Keywords
Second-order visual features, BDNF gene, COMT gene, HTR2A gene, visual scenes, visual perception, emotions, event-related potentials, emotional assessment
Second-order visual features, BDNF gene, COMT gene, HTR2A gene, visual scenes, visual perception, emotions, event-related potentials, emotional assessment
Despite the fact that at this stage of evolution, the human visual system is the main channel for receiving information that performs complex processes of encoding and decoding stimuli, the mechanisms for recognizing and categorizing visual images still remain under-investigated (Babenko et al., 2011; Graham, 2011; Wilson & Gelb, 1984). In particular, second-order visual features – non-local characteristics of the perceived image, which including various options for contrast, orientation, and spatial frequency – are of special interest to human’s visual system researchers (Babenko et al., 2011; Babenko et al., 2016) These features are image regions characterized by spatial heterogeneities of brightness gradients – the first-order characteristics. These heterogeneities are distinguished by the so-called second-order filters, which detect regions with spatial modulations of contrast, orientation, and spatial frequency in the images. First-order visual mechanisms form the basis for subsequent spatial grouping. This is a multi-channel system that performs local analysis of the orientation of the brightness gradients at various spatial frequencies. Second-order visual mechanisms detect spatial changes and represent a certain set of filters.
A number of experimental studies demonstrated that second-order visual mechanisms are selective towards modulations of contrast, orientation (surface curvature) and spatial frequency (depth) and have different cortical localization. The search for second-order stimuli is pre-attentive (Yavna & Babenko, 2012; Yavna et al., 2012).
Second-order features are known to play an important role in identifying images. The studies aimed at studying the role of second-order visual mechanisms in the recognition of emotional stimuli (especially scenes) are of particular importance today. Scenes are complex visual stimuli. Compared to the mechanisms of visual perception of faces and objects, little attention has been devoted to studying the brain mechanisms underlying processing of scenes (Aftanas et al., 2001; Codispoti et al., 2007; Delplanque et al., 2004; Mavratzakis et al., 2016; Olofsson et al., 2008; Pegna et al., 2004). Due to the fact that the human visual system often recognizes the scenes as combinations of images that include various elements, the study of these mechanisms can help us to clarify our understanding of the process of visual perception in vivo.
At the same time, we should take into account the influence of individual characteristics on the processes of visual perception. These may include psychological and biological characteristics. The genetic factors in the individualization of visual perception, i.e. genes that can affect the mechanisms of visual perception of images (including emotional scenes) are the catechol-o-methyltransferase (COMT) gene, the brain derived neurotrophic factor (BDNF) gene, the serotonin receptor gene (HTR2A), etc. In the science periodicals numerous studies examine the associations between these genes and individual psychological characteristics, including emotional intelligence, aggression, anxiety, and neuroplasticity and describe neurochemical processes characteristic to carriers of various genotypes. However, the influence of genes on the processes of visual perception, including the mechanisms for recognizing and evaluating (appraising) emotional visual stimuli (scenes) with the distinct second-order features are poorly described. This fact determined the topic of the present investigation. Let us now consider in more detail the functions of these genes.
BDNF is a key molecule involved in the plastic changes associated with learning and memory. The BDNF stimulates the growth of neurons, axons, and dendrites, the formation of synapses and other processes of neuroplasticity not only in early ontogenesis, but also in the brain of the adult organism (Antal et al., 2010). Changes in BDNF expression in the brain structures involved in memory functions and perception processes (Miranda et al., 2019) are associated with human normal and pathological aging, as well as with mental illness. Together with other neurotrophic factors, BDNF plays an important role in the processes of adaptation of brain neurons to various external impacts influences, as well as in their growth, differentiation, and survival (Popova et al., 2017).
It is established that visual experience in the early postnatal period affects the maturation of the visual cortex. Furthermore, it was investigated the characteristics of maturation and plasticity of the visual cortex in mice with elevated level of BDNF (Huang et al., 1999). Under experimental conditions of visual deprivation, the authors found that BDNF overexpression is sufficient for the development of normal activity of the visual cortex in the absence of visual experience. Meanwhile, a decrease in BDNF expression slows down the development of visual areas of the cerebral cortex under conditions of visual deprivation (Gianfranceschi et al., 2003).
Carriers of the BDNF Val/Val genotype with higher cortical plasticity were characterized by a more thorough processing of visual images. This may be explained by a more detailed analysis of the components of visual images which is associated with a higher activation of occipital areas. There is also some evidence that carriers of BDNF Val/Val genotype with higher neural plasticity perform visual-motor adaptation tasks worse than carriers of Val/Met genotype (Ermakov et al., 2017).
Compared to carriers of the Val/Val genotype, those with the Met allele of the BDNF gene demonstrate higher activation in amygdala and hippocampus when recognizing individuals expressing emotions (Vorobyeva et al., 2019). These data indicate the contribution of the BDNF gene to the formation of brain mechanisms underlying anxiety, as well as its possible role in the recognition of emotional stimuli.
Andreatta et al. (2019) showed that carriers of the Met allele of the BDNF gene have a higher level of anxiety. Loewenstern et al., (2019) confirmed this result, providing the following physiological data: the highly anxious carriers of the Met allele of the BDNF gene have stronger functional associations between the left amygdala and the right dorsolateral prefrontal cortex (anxiety phenotype).
According to (Schofield et al., 2009), carriers of the Met/Met genotype of the BDNF gene had higher verbal recall errors and showed increased P300 latency when performing tasks in the oddball paradigm with corresponding alterations in hippocampal and lateral prefrontal activation, and a localized reduction in hippocampal gray matter. Thus, the Met allele affects the fronto-hippocampal systems involved in the selective processing of stimulus information and memory renewal in healthy individuals. Gajewski et al., (2012) described the impact of the Met/Met genotype of the BDNF gene on fronto-striatal associations. Meanwhile, this genotype was associated with a decrease in the severity of the Stroop effect in elderly people, which was accompanied by an increase in the amplitude of the N450 component during task performance. Researchers concluded that carriers of the Met/Met genotype of the BDNF gene were more resistant to interference. In their earlier work (Gajewski et al., 2010) also suggested that superior memory-based task-switching performance in elderly Met-allele carriers manifested itself in that N2 was increased while the P3 was decreased in Met-allele carriers. Beste et al., (2010) associated the Met allele of the BDNF gene with fewer errors during task performance, as well as with a less time stability of information stored in iconic memory (Beste et al., 2011).
This gene is associated with the dopamine system of the human brain. The enzyme catechol-O-methyltransferase regulates the transmission of impulses in the nervous system, and therefore can affect various mental processes (Serrano et al., 2019). Carriers of the COMT Met allele differentiate emotions and understand facial expression significantly better than those with the Val allele (Gohier et al., 2014b; Weiss et al., 2014). The structural features of this gene are associated with the activity of working memory (Barnett et al., 2011; Dumontheil et al., 2011; Gosso et al., 2008), multitasking ability (Rosa et al., 2010), anxiety (Hashimoto et al., 2007), extraversion (Reuter & Hennig, 2005) level of organization of regulatory (management) functions (Barnes et al., 2011), impulsivity and novelty seeking (Golimbet et al., 2006), and also the tendency to use psychoactive substances (Lovallo et al., 2019).
Individuals with a high enzymatic activity of COMT (the Val/Val genotype of the COMT gene) are better able to deal with stressful situations, because excess dopamine activity is quickly neutralized. On the other hand, individuals with a low enzymatic activity of COMT (the Met/Met genotype of the COMT gene) are more capable to develop complex plans, calculate the probabilities of events and think about their consequences; they have better memory, the ability to synthesize new information, but in a calm environment, in the absence of stress (Stein et al., 2006).
The data on the associations between the COMT gene genotypes and the brain correlates for facial expression recognition indicate that male carriers of the Met allele have higher values of latency of evoked potentials during perceiving faces; women with this genotype have a more pronounced arousal effect (Tamm et al., 2016). Bender et al., (2012) found that the DAT1 and COMT genes affect the same specific time interval (early contingent negative variation, CNV) associated with preparing for movement. According to (Gómez et al., 2003), the generators of this component are located in the frontal cortex (anterior cingulate gyrus) and in the occipital-temporal visual cortex, which corresponds to the expression of the COMT gene. There is evidence for the linkage of the region of chromosome 22 containing the COMT gene with an integrated phenotype that includes visual-motor dysfunction (Alfimova & Golimbet, 2011).
Kondo et al. (2012) discovered the effect of the COMT gene on speech perception reflected in the induced brain activity, as well as the impact of HTR2A gene on the perception of inverted figures. Later, these authors examined associations between COMT genotypes and recognition accuracy, as well as associations between HTR2A genotypes and the response time for recognition (Kondo et al., 2015).
HTR2A has an inhibitory effect in the visual cortex. It is believed to be associated with episodic memory (Reynolds et al., 2006) and emotional intelligence (Kosonogov et al., 2019). Greater activation of the parieto-occipital areas of the brain during positive or negative judgment of stimuli by carriers of the dominant homozygous G/G genotype of the HTR2A gene may be explained theirs detailed assessment of stimulus images. Along with his, carriers of the recessive homozygous A/A genotype had similar activity in the same areas during positive emotional assessment of stimuli, which can be interpreted same way as G/G genotype (Alekseeva et al., 2017; Ermakov et al., 2017).
An analysis of the previous studies showed that knowledge of the role of genes in providing various mechanisms of perception is insufficient for creating a holistic picture. Certain aspects of this issue were investigated in a study conducted by (Nakagami et al., 2013) in marmosets. This results suggest that the patterns of gene expression that are potentially associated with various stages of visual cognition are different in the neurons of each layer of the visual cortex. The regulation of each neuron of primary visual cortex in marmosets is subjected to various regulating impacts when the activity of the retina changes. It should be related to a highly differentiated laminar structure of marmoset visual systems, reflecting the functions of the activity-dependent gene expression. Consequently, HTR2A gene expression depends on the level of activation of the various retinal layers.
Based upon the foregoing, this study aims to examine the associations between BDNF, COMT, and HTR2A genotypes and individual characteristics of the perception of emotional visual stimuli (scenes) with distinct second-order features.
1) Amplitude, spatial, and temporal characteristics of the components of visual event-related potentials in response to emotional visual stimuli (scenes) with distinct second-order features are significantly different in carriers of different BDNF, COMT, and HTR2A genotypes.
2) Characteristics of attribution of emotional valences to visual stimuli (scenes) with distinct second-order features have statistically reliable difference in carriers of different BDNF, COMT, and HTR2A genotypes.
The use of experimental subjects is in accordance with ethical guidelines as outlined in the Declaration of Helsinki. In addition, the design of the experiment, the methodological approach, the conditions of confidentiality and use of oral consent for the subjects was performed according to the Code of Ethics of Southern Federal University (SFU, Rostov-on-Don, Russia) and approved ethically by the Academic Council of the Academy of Psychology and Pedagogy of SFU, on 23 March, 2018. Oral consent for participating in research was approved since the study requires only a small amount information about the participant’s genotype, gender and age and no other additional information. It should be noted that all data were encrypted and stored in the laboratory of the Department of psychophysiology and clinical psychology and only research personal had access to it. Before the start, each participant was informed about the goal of the research, procedure, experimental conditions, and safety of their personal data.
This study involved 34 participants of both genders from 18 to 28 years (27 female), students of Southern Federal University. The study was conducted from April 27, 2018 to July 10, 2018 at the Laboratory of Psychophysiology and Experimental Psychology, Department of Psychophysiology and Clinical Psychology, Academy of Psychology and Education Sciences, Southern Federal University (Rostov-on-Don, Russia). The participants were recruited in person. Students received bonus points in their academic disciplines for their involvement in the study. All participants reported normal or corrected-to-normal vision and gave oral, informed consent before beginning experimentation. For genotype analysis buccal epithelial cells were collected from participants. Genotyping was carried out by the «Biologicheskie resheniya i tehnologii» (Moscow, Russia), where DNA was extracted from clinical material using the Proba-NK reagent kit («ООО DNK-tehnologii», Russia). Thermocycler CFX96 «Touch» (Bio-Rad, USA) was used for real-time polymerase chain reaction (PCR).
For genetic analysis were used genomic DNA preparations obtained from biological samples using DNA extraction kits with the removal of PCR inhibitors and the presence of at least 100 genomic copies in 1 μl. During the genetic examination, the following DNA sections were analyzed:
– COMT catechol-O-methyltransferase gene (Genebank sequence AY341246, mutation 23753G> A, Val158Met, rs4680 code). Possible genotypes: Val/Val, Val/Met, Met/Met;
– BDNF brain neurotrophic factor gene (GenBank sequence NG_011794, mutation 68690G> A Val66Met, rs6265 code). Possible genotypes: Val/Val, Val/Met, Met/Met;
– HTR2A serotonin receptor gene (GenBank sequence, NG_013011, mutation 4692G> A, rs6311 (Tr2)). Possible genotypes: G/G, G/A, A/A.
The genotyping resulted in the following sample composition:
COMT gene: 9 participants (26.47 %) were carriers of Val/Val genotype, 20 (58.82%) – Val/Met and 5 (14.7 %) – Met/Met;
HTR2A gene: 11 participants (32.35 %) were carriers of A/A genotype, 11 (32.35 %) – A/G and 12 (35.29 %) – G/G;
BDNF gene: 23 participants (67.64 %) were carriers of Val/Val genotype and 11 (32.35%) Val/Met.
For this research, 223 images were selected from a database used in previous experiments: 85 of them are conditionally negative, 75 are neutral and 63 are positive (Ermakov et al., 2016; Ermakov et al., 2017; Stoletniy et al., 2020b; Vorobyeva et al., 2016); stimuli are available as Extended data (Kovsh & Yavna, 2020). To obtain stimuli with corresponding characteristics, the computer model of the second-order visual mechanisms developed by (Babenko & Yavna, 2018) was applied. Figure 1 below demonstrates examples of original images and the results of their processing by the model. Stimuli images were generated from contrast modulations in the carrier frequency range from 4 to 32 cycle per image. Resolution of pictures is 1024х768 (slightly more 16°х12° of visual angle on the 100 cm distance). The average luminance of the generated images was equal to 110 of 256 greyscale (30 cd/m2). The background brightness was adjusted to the same level. All 223 selected images were processed and then organized in a stimulus sequence.

Participants were seated in good lit room, facing a computer screen, at viewing distance of 100 cm. A grey background (30 cd/m2) was on the monitor all the time of trial, with and without stimuli. Stimulus image was presented in center of screen and its duration was 500 ms. The interstimuli interval was 500–1500 ms (Figure 2). Stimulus pictures were presented in random order regardless of their emotional valence. Participants were asked to examine the stimuli in terms of what emotions this images evokes in them. When evaluating the stimulus as negative, it was necessary to press the key 1 on the keyboard for negative assessment, 2 for neutral, 3 for positive. The next image appeared on the screen only after the subject evaluated the previous one and the interval between stimuli was completed. Furthermore, participants were instructed to blink only when stimulus ended.
The frequency of responses was analyzed using the REdaS Package for R, version 0.9.3., in order to calculate confidence intervals for average response rate with α = 0.05 (Maier, 2015).
A Neurovisor-136 multi-channel EEG system («Medical Computer Systems», Moscow, Russia) was used to record the EEG from 128 electrodes mounted on elastic cap and referenced on the left and right ears. Electrodes were placed according to 10–5 system (Oostenveld & Praamstra, 2001). EEG signal was digitalized at 1000 Hz, and a bandpass filter of 0.5–50 Hz was applied.
The ERPs were processed using the EEGLAB version 4.11 – an interactive Matlab/Octave toolbox for processing electrophysiological data (Delorme & Makeig, 2004). Before averaging the ERPs, the recordings were filtered and the epochs with eye blinks and other artifact of non-brain origin were rejected. The ERPs were averaged 100 ms before the stimulus onset and 500 ms after it. Baseline was corrected based on 100 ms pre-stimulus period. ERPs were computed and grouped according to type of response that participants gave – negative, neutral and positive and theirs genotype. The ERPs grouped by type of emotional assessment were compared separately for COMT, BDNF, HTR2A samples (For example: COMT x Negative evaluation x Val/Val vs Val/ Met; Val/Val vs Met/Met; Val/Met vs Met\Met etc.). Comparisons were carried out using an unpaired Student’s T-test with p < 0.05. ERPs and topography figures were conducted in EEGLAB. The results from all ERP stimuli are available as Underlying data (Stoletniy et al., 2020a).
This analysis showed that there was no statistically reliable difference in the average response rate in participants with different BDNF gene genotypes regardless type of assessment of target images (Figure 3, upper line). Nevertheless, it should be noted that the ratio of the average number of choices of neutral responses is the reverse of that when choosing negative and positive judgments; the individual participants with the Val/Val genotype chose the neutral response slightly more often than the participants with the Val/Met genotype.
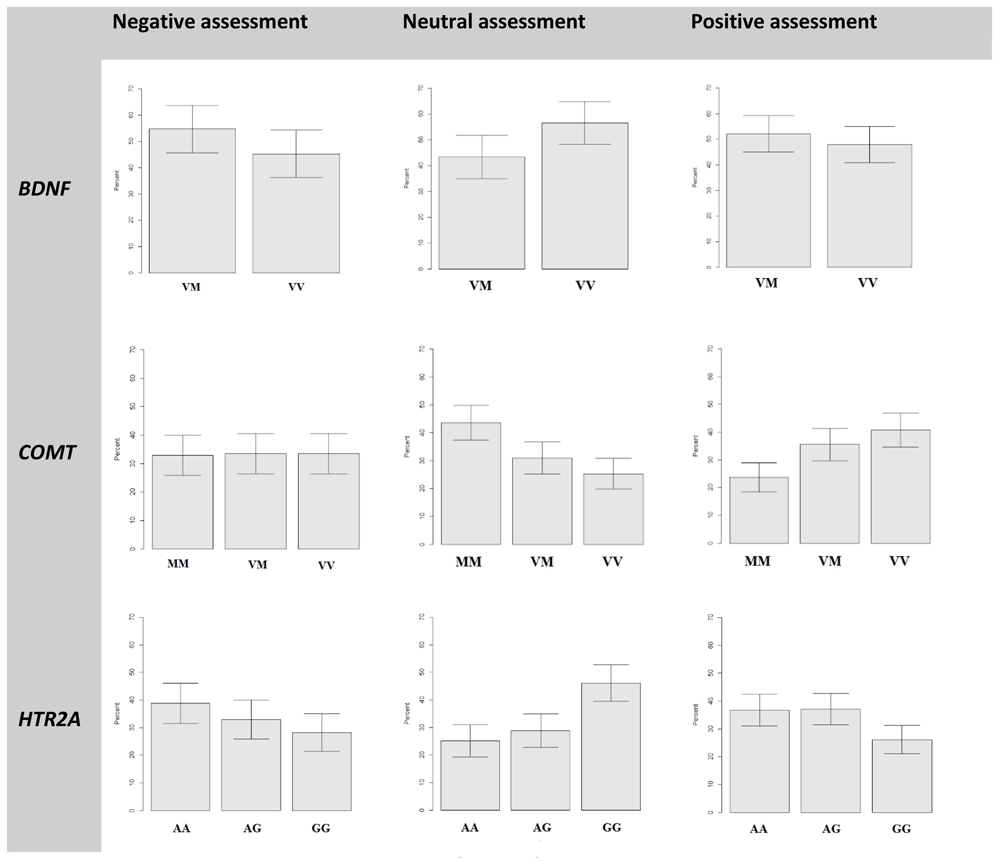
MM- Met/Met genotype, VM – Val/Met, VV- Val/Val for COMT and BDNF genes; AA – A/A, AG – A/G, GG – G/G for HTR2A gene.
Participants with different COMT genotypes chose negative assessments with an average frequency (about 32–33%). There were no significant differences in the frequency of negative responses in the groups. Nevertheless, the distribution of neutral and positive responses is of more interest (Figure 3, middle line). On the one hand, participants with the Val/Val and Val/Met genotype chose the positive assessment of stimuli significantly more often (19% and 13%, respectively) than carriers of Met/Met. On the other hand, the carriers of the Met/Met genotype were significantly often (18% and 13% compared to Val/Val and Val/Met groups) chose to evaluate the images as neutral. There were no significant differences between the average frequency of choosing the neutral and positive responses between the Val/Met and Val/Val groups.
Carriers of different HTR2A genotypes chose negative responses with different average frequency but no statistically reliable difference, because the confidence intervals do not intersect. Although the histogram has a stepwise character from a higher average frequency for carriers of the A/A genotype (39%) to lower frequency for carriers of the A/G (33%) and G/G (28%) genotype (Figure 3, bottom line). A more interesting situation has developed for the neutral and positive types of responses. Participants with the G/G genotype were significantly more likely (46%) to choose the neutral response when evaluating experimental stimuli than those with the A/A (25%) and A/G (29%) genotypes. On the contrary, the G/G group had chosen positive assessment less often (26%), than A/A (37%) and A/G (37%) groups. There was no statistically reliable difference between the average frequency of choosing the neutral and positive responses between the A/A and A/G groups.
BDNF. The comparison of event-related potentials when choosing the negative assessment in the groups of carriers of Val/Met and Val/Val genotypes showed significant differences in the amplitude of the event-related response (p <0.003, t = -3.5) in the time range of 150–230 ms (Figure 4a). As can be clearly seen in the figure, the amplitude of the P2 component of the ERP recorded for negative response in participants with the Val/Val genotype is significantly higher than that of the Val/Met group. When choosing the neutral assessment statistically significant differences was found in latency of 172–227 ms (Figure 4b). The ERP amplitude in the group of carriers of the Val/Val genotype is significantly higher (p < 0.02, t = -1.22) than that of the Val/Met group. Choose of positive response to the stimuli elicited an increase in amplitude of ERP peak in the latency of 133–230 ms. ERP peak for the Val/Val group was significantly higher (p < 0.029, t = -2.36) than that of the Val/Met group (Figure 4c). Table 1 presents the values of the ERPs amplitudes in Oz electrode at a latency of 200 ms for the BDNF group.
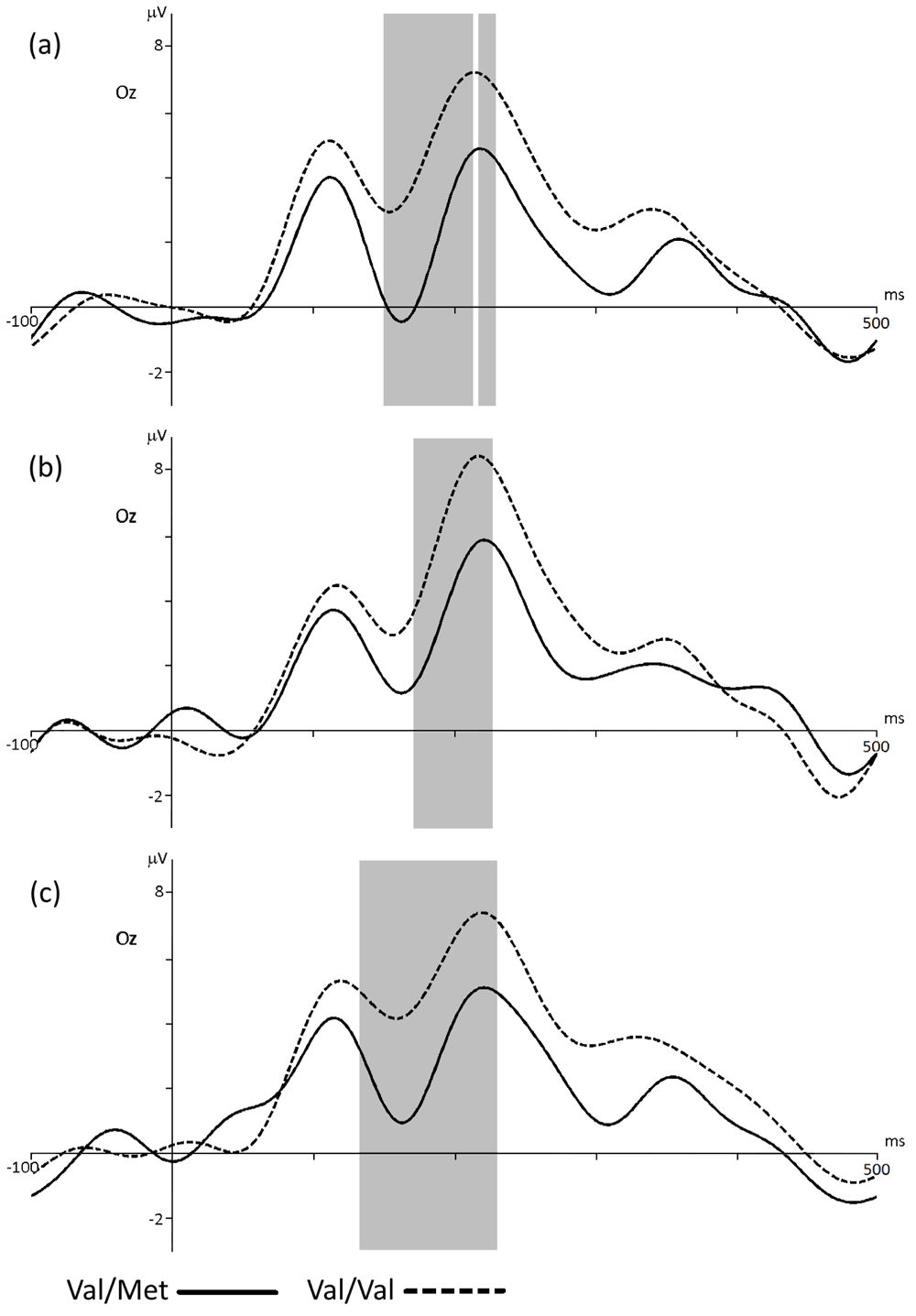
ERP for the groups with Val/Met and Val/Val BDNF genotypes in Oz electrode when choosing the negative (a), neutral (b) or positive (c) assessment of stimuli. The gray bar indicates the latency with statistically significant differences (p < 0.05).
The topography of the potentials distribution shows that the choice of the negative response caused activation in the occipital regions of the cortex. Moreover, it was higher in the group of carriers of the Val/Val genotype of the BDNF gene (Figure 5a). The choice of the neutral assessment caused activation in the occipital regions of the cortex to a greater extent in the group of carriers of the Val/Val genotype (Figure 5b). Nevertheless, activity in the anterior and central areas of the cerebral cortex is somewhat greater in the group of participants with the Val/Met genotype of the BDNF gene. Selection of the positive assessment caused an increase in the amplitude of positive peaks in the region of 140–230 ms in the occipital regions of the cortex, to a greater extent in carriers of the Val/Val genotype of the BDNF gene, as in both previous cases (Figure 5c).
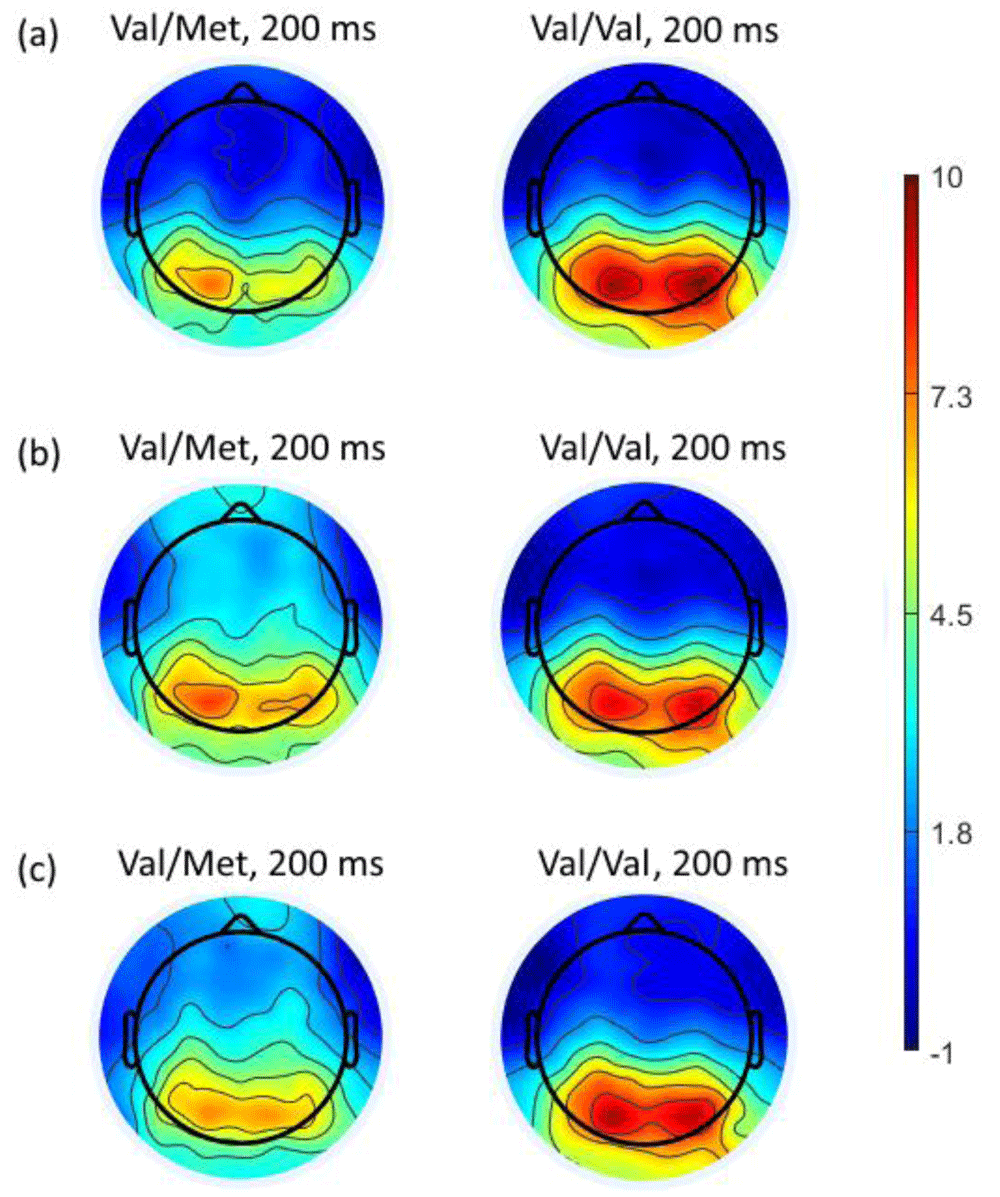
Topography of the distribution of potentials at a latency of 200 ms (where statistically significant differences were found) from onset of stimuli when choosing negative (a), neutral (b) or positive (c) assessment for the Val/Met and Val/Val BDNF genotypes.
COMT. The comparison of ERPs in the groups of participants with the Val/Met and Val/Val genotypes did not show significant differences regardless of assessment type: for negative, p < 0.94, t = -0.06; for neutral, p < 0.59, t = -1.7; for positive, p <0.95, t = -0.10 (Figure 6a–c, solid and intermittent waves on the figures). On the contrary, amplitude of the event-related potentials in participants with the Met/Met genotype is significantly lower compared to Val/Val and Val/Met groups during every type of assessment (Figure 6, a, b, c, dotted wave). For negative evaluation this differences were observed in latency to the 110 to 175 ms (Val/Met - p < 0.03, t = -2.33; Val/Val - p < 0.03, t = 0.6), for neutral in 120 to 170 ms (Val/Met - p < 0.006, t = -3.10, Val/Val - p < 0.03, t = 2.19), for positive - 135 to 155 ms (Val/Met - p <0.02, t = -1.39, Val/Val - p <0.03, t = -2.39). It should be noted that the amplitude of the event-related potential for Met/Met is higher when participants assessed stimuli as the neutral and positive, then negative ones. Table 1 presents the values of the ERPs amplitudes in Oz electrode at a latency of 150 ms for the COMT group.
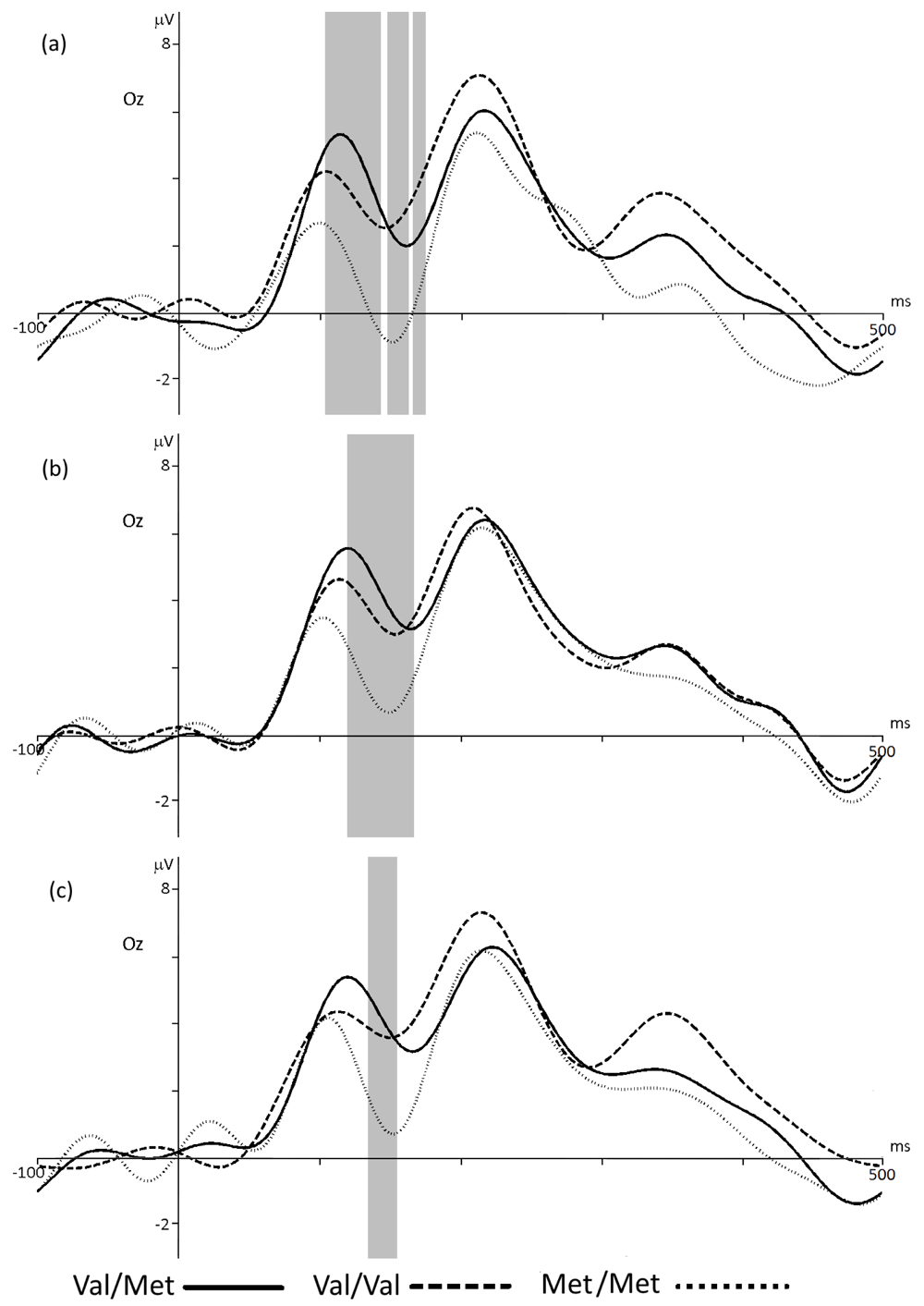
ERP for the groups with Val/Met, Val/Val and Met/Met COMT genotypes in Oz electrode when choosing the negative (a), neutral (b) or positive (c) assessment of stimuli. The gray bar indicates the latency with statistically significant differences (p < 0.05). Significant differences between ERPs in Val/Val and Val/Met groups were not found.
Topography of potentials clearly shows a decrease in activity of occipital regions in the Met/Met group in latency of 150 ms comparing to Val/Met and Val/Val groups. On the contrary Val/Met and Val/Val group showed increase in activity also in the occipital regions of the brain (Figure 7a–c).
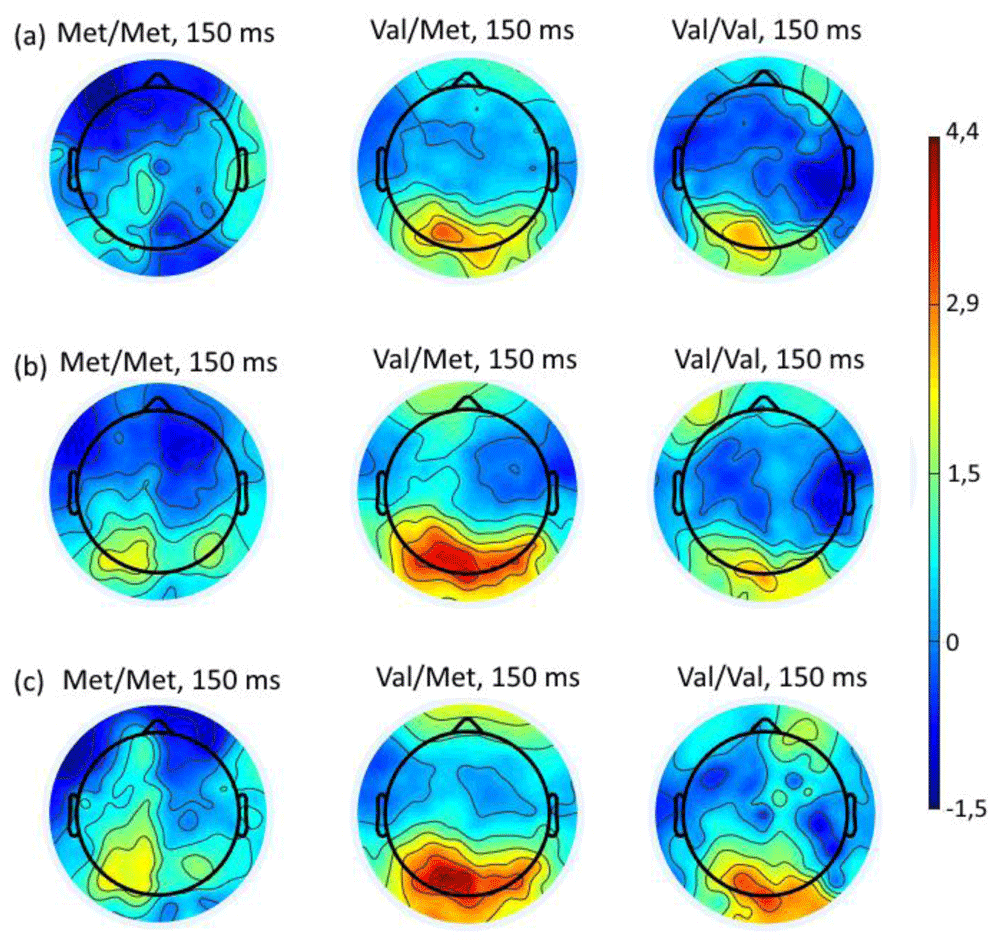
Topography of the distribution of potentials at a latency of 150 ms (where statistically significant differences were found) from onset of stimuli when choosing negative (a), neutral (b) or positive (c) assessment for the Met/Met, Val/Met and Val/Val COMT genotypes.
HTR2A. Comparing the ERPs of A/G and G/G genotype groups showed no statistical difference between them, regardless of assessment type – negative (p < 0.17, t = 1.41), neutral (p < 0.56, t = -0.59) or positive (p < 0.84, t = -0.19). At the same time, chose a negative response type demonstrated that the amplitude of ERP in carriers of the A/A genotype was significantly different from such of the A/G genotype (p < 0.03, t = 2.29) and G / G (p < 0.04, t = 3.12) in the range of 117–133 ms from the onset of stimulus (Figure 8a). Choosing neutral response by carriers of A/A genotype reflected in significant decrease of ERP component’s amplitude in 350–370 ms latency compared to A/G (p < 0.02, t = -2.47) and G/G (p < 0.03, t = -2.25) groups (Figure 8b). Similar to ERP comparison for negative assessment, evaluating stimuli as positive by carriers A/A genotype showed significant increase of ERP amplitude in latency 105 to 124 ms compared to such in A/G (p < 0.01, t = 2.72) and G/G (p <0.03, t = 2.32) genotype carriers (Figure 8c). Table 1 presents the values of the ERPs amplitudes in Oz, Po7 and Poz electrodes at a latency of 120 and 360 ms for the HTR2A group.
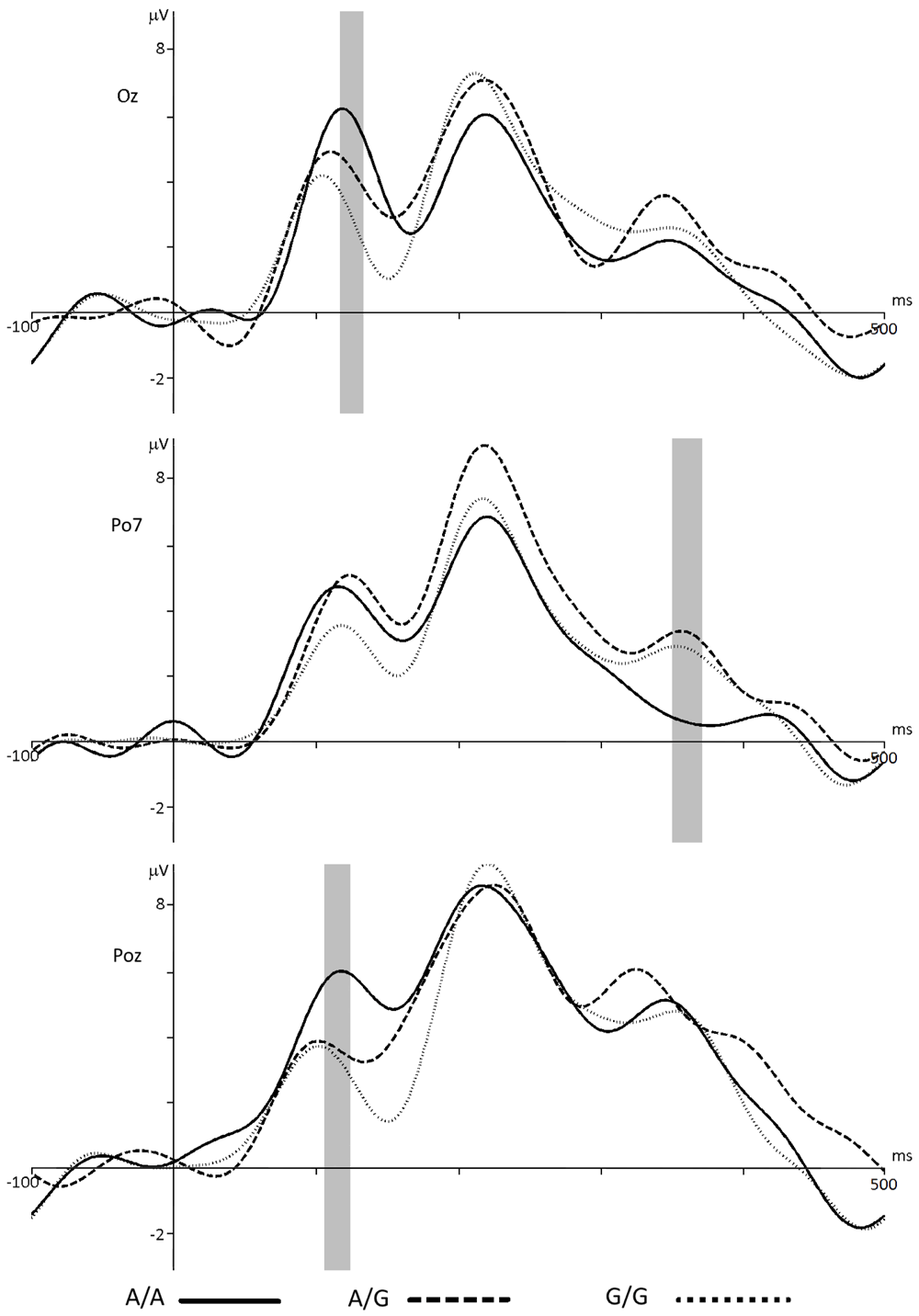
ERP for groups with different HTR2A genotypes in Oz, Po7 and Poz electrodes when choosing negative (a), neutral (b) or positive (c) assessment respectively. The gray bar indicates the latency with statistically significant differences (p < 0.05). Significant differences between ERPs in A/G and G/G groups were not found.
The topography of the distribution of potentials shows a high positivity when choosing negative assessment mainly in the occipital regions of the A/A group and a tendency to decrease of it in same regions in the group of carriers of the A/G genotype, and to a greater extent – in carriers of the G/G genotype (Figure 9a). In the neutral response situation ERP amplitude distribution in carriers of the A/A genotype is somewhat homogeneous over the entire surface of the scalp. However, in carriers of the A/G and G/G genotypes it is much greater in the occipital and parietal regions (Figure 9b). When choosing the positive response, high-amplitude ERPs are localized in the occipital regions. Moreover, the activity in the A/A group is most higher, to a lesser extent it is expressed in the A/G group, and even lower in G/G groups (Figure 9c).
As shown in the Results section, the BDNF Val/Val genotype is associated with a predominance of neutral assessment of visual stimuli processed by second-order filters. Neutral, positive, and negative judgments of visual stimuli are associated with a significantly higher positivity of the P2 component in the occipital electrodes, which may be explained by the fact that compared to the carriers of BDNF Val/Met genotype the carriers of the BDNF Val/Val genotype consumed large brain resources for recognition and differentiation of stimuli, experiencing difficulties in the implementation of these processes. Moreover, brain activity in the anterior and central areas is more pronounced in carriers of the Val/Met genotype. We obtained similar results in previous series of the experiment (during recognition of unprocessed visual stimuli), which may indicate that the Met allele of the BDNF gene is associated with more efficient processes of recognition of emotional scenes (Ermakov et al., 2017). This feature can be explained by a more intense activation of the structures of the limbic system (Vorobyeva et al., 2019) and stronger functional cortico-limbic bonds (Loewenstern et al., 2019), activated during recognition of facial expression in carriers of the Met allele of the BDNF gene.
According to our results, when judging the stimulus as negative, neutral, or positive negativity was reliably expressed in 110–175 ms (i.e., there is a higher, more negative amplitude of the N170 component) in the occipital electrodes in carriers of the COMT Met/Met genotype.
This aspect may be related to the fact that, when evaluating scenes with distinct second-order features, the carriers of the COMT Met/Met genotype primarily pay attention to the facial expression of individuals depicted in the stimulus pictures and assess the emotional valence of the stimulus based on these data (Eimer & Holmes, 2007). However, this process consumes more brain resources to recognize faces emotional expression.
According to a number of studies, carriers of the COMT Met/Met genotype have higher indices of emotional intelligence (Kosonogov et al., 2019), more successfully differentiate and express emotions (Gohier et al., 2014a). Carriers of the COMT Met/Met genotype more decently respond to positive stimuli. The Val/Val genotype is associated with less intense feelings in negative experience, i.e. their level of life satisfaction is less dependent on external events (Kovalenko & Pavlenko, 2009; Shield et al., 2004)
The HTR2A G/G genotype is associated with a significantly more frequent evaluation of stimuli with distinct second-order features as neutral ones, as well as with a significantly rarer assessed of stimuli as positive ones. The A/A genotype is associated with a significantly higher amplitude of the P1 peak, recorded mainly in the occipital regions of the brain when evaluating visual stimuli processed by second-order filters as positive or negative ones; with a significantly lower wave amplitude in the range of 350–370 ms when assessed as emotionally neutral. These results may indicate that carriers of A/A genotype consume more brain resources at the stage of distinguishing of a significant stimulus among insignificant ones, experiencing difficulties in the implementation of this process (difficulties in pre-attentive processing; component P1 is associated with selectivity of attention), and less resources are consumed duding categorization and conscious evaluation of the emotional content of stimuli (Kovalenko & Pavlenko, 2009; Polich, 2012).
The analysis of the electrical activity of the brain in carriers of various genotypes of the BDNF, COMT, and HTR2A genes when choosing different types of responses in attributing emotional valences to visual scenes processed by the filters with the distinct second-order features enabled us to draw the following conclusions.
1) In carriers of the Val/Val genotype the amplitude of the P2 component of visual event-related potentials in the occipital regions of the brain is higher than in carriers of the BDNF Val/Met genotype, regardless of the type of response chosen when evaluating the presented images.
2) The highest ERP amplitude of the three possible assessments (positive, negative, neutral) was found in the response recorded in the neutral evaluation of the presented stimuli; this fact is valid for carriers of the BDNF Val/Val and Val/Met genotypes.
3) Regardless of the type of stimuli, the participants chose responses of different modalities with approximately the same frequency.
1) In the group of participants with the COMT Met/Met genotype for all the three types of responses (regardless the stimuli images) the induced brain activity is characterized by a significant increase in negativity in peak amplitude latency from 120 to 170 ms and occurs mainly in the occipital cortex brain.
2) When carriers of Met/Met assessed the stimuli as negative the amplitude of this early ERP component is showed increasing negativity, compared with that when choosing neutral or positive responses.
3) Met/Met genotype carriers statistically reliably chose neutral assessment more often and positive less often than carriers of other two genotypes.
1) There were no statistically significant differences between the ERP waves in carriers of the HTR2A A/G and G/G genotypes, regardless of the choice of the emotional valences of responses. On the contrary, A/A group had statistically reliable differences in ERPs peaks compared to A/G and G/G genotypes carriers.
2) When choosing negative and positive assessment, carriers of A/A genotype had statistically reliable differences in early latency peak that had large amplitude, compared to A/G and G/G genotypes carriers. Contrariwise, in neutral assessment situation, a statistically reliable differences in ERPs shifted to later positive peak (around 360 ms) that had an increasing negativity in participants with A/A genotype.
3) The localization of activity also has differences in carriers of different HTR2A genotypes. When choosing negative and positive responses, the topography of significant high-amplitude components of the ERP shows their localization in the occipital regions; with a late latent significant ERP when choosing a neutral type of responses, increased activity also affects the parietal areas (which is more reliable to carriers of the A/G and G/G genotypes compared to carriers of the A/A genotype).
4) G/G genotype carriers were significantly more often chose neutral response and less often positive response for stimuli than carriers of other two genotypes.
In this study, associations between the BDNF, COMT, and HTR2A genotypes and individual characteristics of the perception of emotional visual stimuli with distinct second-order features were investigated. The findings of the study confirmed both hypotheses of the research. Furthermore, the results lead to conclusion that the images preserving second-order features, where all the homogeneous areas are removed, contain all the necessary information for their successful recognition. This may be explained by the work of autonomous mechanisms operating at a pre-attentive stage of visual processing (Babenko & Yavna, 2018). At the same time, various genotypes are associated with varying degrees of success of the pre-attentive and conscious stages of processing emotional visual stimuli (scenes), which confirms the hypothesis that there are genetic factors that individualize the mechanisms of visual perception.
Open Science Framework: EEGLAB datasets for study of event-related potentials during categorization of emotionally-charged and neutral images with distinct second-order features in carriers of polymorphisms of the COMT, HTR2A, BDNF genes. https://doi.org/10.17605/OSF.IO/PDHGZ (Stoletniy et al., 2020a).
This project contains the follow underlying data:
1_Negative estimation (ERP datasets recorded when participant chose negative assessment of stimuli).
2_Neutral estimation (ERP datasets recorded when participant chose neutral assessment of stimuli).
3_Positive estimation (ERP datasets recorded when participant chose positive assessment of stimuli).
Data Characteristics.xlsx (detailed description of datasets files).
Data Description.docx (detailed description of experiment condition and datasets).
Open Science Framework: Stimuli database for studying emotional assessment of emotionally-charged images with distinct second-order features. https://doi.org/10.17605/OSF.IO/WY2VF (Kovsh & Yavna, 2020).
This project contains all visual stimuli provided for this study, alongside a data description list of the image features.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Experimental psychology, psychophysiology.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 20 Aug 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)