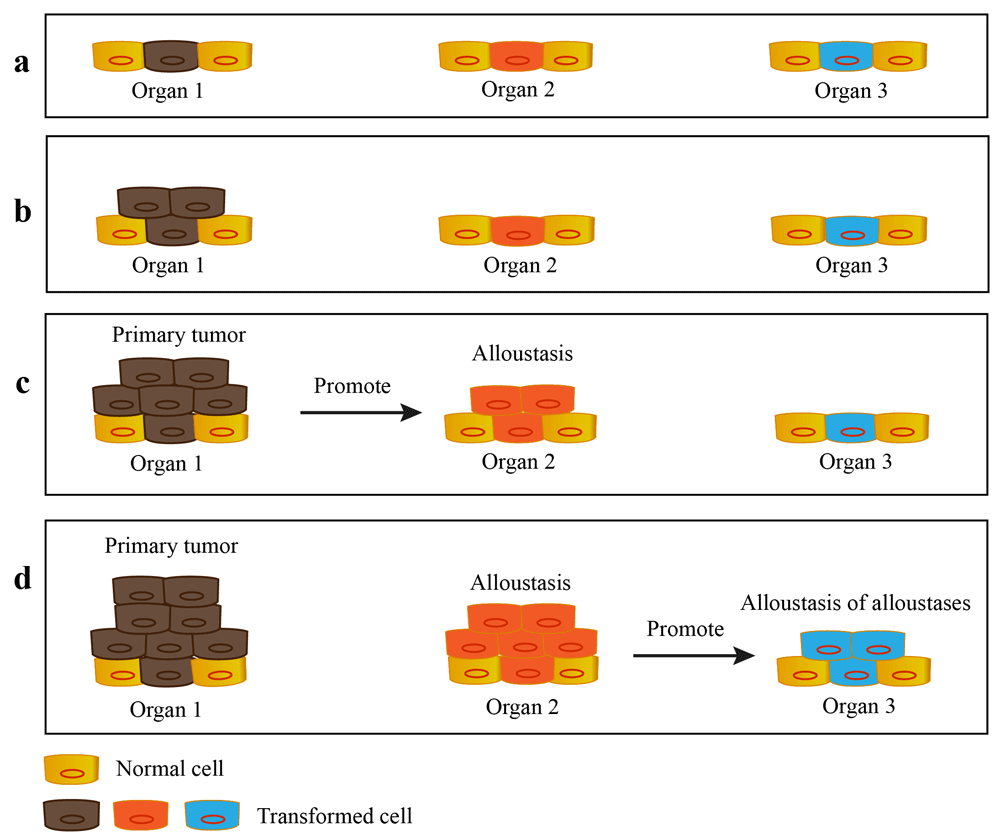
a. The transformed cells arise from multiple organs of the patient. b. The special transformed cell acquires priority selection. c. The selected transformed cell continues to grow as primary tumor. The outgrowth of primary tumor promotes the formation of alloustasis. d. The outgrowth of alloustasis promotes the formation of alloustasis of alloustases.
As I mention before, cancer is a selective and evolutionary process. It is possible that there are multiple transformed cells arising from multiple sites among various organs. These transformed cells will influence the host environment of the whole body and directly or indirectly cooperate with each other to promote the progression of primary tumors. Finally, only part of them acquire the ability to continue growing as malignant lesions (Figure 3) which is observed clinically as a result of a ‘field effect’ (Freireich et al., 2005).
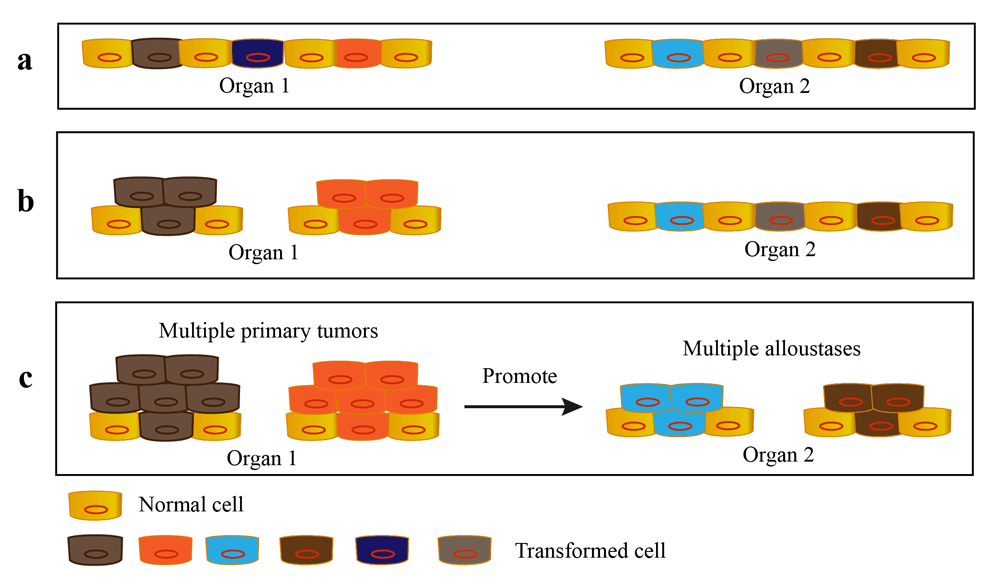
a. The transformed cells arise from multiple sites of different organs. b. Multiple transformed cells into the same organ are selected out and continue to grow as multiple primary tumors. c. The outgrowth of multiple primary tumors promotes the single or multiple alloustasis.
According to the OSD model, the particular pre-cancerous cells are kept evolutionary and equally selected by host environment of the whole body prior to the formation of primary tumor. Moreover, we propose that the growth of malignant lesions (including primary tumors and alloustasis) is dynamically regulated by the fluctuation of host environment in some patient. After the outgrowth of malignant lesions, they may even be destroyed by their local environment (Figure 4). This can be applied for explanation of the CUP behavior and phenomena of regression of human cancers without treatment (spontaneous regression) which is first reported more than 100 years ago and is well documented for many types of cancer, albeit with low frequency (Harada et al., 2010; Kumar et al., 2010, Onuigbo, 2012, Strub et al., 2013).
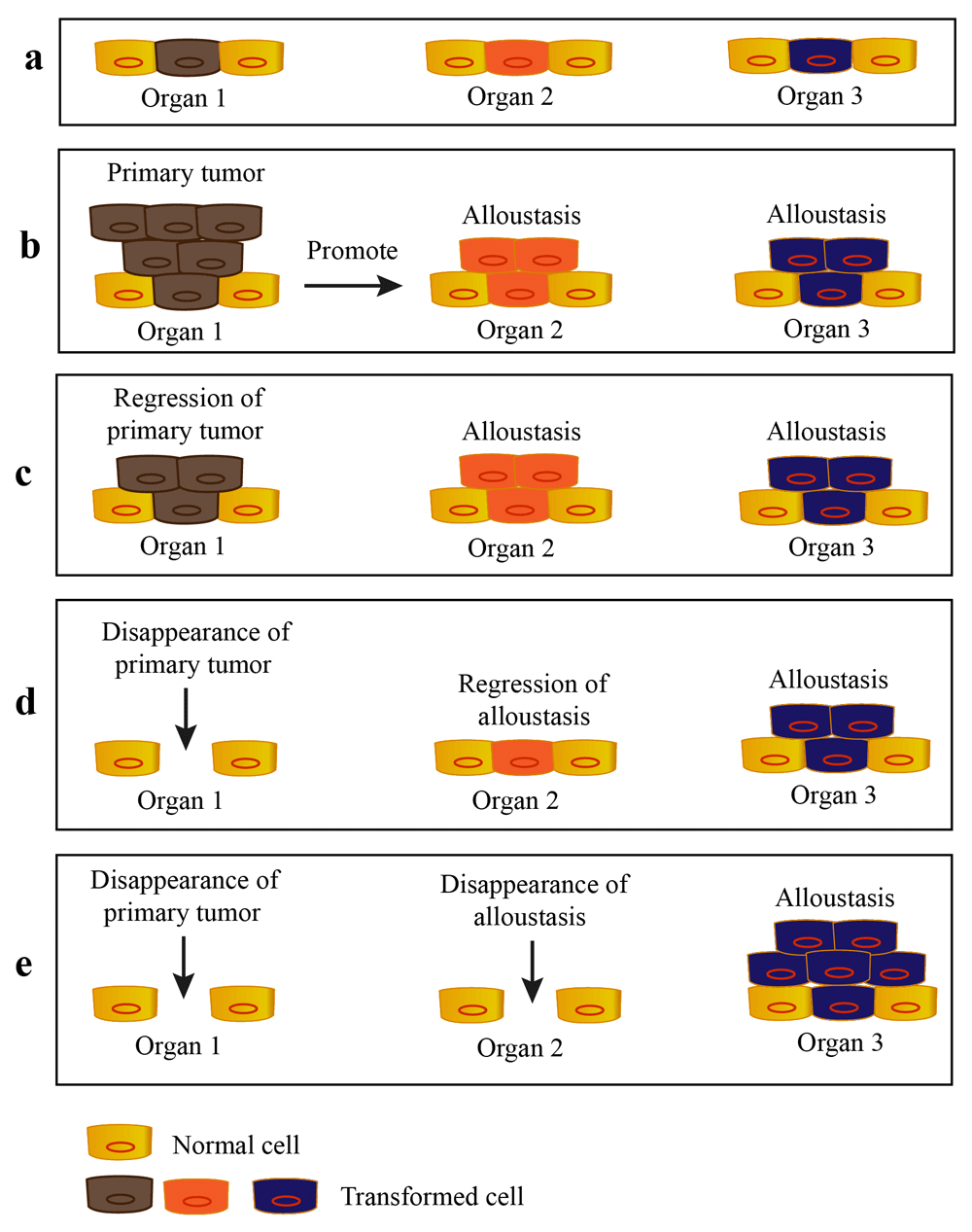
a. The transformed cells arise from multiple organs of patient. b. The outgrowth of primary tumor promotes the formation of alloustasis. c–e. The malignant lesions of patient are dynamically regulated by the host environment and their own microenvironment. The primary tumor and alloustases are gradually eliminated or even promoted by the host.
Here I challenge the prevalent hypothesis of cancer metastasis, and consider that, in some patients, the ancestral cells of secondary tumors do not necessarily have to be cancer cells originating from a primary site. I propose a complementary OSD hypothesis to indicate that some tumors formed in situ into secondary sites (defined as alloustases) resulting from clonal selection and evolution for adapting the dynamic environment (tumor microenvironment and host environment of the whole body).
According to the OSD hypothesis, the relationship of primary tumors and alloustases is mutually independent but interrelated. In a sense, the origin of alloustasis seems like that of a primary tumor. However, the hypothesis highlights that the progenitors of alloustases differ from those of the primary tumors and the progress of alloustasis is promoted by the formation of primary tumor. Meanwhile, the primary tumors and alloustases may exhibit parallel evolution, and they will cooperate or even compete with each other during the progress of cancer.
I also emphasize the central significance of environment. The local microenvironment and systemic host environment of malignant lesions (both primary tumors and alloustases) are not stable but rather very dynamic. Hence the cancer lesions may be diminished or eliminated by the environment. Notably, we consider cancer alloustasis as a systemic disease. In that case, I think it is necessary to treat cancer as a systemic illness. More attention should be focused on systemic treatment and prevent of cancer as they relate to etiology. Perhaps the identification of systemically-acting carcinogens based on the systemically therapeutic strategies might be targeted clinically and would have a substantial impact on overall cancer mortality.
No data is associated with this article.
This work was supported by the National Natural Science Foundation of China (No. 81572879).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the opinion article discussed accurately in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
Partly
Are arguments sufficiently supported by evidence from the published literature?
Partly
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
Partly
References
1. Klein CA: Parallel progression of primary tumours and metastases.Nat Rev Cancer. 9 (4): 302-12 PubMed Abstract | Publisher Full TextCompeting Interests: I am member of the scientific advisory board of HiberCell, New York.
Reviewer Expertise: cancer biology, metastasis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 03 Jan 19 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)