Keywords
Clear cells, gastric cancer, gastrectomy
Clear cells, gastric cancer, gastrectomy
Clear cell carcinoma (CCC) generally develops in organs originating from the Mullerian system, such as the lower urinary and female genital tracts1. Its occurrence in the gastrointestinal tract is uncommon, and cases occurring in the stomach are exceedingly rare2. Clear cytoplasm, and hence clear cells, are the result of intracellular accumulation of glycogen, lipid, water, or mucin3. Due to its rarity, the clinicopathological and biological behaviors of this entity remain unclear.
Until now, only a few cases of gastric CCC have been reported in the English literature. These reports generally included gastric carcinoma with focal clear cell changes. We report herein the third case of a pure gastric CCC.
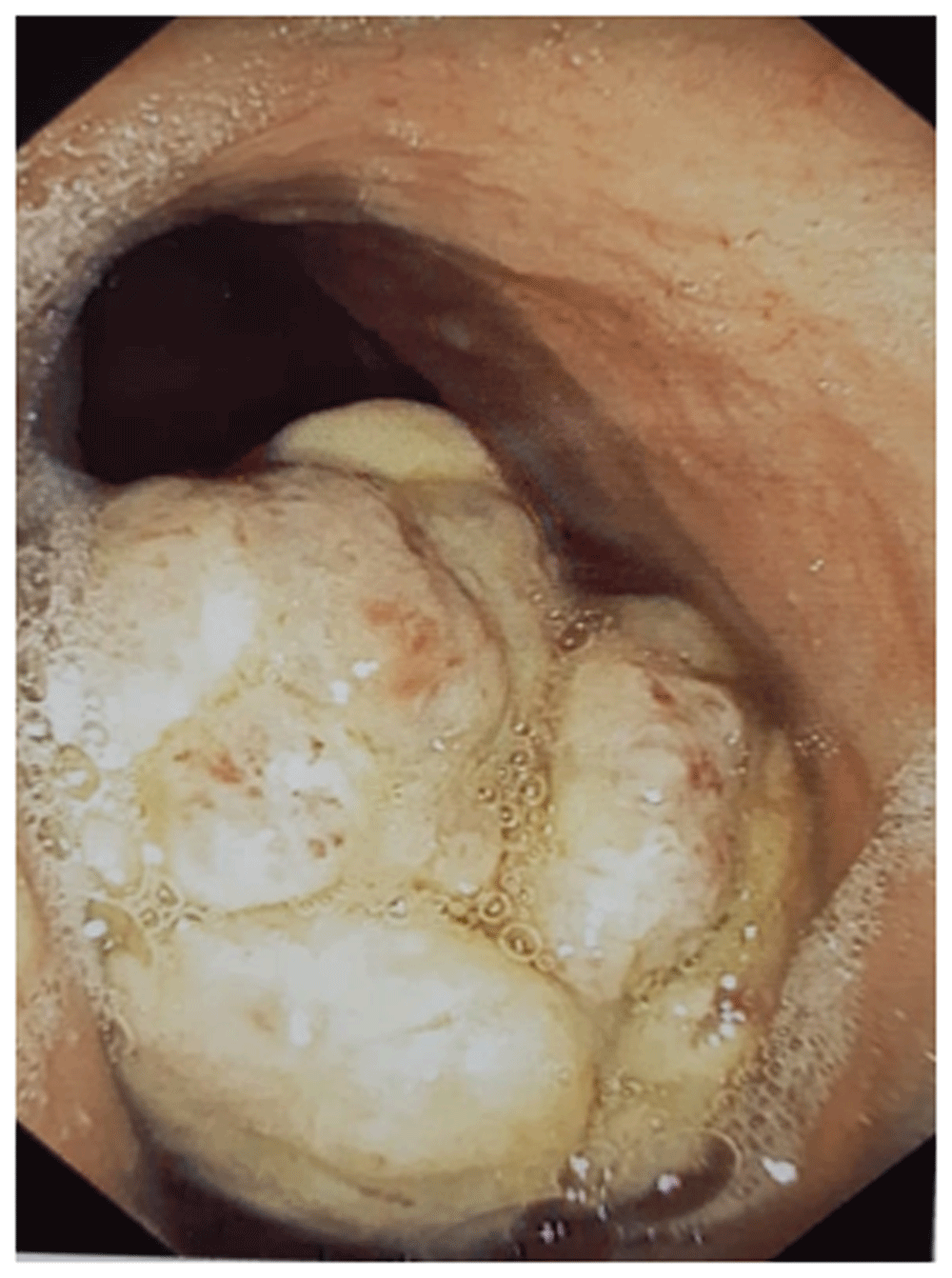
We report the case of a 51-year-old north African woman, who presented with a history of epigastric pain of four months. The abdominal examination found mild epigastric tenderness but no palpable mass. The upper gastrointestinal endoscopic examination revealed a polypoid tumor of the greater curvature of the stomach measuring 6×4 cm (Figure 1). Biopsies were performed using digestive endoscopic biopsy forceps. The anatomopathological examination showed a poorly differentiated carcinoma with a signet-ring cell component. No immunohistochemical examination was performed.
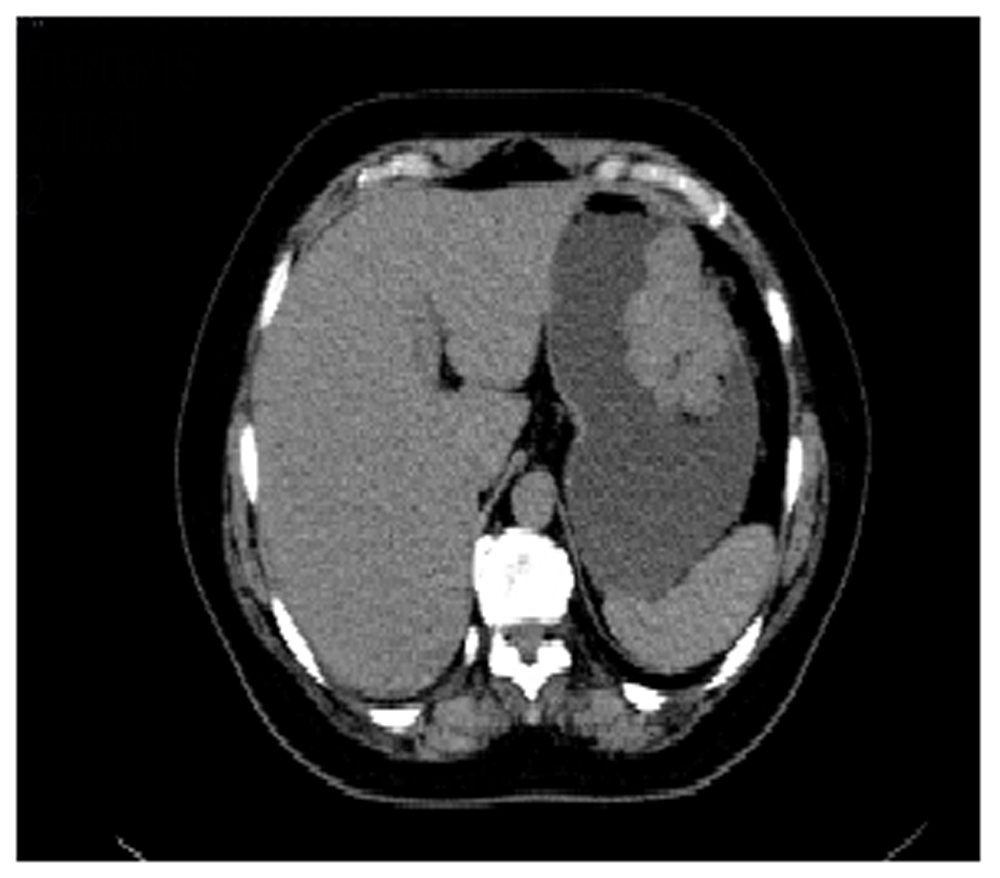
A CT scan of the thorax and abdomen, performed as part of the extension assessment, showed a pedicled budding mass with endoluminal development of the vertical part of the greater gastric curvature measuring 6 × 4 × 5cm (Figure 2). Otherwise, it did not show evidence of any renal tumor or hepatic or pulmonary localization.
In order to reduce tumor volume and improve the R0 resection rate, the patient received four courses (one course per two weeks) of perioperative chemotherapy with 5-fluorouracil (2600mg/m2), folinic acid (350mg), oxaliplatin (85mg/m2) and docetaxel (50mg/m2) according to the FLOT regimen, administrated through a totally implantable venous access port via the internal jugular left vein. Then the patient underwent distal subtotal gastrectomy with a manual Roux-en-Y esophagojejunostomy, via a midline incision. This was the procedure of choice of the clinical lecturer who performed the intervention in case of proximal gastric cancer.
Gross examination revealed an ulcerated polypoid tumor with endoluminal development. The histological exam showed an invasive tumor arranged in lobules, clusters, and nests within a highly vascularized stroma (Figure 3a). There were necrotic changes. Tumor cells had abundant clear cytoplasm and well-defined cytoplasmic borders. The nuclei had marked atypia and prominent eosinophilic nucleoli (Figure 3b). The tumor infiltrates to the subserosa without serosal invasion. Moreover, we noted the absence of vascular emboli, perineural tumor invasion and lymph node metastasis. Periodic acid-Schiff and alcian blue stains were negative. An immunohistochemical study was performed to rule out a renal origin. The tumor cells were negative for CD10 and vimentin. They were positive for cytokeratin with diffuse cytoplasmic and membranous staining. The diagnosis of primary gastric CCC in its pure form was made.
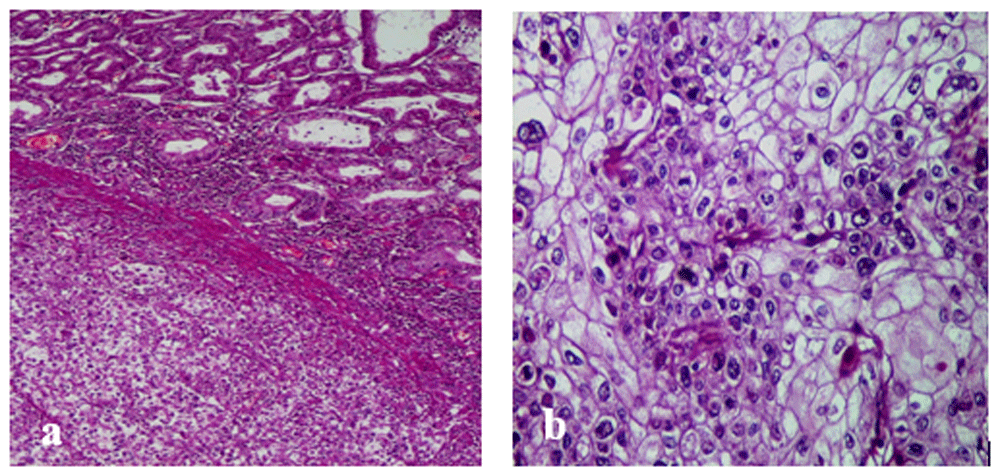
(a) Clear cell gastric carcinoma (top right: normal gastric mucosa; bottom left: gastric carcinoma; He x 100). (b) Tumor cells are clear with marked nuclear atypia and numerous mitotic figures.
The postoperative course was uneventful and the patient was discharged on the fifth postoperative day on analgesic treatment and low-molecular-weight heparin for thirty days. The patient received four courses of adjuvant chemotherapy (FLOT regimen). The CT scan done after six months showed no local or distant recurrence.
A CCC can develop in various organs, and the most common sites are the kidneys and the female genital tract1. Its occurrence in the gastrointestinal tract is uncommon. Only few case reports or small series have been described in the colon4, pancreas5, and the biliary system6.
Even though the presence of clear cell changes in gastric carcinoma has been reported in 8.5 % of cases7, the pure form of CCC of the stomach is an extremely rare oncologic entity. There is no specific reference to CCC in the latest WHO classification of gastric carcinoma. This entity has not been well documented, with only limited literature available on the topic.
Regarding the clinical characteristics, Kim et al.7 have demonstrated, in a large cohort study, that gastric carcinomas with clear cell changes were associated with younger age and tended to be located in the gastric antrum. However, Ghotli et al.8 showed that gastric CCC had a predilection for the gastroesophageal junction. Moreover, these tumors are polypoid and histologically characterized by a tubulo-papillary pattern. These features are consistent with the characteristics of the tumor in our case, which was polypoid and located in the vertical part of the greater gastric curvature.
What makes this case remarkable is that the present tumor is made of 100% clear cells. This situation is different from previously reported case series, in which only a portion of the adenocarcinoma showed clear cell cytoplasm. Ghotli et al.8 reported 12 cases and defined the CCC as adenocarcinoma composed of more than 10% of clear cells. Kim et al.7 reported 65 cases and defined CCC as a carcinoma composed of more than 5% of clear cells. To the best of our knowledge, only Terada9 and Yamada et al.10 have reported the pure form.
It has been shown that the presence of clear cell changes is an independent indicator of poor prognosis7 since it is associated with advanced depth of invasion, presence of lymphovascular tumor emboli, and lymph node metastases, compared to gastric adenocarcinoma without clear cell changes.
Advances made in techniques used for pathological examinations and immunohistochemistry made the diagnosis of gastric CCC easier. Immunophenotypically, it has been demonstrated that CCC carcinoma shows overexpression of cyclin D18. It has also been noted that clear cell tumors of the stomach may produce alpha-fetoprotein (AFP) in the serum and within the tumor11. In our case, AFP was not measured.
Recently, hepatocyte nuclear factor-1b (HNF-1b) has been accepted as a unique biomarker of CCC for tumors of the female genital tract, bladder, and pancreas5,12,13. In the stomach, carcinomas with clear cell changes also show increased positive immunostaining of HNF-1b as it has a role in cellular glycogen synthesis7. Nevertheless, until now, there has been no reports about the role of HNF-1b in gastric adenocarcinomas.
Due to its rarity, there are no therapeutic guidelines for CCC. It is managed like conventional gastric carcinomas, and its surgical treatment depends on its localization. In our case, the tumor was located in the vertical part of the great gastric curvature and necessitated total gastrectomy.
Pure primary CCC of the stomach are exceedingly rare and are associated with a poor prognosis. Immunohistochemistry is the cornerstone of the diagnosis of these tumors to rule out metastases from a renal CCC.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: pathology, immunooncology
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medical human genetics, clinical genetics, cytogenetics, molecular diagnosis, biochemical genetics, birth defects and teratology, and genetic counseling.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 25 Aug 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)