Keywords
Colon, schwannoma, colonic neoplasms, colectomy
Colon, schwannoma, colonic neoplasms, colectomy
Schwannomas are a common type of tumor of peripheral nerve in adults which originate in Schwann cells. These tumors mainly present along the peripheral nerves and are rarely identified in the gastrointestinal (GI) tract1,2. GI tract schwannomas develop most frequently in the stomach (83%), and less frequently in the small intestine (12%), colon and rectum3. Sigmoid colon schwannomas are very rare and only 28 cases have been reported so far3,4. Most colon schwannomas are incidentally identified as submucosal tumors on screening colonoscopy1,5. Colonoscopic biopsies alone usually provides limited information and definite diagnosis is made after surgical resection1. Most of the colon schwannomas are benign and surgical resection with adequate free resection margins is the treatment of choice6. Here we present a case of sigmoid colon schwannoma and discuss the clinical features of the disease with a literature review.
A 66-year-old Asian female patient, who was a housewife, visited a local clinic for a routine screening colonoscopy in mid-January 2018. During the colonoscopy, a submucosal tumor sized about 4cm was identified at the sigmoid colon (Figure 1) and biopsy was performed. The microscopic exam of the biopsied specimen showed an ulcerated lesion with a proliferation of fibroblast-like spindle cells beneath the ulcer, which was insufficient for a definite diagnosis.
The patient was referred to our hospital at the end of January 2018. She presented no specific symptom and physical examination showed no specific finding. She had a history of hypertension and a benign breast mass. She had a positive family history of cancer: her father had gastric cancer, and her uncle had lung cancer.
An abdominopelvic computerized tomography (CT) scan revealed 4.4cm sized well-circumscribed, well-enhanced, round-shaped mass in the sigmoid colon, which was slightly heterogenous inside. No intraabdominal metastasis was identified (Figure 2A–2B). Chest CT scan showed no intrathoracic metastasis. Carcinoembryonic antigen (CEA) level was 1.33ng/mL, which was within a normal range (0.0–5.0ng/mL). Other laboratory test results were also within normal ranges. Differential diagnosis was 1) gastrointestinal stromal tumor (GIST); and 2) neuroendocrine tumor (NET).
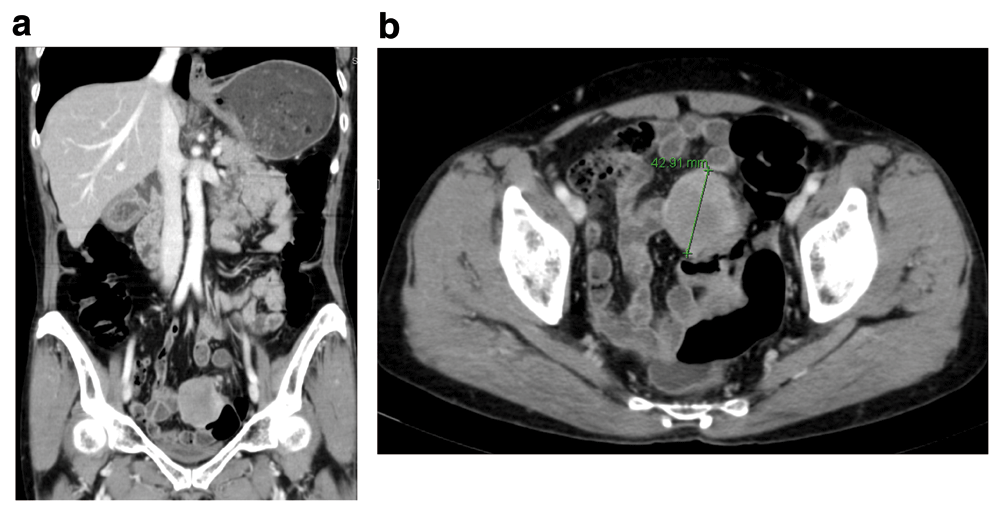
A well-circumscribed, well-enhanced, round-shaped mass was identified at the sigmoid colon. (A) Coronal view. (B) Axial view.
The patient underwent a laparoscopic anterior resection. On laparoscopic exploration, an extruding mass was identified at the anterior wall of the sigmoid colon and no metastasis was observed. The sigmoid colon was mobilized and the inferior mesenteric artery was low ligated. Sigmoid colon resection with end-to-end anastomosis was performed.
On examining the resected specimen, about 4.5 × 4.0cm sized round mass was observed on the surface of the serosa and there was no tumor infiltration to the serosa (Figure 3A–3B). The tumor was located 7cm from the proximal resection margin and 4cm from the distal resection margin. On sections after fixation, the cut surface showed a yellowish mass (4.2×3.2cm), which was abutting on the circumferential resection margin. The mass was relatively well-demarcated without encapsulation (Figure 4). On hematoxylin and eosin (H&E) stain, the tumor was composed of spindle cells with low nuclear atypia, with nuclear palisading growth pattern, and lymphoid cuffing surrounding tumor cells were identified (Figure 5A–5C). Mitosis was rarely observed (1/50 in high-power field). The remaining mucosa and serosa were grossly unremarkable. The resection margins were free from tumor. Lymph node metastasis was zero in 13 regional lymph nodes. On immunohistochemical analysis, s-100 was strongly positive in tumor cells; otherwise, c-kit, CD34, and SMA were negative (Figure 6A–6D). Finally, the diagnosis was a benign schwannoma of the sigmoid colon.
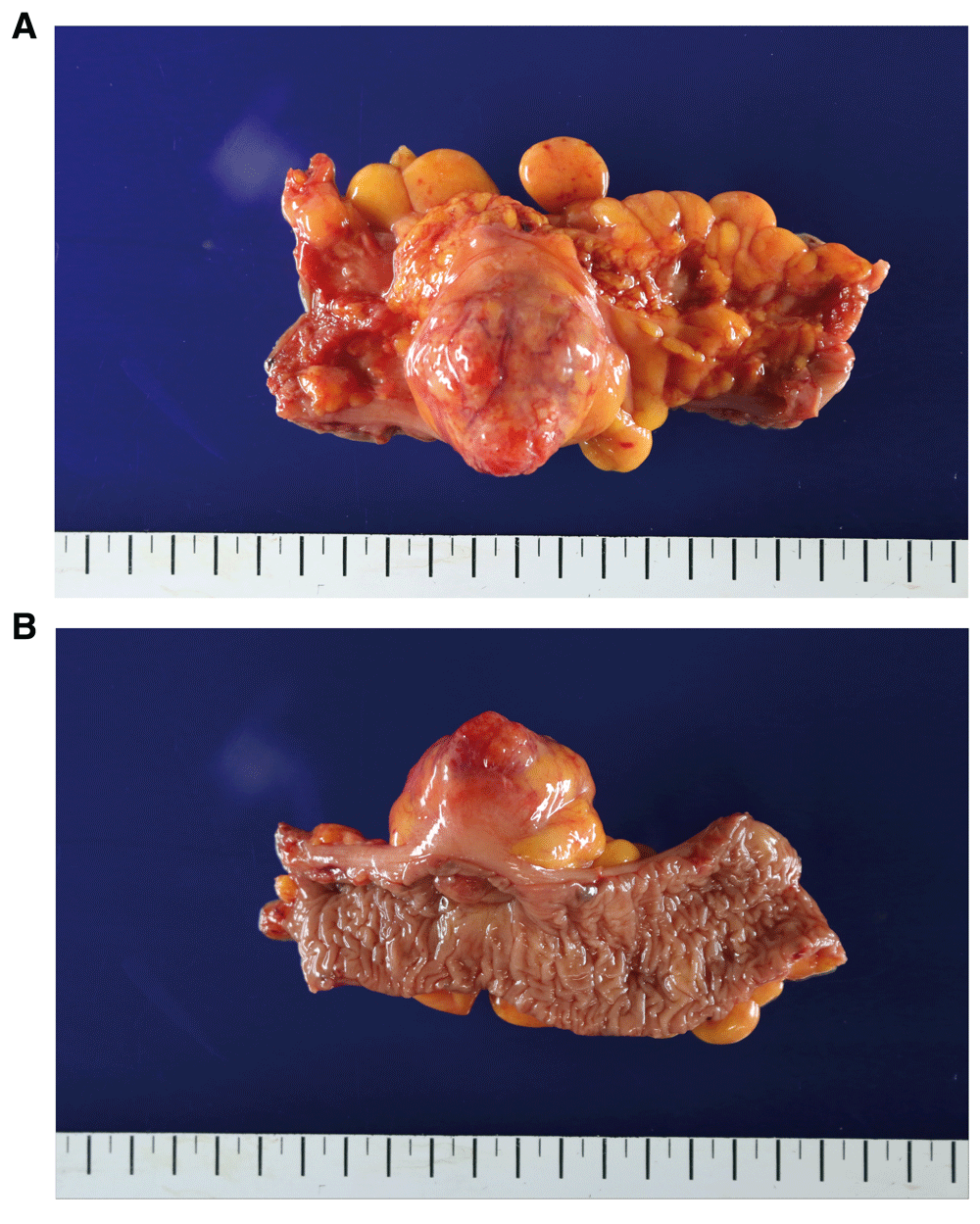
(A) 4.5 × 4.0cm sized round, protruding mass was observed on the surface of the serosa. (B) The photo was taken from the mucosal side.
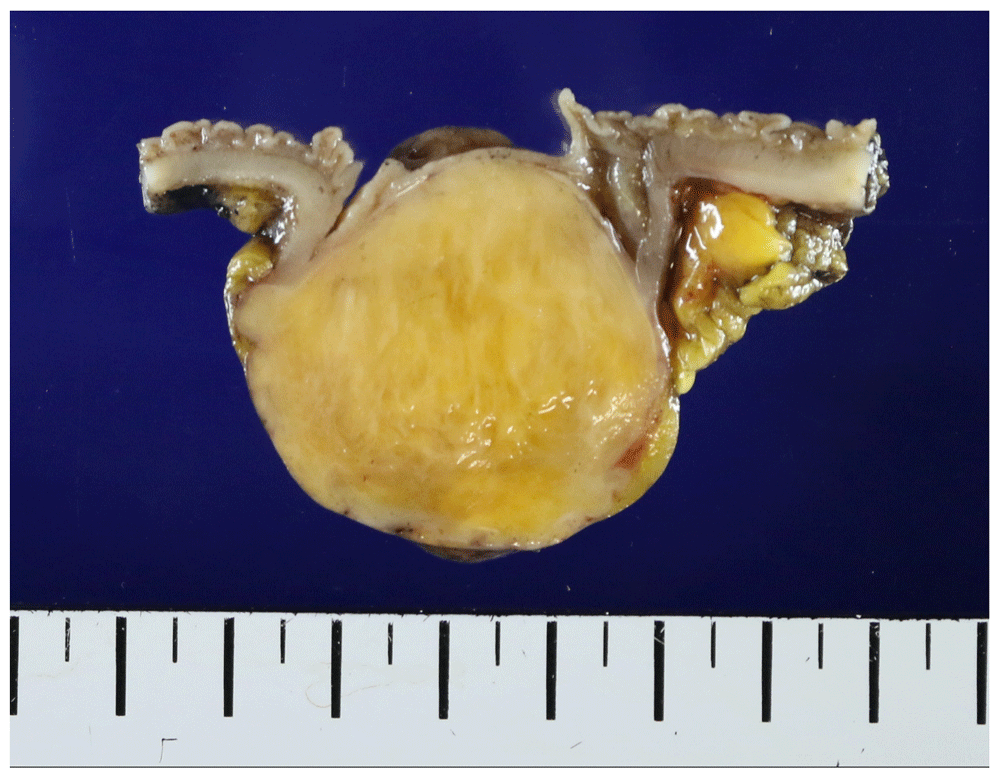
Relatively well-demarcated yellowish mass without encapsulation is shown.
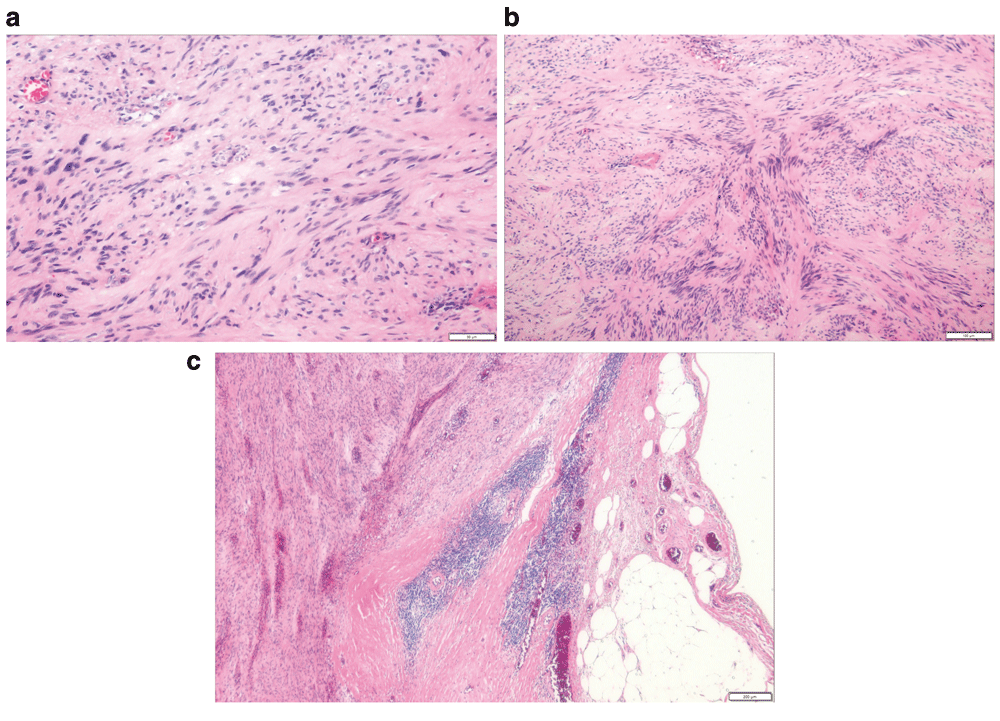
(A) The tumor cells are composed of spindle cells with low nuclear atypia. (B) Nuclear palisading growth pattern is shown. (C) Lymphoid cuffing surrounding tumor cells is shown.
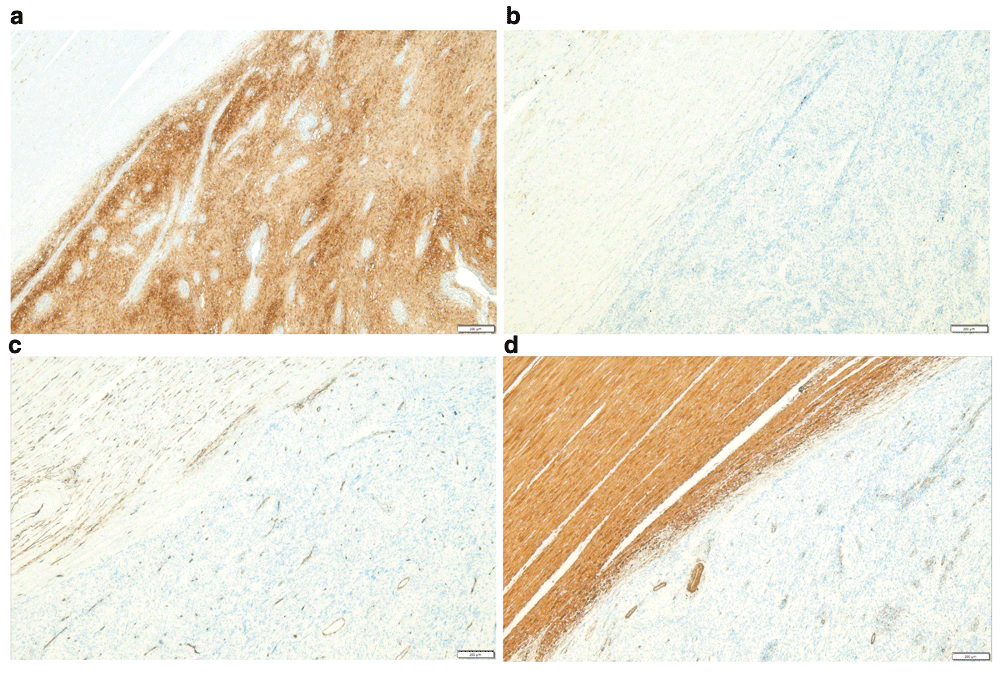
(A) S-100 is diffuse, strong positive in tumor cells. (B) C-KIT is negative in tumor cells. (C) CD34 is negative in tumor cell, but normal vessel structures were stained. (D) SMA is negative in tumor cells, but normal smooth muscle in the proper muscle layer is stained.
The patient recovered from surgery uneventfully and was discharged on postoperative day 5. When she visited the out-patient clinic two weeks after discharge, she did not present any complication. No postoperative adjuvant therapy was performed.
Schwannomas are peripheral nerve sheath tumors which rarely develop in GI tract1,2. GI tract schwannomas represent about 2–6% of all mesenchymal tumors2,3.
For the first time, Daimaru et al. clarified the entity of the nerve sheath tumors developing in the GI tract and proposed these tumors to be designated as “benign schwannoma of the GI tract” in 19887. Lymphoid cuffing, benign nuclear atypia and positive immunostaining for S-100 protein were the distinct features of the schwannoma of the GI tract, which distinguish the schwannoma from other spindle-cell stromal tumors of smooth muscle origin7. Until the early 1990s, most GISTs traditionally had been classified as smooth muscle tumors8. Ueyama et al. suggested that most of the GIST had smooth muscle differentiation and excluded schwannomas from the GIST8.
Currently, GI tract schwannomas are classified as non-epithelial tumors of which disease entity is clearly distinct from leiomyomas, leiomyosarcomas, gastrointestinal autonomic nerve tumors (GANTs) and GISTs2,9. And GI schwannomas are considered distinguished from conventional soft-tissue schwannomas and CNS schwannomas2.
GI schwannomas are mostly identified in the stomach and less frequently seen in colon, rectum, small intestine or esophagus2,3. They are most frequently diagnosed among people in their sixties and the incidence rates are identical for males and females3,9,10. Most of them are incidentally identified during screening endoscopy or imaging studies because they are usually asymptomatic. However, just like any other GI tumors, they can present some clinical symptoms such as abdominal pain, tenesmus, rectal bleeding or melena1. Sometimes these tumors manifest as colonic obstruction or intussusception4,9,10.
Endoscopically, these tumors usually present as a submucosal tumor with smooth mucosa or with mucosal ulceration1–3. On CT scans, the tumors usually present as exophytic masses with homogeneous enhancement and cystic change, necrosis, or calcification within tumors are uncommon2.
A preoperative diagnosis is challenging because endoscopic mucosal biopsy usually provides limited information to differentiate them from other mesenchymal tumors of GI tract such as GISTs, NETs, leiomyomas, or leiomyosarcomas3. In Inagawa’s study, only 15% of the colon schwannomas were diagnosed on preoperative endoscopic biopsy11; in Bohlok’s study, 24% of the colorectal schwannomas were diagnosed preoperatively3.
Diagnosis is confirmed pathologically with immunohistochemical analysis. Histopathological features of schwannomas are mainly elongated bipolar spindle cells with variable cellularity and sometimes peripheral cuff-like lymphocyte infiltration is exhibited around the tumor, which helps to differentiate schwannomas from other spindle-cell tumors like fibromas or leiomyomas5,7,11. Schwannomas can be distinguished from other smooth-muscle tumors by strong s-100 positivity in immunohistochemical analysis11–13. Additionally, CD34 or c-kit protein is useful to distinguish the schwannomas from GISTs11–13. Schwannomas are S-100 positive, but CD-34 and c-kit negative; most GISTs are s-100 negative, but CD-34 and c-kit positive.
Prognosis is generally promising because most of the GI schwannomas are benign and malignant potential is low1–3. However, even though many researchers reported the benign features of GI schwannoma, some of these tumors present local recurrence or distant metastasis. In Bohlok’s study, 3 (3.1%) out of 93 cases of colorectal schwannomas were malignant3. High mitosis rate, high Ki-67 index, and large tumor size are considered to be associated with malignancy3.
Complete surgical resection obtaining free resection margins is the best therapeutic option1,3, because tumor recurrence is generally owing to incomplete surgical resection with inadequate margins2. In some limited cases, patients can be treated by endoscopic resection or transanal resection without undergoing radical surgery3.
Adjuvant therapies are not commonly recommended if surgical resection achieving free margins is completed5,14. Currently, limitation of our knowledge is that there is no consensus for subsequent treatment after surgical resection in case of malignant transformation5.
Sigmoid colon schwannomas are very rare colonic neoplasms. To our knowledge, only 28 cases of sigmoid colon schwannomas have been published. Because of its rarity and challenge to diagnosis, review of the clinical features of the disease with a presenting case would be of help for physicians and surgeons. We believe that our study presents the clinical manifestations including endoscopic, imaging, histopathologic and immunohistologic findings of this rare disease with a thorough literature review and it provides guidance in diagnosis and treatment of the disease. Limitation of this study is that the treatment strategy for metastatic diseases was not suggested because only very limited cases were reported and no consensus exists for now.
Sigmoid colon schwannomas are usually found incidentally during screening colonoscopy and present as submucosal tumors. Preoperative diagnosis is challenging because clinical manifestations, as well as colonoscopic and CT findings, are nonspecific. No specific tumor marker exists either. Histopathologically, the tumors consist of spindle cells. However, colonoscopic biopsies have limitations in terms of a definite diagnosis and differential diagnosis includes schwannoma, GIST, NET, leiomyoma, leiomyosarcoma, etc. Conclusive diagnosis can be made by confirming s-100 proteins in immunohistochemical analysis and mostly confirmed post-surgically. Complete surgical resections with adequate free margins are required because although the majority of the diseases are benign, some are reported to be malignant. There is no consensus for adjuvant chemotherapy.
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Breast cancer, thyroid cancer, endoscopy, ERCP
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Gastrointestinal Pathology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 13 May 19 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)