Keywords
Diagnosis related group, DRG, Medicare, inpatient, hospital, reimbursement, payment
Diagnosis related group, DRG, Medicare, inpatient, hospital, reimbursement, payment
The Centers for Medicare and Medicaid Services (CMS) reimburses most inpatient medical and surgical discharges in the U.S. under a capitated framework known as the inpatient prospective payment system (IPPS)1. Upon discharge, a patient is assigned to a medical severity diagnosis-related group (MS-DRG) based on the nature of the patient’s reason for hospitalization as well as any associated complications or comorbidities. Thus, providers are paid a fixed (capitated) rate for inpatient discharges, irrespective of the intensity of the services provided or the specific consumables (drugs or devices) utilized. This stands in contrast to ambulatory settings, where most products and services are reimbursed individually on a line-item basis.
CMS adjusts the payment rates for MS-DRGs annually2. Each MS-DRG is assigned a weight based on providers’ submitted costs, which is intended to reflect the average resources expended to care for a patient in that MS-DRG, independent of region- and institution-specific factors. CMS also defines standardized base payment rates for labor, non-labor, and capital costs, which are also adjusted annually. These weights and standardized rates are used, together with institution-, region-, and case-specific modifiers, to determine a particular hospital’s reimbursement for a discharge under a particular MS-DRG.
Hospital administrators use Medicare base payment rates as a significant input into their decision to adopt new products for inpatient care3, and these rates may also influence manufacturers’ investment activities across various clinical areas. However, we have not observed in the literature a comprehensive examination across MS-DRGs of how these rates have changed over time. In this descriptive study, we analyzed base payment rates from 2009 to 2018 to explore the extent to which reimbursement for individual MS-DRGs has (or has not) kept pace with inflation during this period.
We identified all MS-DRGs in continuous existence from fiscal year 2009 (the first full year after the transition to severity-based coding and cost-based reimbursement was completed4) to fiscal year 2018, and assigned them to major diagnostic categories (MDCs), using CMS definitions and annual IPPS “Final Rule” data tables (e.g., these data from fiscal year [FY]2018). For years in which correction notices were issued, we used the latest (corrected) data. In order to focus on MS-DRGs with the largest clinical and budgetary relevance, we limited our analysis to those with over $100 million in total Medicare spending in 2015 (per CMS data). Our final analysis set was comprised of 211 MS-DRGs (of the 761 MS-DRGs in existence in 2019), distributed across 21 MDCs (including “pre-MDC” and unassigned/unclassified). Our complete data file is available as Extended data.
For each year, we used available data provided by CMS to construct a “base reimbursement rate” for each MS-DRG, adapting methods described elsewhere5. We first obtained the weight of each MS-DRG from that year’s Final Rule tables (e.g., Table 5 in the FY2018 document). We then calculated the sum of the standardized labor- and non-labor-related amounts (Table 1A of the FY2018 document, “meaningful use” data), plus the capital amount (Table 1D of the FY2018 document) for that year. We then multiplied this total dollar amount by the MS-DRG’s weight to obtain a value for the base reimbursement rate in nominal dollars.
We adjusted each MS-DRG’s base reimbursement rate from nominal dollars to constant (January 2009) dollars by using the CPI-U calculator provided by the Bureau of Labor Statistics.
For each MS-DRG, we determined the “best fit” compound annual growth rate (CAGR) (r in the best-fit exponential line equation [y = a(1 + r)x]), which we refer to as the “best-fit CAGR” in the remainder of this article. Compared with standard CAGR calculations, which use just the starting and ending reimbursement rates, this approach takes into account all of the observed values over the time period. We also calculated each MS-DRG’s “expected” reimbursement rate by year, based on the derived slope and intercept of its best-fit line, in order to calculate the coefficient of determination (R2) between the calculated best-fit exponential equation values and the observed reimbursement rates.
We examined differences in best-fit CAGR between subsets of MS-DRGs with the Kruskal-Wallis test (on MDCs containing five or more MS-DRGs), and confirmed statistically significant results with post hoc pairwise comparisons (Dunn test with Bonferroni correction). All mathematical calculations and statistical analyses were performed using Microsoft Excel (see here and here).
From FY2009 to FY2018, inflation-adjusted MS-DRG base reimbursement rates had a median best-fit CAGR of -0.26% (interquartile range [IQR], -0.89% to 0.47%) (Table 1)a. Over the analyzed time period, 59.2% (125/211) of MS-DRGs had negative best-fit CAGRs. In bivariate models, the variability in the best-fit CAGRs of individual MS-DRGs was not explained by either the 2015 Medicare spending level or 2018 inflation-adjusted base reimbursement rate (Figure 1; R2 < 0.02 for each).
Major diagnostic categories (MDCs) containing fewer than five medical severity diagnosis-related groups (MS-DRGs) are not independently reported or analyzed.
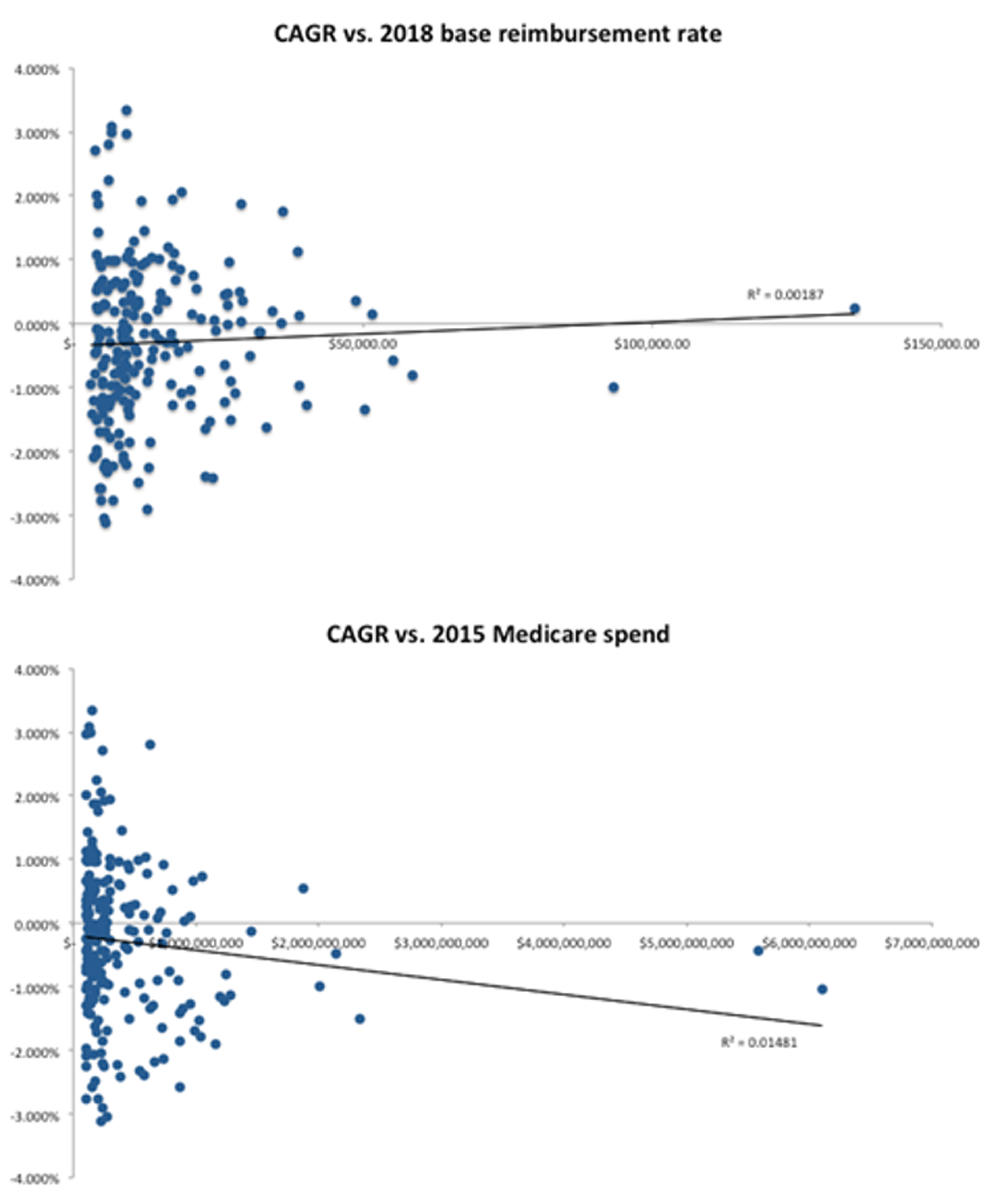
Each circle represents one medical severity diagnosis-related group. Best-fit line with R2 is shown in each scatter plot.
Medical MS-DRGs exhibited a more negative median best-fit CAGR than surgical ones (-0.57% vs. -0.14%; adjusted H=5.631, df=1, p=0.018 via Kruskal-Wallace). A total of 66% of medical MS-DRGs exhibited negative best-fit CAGRs (71/108), compared with 52% (54/103) of surgical ones.
In total, 11 MDCs were comprised of five or more MS-DRGs, and thus were suitable for further analysis. We found a statistically significant difference in median best-fit CAGRs between MDCs (adjusted H=63.128, df=10, p=9.23 × 10-10 via Kruskal-Wallace). Upon pairwise analysis (Dunn with Bonferroni correction), we determined that the best-fit CAGRs of MS-DRGs for musculoskeletal diagnoses (MDC 08) were statistically significantly larger than that of MS-DRGs for nervous, respiratory, digestive, or infectious diseases (MDCs 01, 04, 06, and 18, respectively) (Table 2).
Corrected P values for post hoc pairwise comparisons of growth rates for best-fit compound annual growth rates by MDC. Statistically significant results (P<0.05) indicated in italics.
One plausible explanation for differences in median best-fit CAGRs could be the relative fraction of surgical MS-DRGs (which have higher growth) in different therapeutic areas; indeed, the musculoskeletal MDC is substantially enriched for surgical MS-DRGs (25/29 (86%), compared with 103/211 (49%) in the overall sample). Thus, we re-analyzed median best-fit CAGRs across MDCs after first separating medical MS-DRGs from surgical ones. There were eight MDCs comprised of 89 surgical MS-DRGs, and seven MDCs comprised of 84 medical MS-DRGs, suitable for analysis (i.e., comprised of five or more MS-DRGs).
Looking only at surgical MS-DRGs, we found a statistically significant difference in median best-fit CAGRs between MDCs (adjusted H=32.465, df=7, p=3.3 × 10-5 by Kruskal-Wallace), and on pairwise analysis, we confirmed that the median best-fit CAGR for surgical MS-DRGs for musculoskeletal diagnoses (MDC 08) was statistically significantly larger than that for digestive and infectious ones (MDCs 06 and 18, respectively) (Table 3). Looking only at medical MS-DRGs, we failed to identify a statistically significant difference in median best-fit CAGRs between MDCs (adjusted H=11.129, df=6, p=0.084 by Kruskal-Wallace).
Corrected P values for post hoc pairwise comparisons of growth rates for best-fit compound annual growth rates by MDC. Statistically significant results (P<0.05) indicated in italics.
| MDC 04 | MDC 05 | MDC 06 | MDC 07 | MDC 08 | MDC 11 | MDC 18 | |
|---|---|---|---|---|---|---|---|
| MDC 01 | 6.8 | 17 | 3.9 | 21 | 1.6 | 7.9 | 1.2 |
| MDC 04 | 1.8 | 25 | 12 | 0.063 | 1.0 | 12 | |
| MDC 05 | 0.40 | 11 | 0.92 | 11 | 0.11 | ||
| MDC 06 | 8.8 | 0.0028 | 0.33 | 13 | |||
| MDC 07 | 1.2 | 5.4 | 3.2 | ||||
| MDC 08 | 18 | 0.0012 | |||||
| MDC 11 | 0.089 |
Under the IPPS, Medicare annually adjusts base payment rates for inpatient discharges based on providers’ reported costs. In this descriptive study, we observed that the majority of MS-DRG base payment rates have failed to keep pace with inflation. We also observed that reimbursement rates for medical discharges have declined more rapidly than those for surgical ones, and within surgery, reimbursement for orthopedics-related discharges has grown more rapidly than reimbursement for gastrointestinal or infectious disease discharges.
The IPPS is intended to contain health care spending by encouraging hospitals to reduce costs and increase efficiency, and it has been largely effective at achieving that aim6. That success is a double-edged sword, however. Capitated payments encourage hospitals to adopt new technologies that boost efficiency or directly enable overall cost savings, and discourage them from purchasing those that accomplish neither of these aims; however, that latter group may include products that improve patient care in ways that are not clearly reflected on institutions’ financial balance sheets, while increasing hospitals’ costs7–9. The extent to which declining reimbursement rates for many MS-DRGs further exacerbates these behaviors warrants further study.
Federal programs and policies exist to help offset expenses that exceed MS-DRG payments, but available evidence suggests their impact is limited10. Medicare’s new technology add-on program (NTAP) is only available for products that meet stringent criteria for economic and clinical impact, and only 19 drugs and devices in total were approved for supplemental payments through this mechanism from 2001 to 201511. Thus, in many cases, particularly for technologies with an intermediate level of added cost and benefit, increased expenses for inpatient discharges are borne by providers without additional compensation beyond that derived from the MS-DRG reimbursement formula.
In addition to affecting hospitals’ behavior, declining reimbursement rates may reduce the economic incentive to develop new drugs or devices for inpatient care. Numerous empirical economic studies (including those cited here and here) in the pharmaceutical industry have shown a positive relationship between R&D spending (and output) and market opportunity size12–15. As noted above, this phenomenon may be particularly significant for new products that provide benefits to patients and hospitals that are difficult to quantify, as well as those that fail to meet the clinical and financial criteria to quality for add-on payments. Further work, building on the descriptive analysis presented here, is warranted to examine in more detail the relationship between new product development for inpatient care and reimbursement growth rates.
An important consideration regarding the interpretation of MS-DRG payment trends relates to the distinction between gross (top-line) reimbursement, which is measured in this study, and net (bottom line) profitability. It is entirely possible that some MS-DRGs with flat or declining reimbursement can still be highly profitable compared to others with increasing payment levels, if hospital costs can be disproportionately reduced. In theory, profitability, not reimbursement, should dictate the attractiveness of developing and purchasing drugs and devices for inpatient use under a given MS-DRG. In practice, however, we believe payment levels do, in fact, influence the perceptions—and, ultimately, the behaviors—of manufacturers and hospitals. Anecdotal evidence suggests that hospitals rarely have insight into the profitability of specific inpatient procedures (illustrated here and here), and often base purchasing decisions on payment levels16. Similarly, in our professional experience with medical device executives, we have found that calculations of reimbursement rate trends like the ones performed here are a key component of investment decisions around developing and commercializing inpatient care products.
This work has some additional limitations. First, the base reimbursement rates for each MS-DRG we determined do not precisely reflect the amounts that particular institutions receive, due to adjustments for factors like low socioeconomic status in a hospital’s catchment area and the institution’s involvement in graduate medical education. Thus, a specific hospital may experience different MS-DRG reimbursement rates and growth trends than the ones calculated here. Second, reimbursement rates by private payers may not follow exactly the same trends as those described here. Non-Federal payers typically reimburse at rates above Medicare17, but trends for individual MS-DRGs have not been comprehensively examined. Third, since 2015, some Medicare providers have opted to participate in bundled payment programs for inpatient and post-hospitalization care in some clinical areas instead of the IPPS, so their reimbursement rates for some MS-DRGs may not be accurately reflected by the data analyzed here. This is particularly relevant for surgical MS-DRGs for musculoskeletal discharges (MDC 08), for which we observed larger reimbursement growth than other surgical MS-DRGs, because this MDC includes many procedures that are now covered by bundled payments. In summary, although the data presented here are generally applicable, the experience of a particular hospital depends on factors specific to its payer mix, geography, and other factors.
Notwithstanding its limitations, our analysis is the first to calculate trends in inflation-adjusted Medicare base reimbursement rates for inpatient discharges across individual MS-DRGs. We show here that since 2009, these reimbursement rates have declined for a substantial fraction of MS-DRGs, particularly for medical discharges as compared with surgical ones, and that reimbursement for musculoskeletal discharges appears to have been relatively spared compared with some other surgical domains. To the extent that manufacturers and hospitals use these rates to guide their investment decisions, the IPPS may unintentionally promote or disincentivize innovation and commercial uptake in specific clinical areas. The relationship between these reimbursement trends, new product development, and market adoption in inpatient care warrants further study.
MS-DRG weights, standardized reimbursement amounts, and 2015 total Medicare payments by MS-DRG were obtained from Final Rule tables for 2009-2018 from the website of the Center for Medicare and Medicaid Services (CMS).
These data are publicly available from CMS, without any restrictions.
Open Science Framework: MS-DRG data and analysis. https://doi.org/10.17605/OSF.IO/FKMYA.
This project contains Supplemental File 1: “MS-DRG data and analysis”
Extended data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
We thank Julie Lin for helpful comments, and Emily Villas for assistance with data collection.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Health economics, health care payment reform.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Evaluating Medicare’s various PPS policies
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 30 Apr 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)