Keywords
Aloe vera oil, Ozone Therapy, Full-thickness skin defect, macrophage count, epithelization
Aloe vera oil, Ozone Therapy, Full-thickness skin defect, macrophage count, epithelization
Soft tissue wound refers to the damage or loss of tissue continuity, possibly caused by internal factors such as drugs, changes in circulation and metabolic processes, infection, and oxygen transportation failures. External factors include extreme temperatures, injuries, radiation, and the presence of chemical substances1. The body's physiological response, in terms of wound healing, results from various interrelated processes, including coagulation, inflammation, deposition, extracellular matrix differentiation, fibroplasia, epithelialization, contraction, and remodeling1. Furthermore, the remediation course is mediated by various cells, cytokines, matrices, and growth factors, through three phases - inflammation, tissue proliferation, and remodeling2. The process of acute cutaneous wound healing consists of three stages. The first stage is the inflammatory phase, which occurs following the injury and lasts until day-3. The second stage is the proliferation phase that generally takes place from day -7 until day-14, and the third stage is the remodeling phase that can last for several months or even years. The result of a previous study explained that only at specific doses did ozonated oil show a significant increase in the initial phase of wound healing3.
Macrophages play critical roles in all phases of adult wound healing, which are inflammation, proliferation, and remodeling. As wounds heal, the local macrophage population transitions from predominantly pro-inflammatory (M1-like phenotypes) to anti-inflammatory (M2-like phenotypes). Thus, local macrophages retain pro-inflammatory characteristics4.
Aloe vera is a thorny plant that grows in tropical and subtropical climates. It has been used for millennia to improve cutaneous wound healing, with this now being known to be due to its ability to increase the proliferation and migration of fibroblasts and human primary epidermal keratinocytes (HPEKs) flow cytometry analysis revealed that cell surface expressions of β1-, α6-, β4-integrin, and E-cadherin increased in HPEKs treated with Aloe vera gel. These increases may contribute to cell migration and wound healing5. Many active ingredients, including vitamins, enzymes, minerals, sugars, lignin, salicylic acids, and amino acids, have been identified from these species. However, therapeutic effects have not correlated well with each component. Recently, a study exploring the biological effects of a single element from Aloe species was conducted. Choi et al. reported that a 5.5 kDa glycoprotein isolated from Aloe vera gel by activity-guided sequential fractionation was found to enhance keratinocyte proliferation and migration, and thereby accelerate wound healing5. However, it is currently believed that biological activities associated with Aloe arise from the synergistic actions of multiple compounds rather than from a single component5.
Ozone therapy is an alternative medicine with disinfecting properties and strong oxidative stress induction capacity. These characteristics have been implicated in the stimulation of protective mechanisms for cells and organs by increasing the efficacy of endogenous oxygen-free radicals scavenging6. Moreover, ozone therapy inactivates bacteria and damages cell walls through the oxidation of phospholipids and lipoproteins, and also inhibits fungal growth, damages viral capsids, and interferes with the bacterial reproduction cycle7. This therapy could be applied as an adjuvant or alternative to standard treatments in patients with various types of wounds, particularly when used topical6. Previous studies have highlighted the benefits of ozonated sesame oil in wound healing7.
Therefore, this study aimed to evaluate the effect of Aloe vera oil provided with different dosages of ozone in accelerating the healing rate comparing two-phases; inflammation ( day 3) and proliferation (day 7) of full-thickness defect wounds in Sprague Dawley rats as an adjuvant treatment based on macrophage count and new epithelialization length.
This was an experimental study using a post-test only control group design. The outcome data were recorded after the duration of the treatment. A total of 50 adult male Sprague Dawley rats were distributed randomly into ten groups, with five in each. A total of ten groups were created, where the controls, P1 (received Aloe vera oil) and P2 (received gentamicin), respectively, for three days. Furthermore, P3, P4, and P5 was administered ozonated Aloe vera Oil at a dose of 600, 1200, and 1800 mg/ml of ozone, respectively for three days. Therefore, P6 (received Aloe vera oil) and P7 (received gentamicin), respectively, for seven days, while P8 P9 and P10 received ozonated Aloe vera Oil at a dose of 600, 1200, and 1800 mg/ml ozone, respectively for seven days. A full-thickness defect measuring 1 cm in diameter was created on the back of the rat. The rats were sacrificed in each group on the third (P1, P2, P3, P4, P5) (inflammation stage of wound healing ) and seventh day (P6, P7, P8, P9, P10) (proliferation stage wound healing), respectively, to obtain the macrophage count and length of new epithelialization (Figure 1).
The 50 adult male Sprague Dawley rats from a local laboratory-grade rat breeder, with a pure-breeding status, weighing 250 ± 50 grams and lodged in individual stainless-steel laboratory-grade rat cages at room temperature of 28.0 ± 2.0°C, which was monitored with a digital thermometer. The rats were acclimated in the laboratory for one week. Twelve hours light was provided with from 06.00–18.00 and darkness from 18.00–06.00 with allowed access food (BioFeed pellets, manufactured by Karunia Kasih Abadi, Klaten, Indonesia) and water supply (clean drinkable water). These conditions were to ensure the elimination of stress, and feeding was conducted ad libitum. Each cage was inhabited by two to three rats according to the cage size. We did not mix rats from different groups in one cage. We routinely assessed the clinical and behavioral status of the rat every day during routine checks to prevent any clinical or behavioral changes in the laboratory rat. No rats were found dead, having decreased appetite/growth, or exhibited behavioral changes during the study period.
A previous study6 involved the application of ozonated sesame oil (8 microliters) to a wound measuring 3.5 mm in diameter, we employed the same method. The doses used in this investigation were 50.6, 101.3, and 205.5 mg/ml of ozonated Aloe vera oil; hence the amount of ozone provided to each 3.5 mm wound was 0.405 mg, 0.81 mg, and 1.62 mg, respectively. Furthermore, the ozonated oil (Cap) capacity was determined using the formula: Cap = C x FRate x T, where Concentration (C) was set at 45 mg/L, and FRate(Flow rate) at 1.5 liters/minute, while Timeincludes 30, 60 and 120 minutes. The manufacture and testing of ozonated Aloe vera were carried out at the Plasma Research Center (PRC), Diponegoro University, Indonesia. The tools used for making ozonated oil were ozone generators (ozone generator manufactured in-house by Plasma Research Center, Diponegoro University) and magnetic stirrers. The ozone outlet is connected to an anti-oxidation hose with a diffuser, which served to increase the effectiveness of ozone absorption in the oil. Chemical characterizations (acidity value (AV), iodine value (IV); and peroxide value (PV)) of ozonated Aloe Vera oil samples have also been performed. As for PV evaluation, it was determined using both iodometric titrations according with the official monograph of the European Pharmacopoeia as well as placing the sample at reflux for 60 minutes18. Physical characterization has been performed by viscosity measurements (Viscomate VM-10AL, CBC Europe) at both the temperatures of 22 and 35_0.2 1C. A total of 40 ml Aloe vera oil was used in this experiment. Previous studies have used three ozone concentration – low, medium and high – which we replicated6. In this experiment the ozone concentration used were 600mg/ml as the low PV concentration; 1200 mg/ml for medium, and 1800 mg/ml as the high PV concentration. Table 1 shows the peroxide value (PV) concentration in every ozone dose.
Full-thickness wounds of one-centimeter diameter were made on the back of the rat (Figure 2). The procedure involved anesthetizing the Rats with a mixture of Ketamine (100 mg/mL; Ketamin Cas Number: 6740-88-1 Ikapharmindo, Indonesia) -Xylazine (20 mg/mL; Xyla Cat. No.: IX2 Interchemie werken "De Adelaar" BV Local Distributor: PT Tekad Mandiri Citra). A Mixture dose of 80 mg/kg Ketamine; 10 mg/kg Xylazine via the intraperitoneal route, followed by the careful placement of the rat in the left lateral decubitus position. The hair on the dorsum area was shaved, and povidone-iodine was used to clean the skin, followed by ethanol 70%. The wound area was marked, and the lesion was made by cutting the skin using a scalpel to the base of the panniculus carnosus. The fatty and connective tissues were cleaned, and the site irrigated with normal saline (if necessary), before wrapping with a band-aid. Furthermore, the non-adhesive cottonoid was applied to covering the wound. The rats were then placed in a clean and warm cage with adequate lighting to recover from the anesthesia.
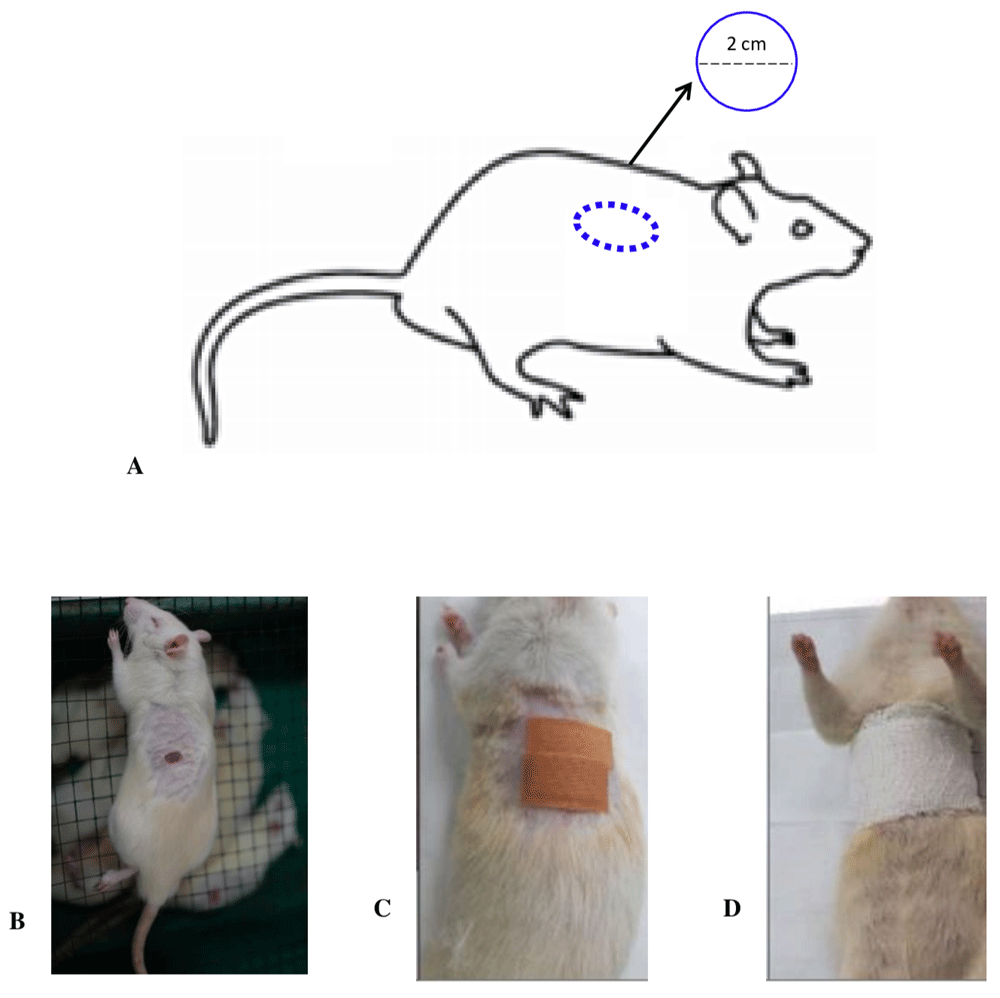
A: Image of the wound creation process on the back of rat; B: Example of full-thickness defect wound (round shape in this study); C: Placement of non-adhesive segment of Hansaplast plaster on wound model; D: Elastomull bandage was used to wrap the thorax and dorsum area of rat, and tightness was adjusted to ensure proper breathing (adequate chest expansion) and movement of extremities before returning to the cage.
Eight microliters of either untreated Aloe vera oil or Ozonated aloe vera were applied twice a day (every 12 hours) on the wounds, and 1 cm gentamycin ointment was used on the control group.
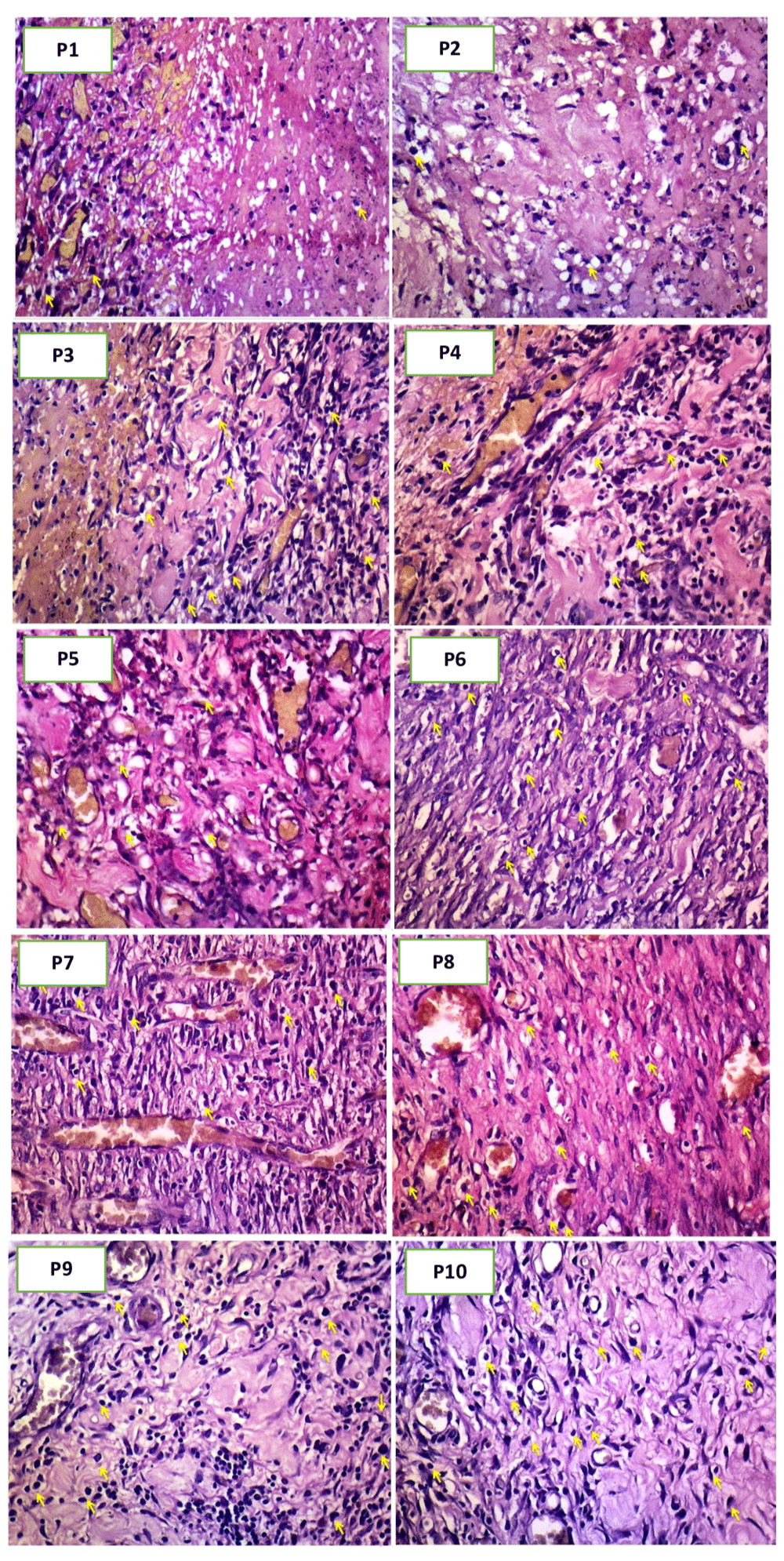
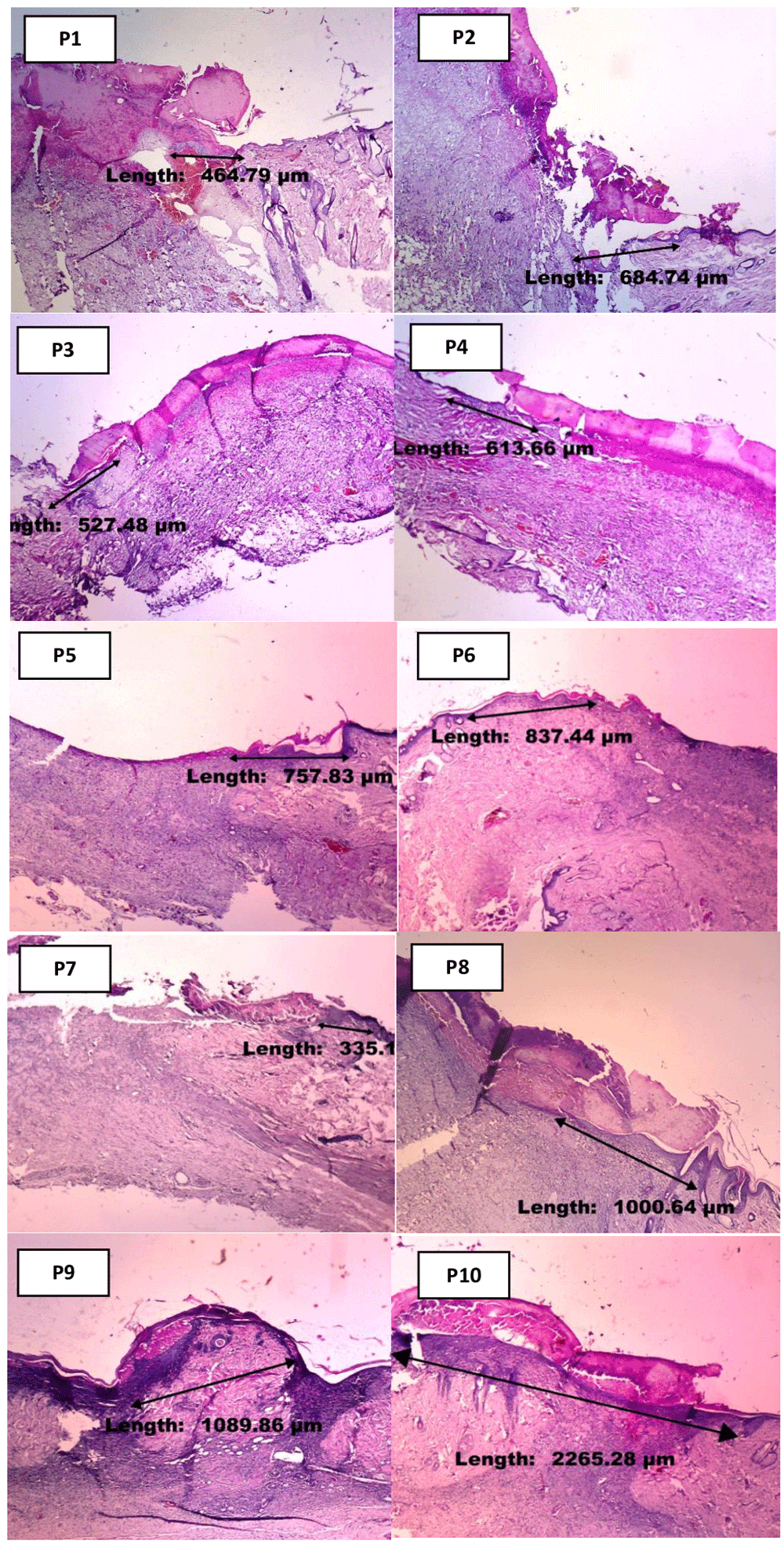
Surgical excision of the wounded area was performed at days 3 and 7 in each group. Skin tissue was immersion fixed in 10% NBF (neutral-buffered formalin) (Medici, medical labware, Jakarta Indonesia. Cat. NBF-19L) for 24 hours at room temperature. Sections (4 mm) were deparaffinized in xylene (Xylene Cath No 1.08661.2500 MERCK Merck Millipore Germany) and rehydrated in alcohol (Dipanax, Cath No 20501021606, Tangerang, Indonesia) gradients. Hematoxylin and eosin (H&E) staining (Hematoxylin Solution, Harris Modified, MFCD00078111 Merck Germany; Eosin Y Solution, Product No HT110132Sigma-Aldrich USA) were performed for histology9. The macrophage count was assessed microscopically, using a 100x magnification binocular microscope. The length of new epithelialization was examined by mason staining to the paraffin block10, followed by the measurement of epithelial tissue thickness with a microscope, under 40x magnifications. The results were then documented/photographed with optics (Miconas, Indonesia).
This research was approved and declared ethically feasible by the Ethics Commission of the Public Health Faculty, Diponegoro University Semarang, with ethical clearance number 132/EC/H/KEPK/FK-UNDIP/X/2019. All efforts were made to alleviate harm to animals by administering anesthesia to all of the study animals before full-thickness wound model creation, keeping the animals in a well-maintained cage, and ensuring graceful termination of animals before we took tissue samples for a histopathology examination.
Data analysis was performed using SPSS 21 version. All data were analyzed using descriptive statistics. Normality tests were performed using the Shapiro wilk test. Homogeneity testing and one-way ANOVA were performed to compare the control with the treatment groups. Post hoc Games-Howell was used to determine the difference between each variable. This process was conducted to investigate the presence of significant differences, and p-value <0.05 was considered statistically significant.
Table 2 shows the differences in the mean macrophage count of treatment groups 1-10, and the highest mean value was recorded on day-3, in P5(administered 1800 mg of ozonated Aloe vera oil) as 131.96. Meanwhile, the lowest (79.96) was measured on day-3, in P2, with gentamicin treatment (Figure 3). The result of the data normality test shows the absence of normal data distribution (p=0.028 or p<0.05). Hence, data transformation was performed to generate normally distributed data (with a p-value range of 0.065 to 0.884).
| Group | Mean ± SD | Median (min – max) | p | Transf. |
|---|---|---|---|---|
| P1 | 93.08 ± 20.89 | 85.6 (78.4 – 129.6) | 0.028 | 0.065* |
| P2 | 79.96 ± 11.93 | 78.2 (63.0 – 95.8) | 0.848* | 0.759* |
| P3 | 88.04 ± 34.18 | 80.2 (40.6 – 131.8) | 0.864* | 0.521* |
| P4 | 108.92 ± 13.49 | 106.2 (96 – 131.8) | 0.117* | 0.186* |
| P5 | 131.96 ± 25.95 | 141.4 (102.2 – 161.8) | 0.395* | 0.325* |
| P6 | 69.08 ± 13.08 | 68.6 (54.8 – 87.4) | 0.834* | 0.884* |
| P7 | 90.28 ± 36.21 | 78.6 (56.6 – 132.6) | 0.170* | 0.216* |
| P8 | 90.28 ± 10.67 | 90.2 (73.4 – 102) | 0.553* | 0.401* |
| P9 | 85.96 ± 17.71 | 78.2 (72 – 116) | 0.104* | 0.201* |
| P10 | 83.16 ± 25.30 | 94.8 (51.2 – 108.0) | 0.276* | 0.222* |
All normally distributed data were tested using the one-way ANOVA to ascertain the presence of significant differences in mean macrophage count between the ten groups. Table 3 shows that the data obtained were not homogenous, at p=0.028, or p<0.05. However, one-way ANOVA test resulted in a p-value of <0,001, indicating the presence of a significant difference between groups. Therefore, a Post hoc Games-Howell test was then conducted to observe the variation in the mean macrophage count of two groups. Table 4 shows the results of the Post hoc Games-Howell test, and a significant difference (p<0,05) was observed between the group provided with gentamicin (P2) and 1800 mg/ml of ozonated Aloe vera oil (P5) on day-3, at 79.96 and 131.96, respectively. Also, there was substantial variation between the groups administered 1200 mg/ml (P4) and 1800 mg/ml (P5) of ozonated Aloe vera oil sacrificed on day 3, with those euthanized Aloe vera oil on day 7 (P6), at 168.92, 131.96 and 69.08, respectively.
| Group | Mean ± SD | P | Homogenous |
|---|---|---|---|
| P1 | 93.08 ± 20.89 | 0.029* | 0.028 |
| P2 | 79.96 ± 11.93 | ||
| P3 | 88.04 ± 34.18 | ||
| P4 | 108.92 ± 13.49 | ||
| P5 | 131.96 ± 25.95 | ||
| P6 | 69.08 ± 13.08 | ||
| P7 | 90.28 ± 36.21 | ||
| P8 | 90.28 ± 10.67 | ||
| P9 | 85.96 ± 17.71 | ||
| P10 | 83.16 ± 25.30 |
| Group | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 0.935 | 1.000 | 0.807 | 0.293 | 0.429 | 1.000 | 1.000 | 0.999 | 0.996 |
| P2 | – | 1.000 | 0.107 | 0.046* | 0.898 | 1.000 | 0.891 | 0.999 | 1.000 |
| P3 | – | 0.899 | 0.580 | 0.992 | 1.000 | 1.000 | 1.000 | 1.000 | |
| P4 | – | 0.755 | 0.038* | 0.911 | 0.399 | 0.410 | 0.658 | ||
| P5 | – | 0.015* | 0.569 | 0.140 | 0.125 | 0.293 | |||
| P6 | – | 0.968 | 0.306 | 0.711 | 0.988 | ||||
| P7 | – | 1.00 | 1.000 | 1.000 | |||||
| P8 | – | 1.000 | 0.997 | ||||||
| P9 | – | 1.000 |
Table 5 shows the variation in the mean length of new epithelialization between the ten treatment groups, with the highest value of 2451.40, recorded in group P10 provided with 1800 mg/ml of ozonated Aloe vera oil, on day-7. Meanwhile, the least (799.49) was reported on day-3, in group P3 administered 600 mg/ml of ozonated Aloe vera oil (Figure 4). The data normality test result shows the normal distribution in the ten treatment groups, with p-value ranging from 0.083 to 0.970. Therefore, all normally distributed data were tested using the one-way ANOVA to investigate any significant differences in mean macrophage count between the ten groups. Based on the normality test, Table 6 demonstrated non-normal distributed data, with p=0.013 or p<0.05. However, one-way ANOVA test showed a p-value of <0.001, indicating the absence of any significant differences between treatment groups. Therefore, a Post hoc Games-Howell test was conducted to evaluate the variation in the mean length of new epithelialization between two groups.
| Group | Mean ± SD | Median (min – max) | p |
|---|---|---|---|
| P1 | 1179.20 ± 235.65 | 1142.72 (950.12 – 1523.65) | 0.606* |
| P2 | 1175.14 ± 343.37 | 1111.27 (804.10 – 1563.76) | 0.370* |
| P3 | 799.49 ± 272.93 | 789.97 (466.58 – 1141.83) | 0.907* |
| P4 | 1487.41 ± 871.70 | 1230.43 (699.53 – 2962.25) | 0.182* |
| P5 | 981.11 ± 236.15 | 871.14 (769.91 – 1275.64) | 0.158* |
| P6 | 1160.88 ± 276.51 | 1078.29 (867.30 – 1595.27) | 0.636* |
| P7 | 1367.64 ± 105.20 | 1314.88 (1254.88 – 1506.94) | 0.427* |
| P8 | 2074.48 ± 531.74 | 1981.17 (1333.25 – 2604.28) | 0.462* |
| P9 | 1984.09 ± 653.72 | 1675.41 (1397.27 – 2726.21) | 0.083* |
| P10 | 2451.40 ± 296.22 | 2303.98 (2157.46 – 2866.08) | 0.403* |
| Group | Mean ± SD | p | Homogenous |
|---|---|---|---|
| P1 | 1179.20 ± 235.65 | <0.001* | 0.013 |
| P2 | 1175.14 ± 343.37 | ||
| P3 | 799.49 ± 272.93 | ||
| P4 | 1487.41 ± 871.70 | ||
| P5 | 981.11 ± 236.15 | ||
| P6 | 1160.88 ± 276.51 | ||
| P7 | 1367.64 ± 105.20 | ||
| P8 | 2074.48 ± 531.74 | ||
| P9 | 1984.09 ± 653.72 | ||
| P10 | 2451.40 ± 296.22 |
The result of the Post hoc Games-Howell test (Table 7) shows a substantial increase in the length of new epithelialization, particularly in groups treated using 1800 mg/ml of ozonated Aloe vera oil (P10) for seven days (2451.40 μm), compared to those administered Aloe vera oil therapy (P6) and gentamicin therapy (P7), at 1160.88 µm and 1367.64 µm, respectively. Furthermore, ozonated Aloe vera oil therapy proved effective in each group of Sprague Dawley rats with full-thickness skin defect, based on the increased epithelialization.
| Group | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 1.000 | 0.447 | 0.996 | 0.920 | 1.000 | 0.798 | 0.168 | 0.390 | 0.002* |
| P2 | – | 0.665 | 0.997 | 0.978 | 1.000 | 0.942 | 0.191 | 0.425 | 0.005* |
| P3 | – | 0.773 | 0.967 | 0.579 | 0.079 | 0.043* | 0.130 | <0.001* | |
| P4 | – | 0.928 | 0.994 | 1.000 | 0.928 | 0.981 | 0.485 | ||
| P5 | – | 0.970 | 0.185 | 0.082 | 0.220 | 0.001* | |||
| P6 | – | 0.825 | 0.163 | 0.382 | 0.002* | ||||
| P7 | – | 0.312 | 0.601 | 0.008* | |||||
| P8 | – | 1.000 | 0.897 | ||||||
| P9 | – | 0.869 |
This process occurred during the wound healing process with gentamicin (P7), with no significant difference (1367.64 µm), compared to P6 treated with Aloe vera oil (1160.88 µm), P8 with 600 mg/ml of ozonated Aloe vera oil (2074.48 µm), and P9 with 1200 mg/ml of ozonated Aloe vera oil (1984.09). Therefore, the administration of ozonated Aloe vera oil at specific doses has a significant positive effect on the healing response of full-thickness defect wounds in Sprague Dawley rats, leading to faster new epithelialization length development.
The process of acute cutaneous wound healing generally consists of three stages, including the inflammatory phase experienced immediately after the injury, which extends to day-7. The second stage is proliferation, observed to day-14, and the third is the remodeling phase, known to last for several months or even years6. Previous studies have demonstrated a significant increase in the initial phase of wound healing in response to moderate doses of ozonated oil (1500 mg/dl)6. The current study showed the effectiveness of 1800 mg/ml ozonated Aloe vera oil in promoting the wound healing response of full-thickness defects in Sprague Dawley.
According to Valacchi et al.6, ozone is an efficient bactericidal, anti-fungi, and anti-viral agent, hence the propensity to reduce infection and improve skin unification during wound healing. The effect of ozonated oil on wound closure rate depends on the peroxide content6, as ozone possesses powerful oxidative characteristics in both gas and liquid phases, therefore producing microbicidal effects needed to damage the cell wall and cytoplasmic membrane of bacteria and fungi. This cascade of events leads to increased cell permeability, consequently facilitating the entry of ozone molecules11.
The use of ozonated oil also prevents superinfections and stimulates tissue reconstruction through increased cell proliferation and the formation of new vascularization6. Also, an increase in oxygen tension at the wound site elevates the tendency of granulation tissue formation, which has implications on the wound closure rate4. The augmented expression of transforming growth factor-beta (TGF-beta) and vascular endothelial growth factor (VEGF), known to play critical roles in the wound healing process, has also been achieved in clinical and experimental wound healing research with ozone therapy12.
The results of data analysis showed differences in the mean macrophage count between groups treated with 600, 1200, and 1800 mg/ml of ozonated Aloe vera oil and the control, administered Aloe vera oil or gentamicin alone. The evaluation parameter was higher in groups provided with 1200 mg/ml and 1800 mg/ml of ozonated Aloe vera oil for three days compared to those given gentamicin for three days and Aloe vera oil for seven days. This outcome was probably due to the increased oxidative role of ozone during the early phase of the inflammatory process13.
Ozone has been widely recognized as an anti-viral, bactericidal, and anti-fungal agent, and used in clinical therapy for its beneficial wound healing effects. This element is not able to penetrate the cell but is able to bind unsaturated fatty acids, and consequently form reactive oxygen species (ROS)6.
One form of ROS compound is hydrogen peroxide, known as a non-radical oxidant and implicated as an ozone mediator in several biological processes, including wound therapy14. Besides, ROS plays a crucial role in these processes by decreasing blood flow and forming a thrombus.
ROS are known to assist in the transportation of neutrophils through the blood vessels, therefore serving as a protection against bacteria by signaling the immunocytes, including monocytes, to fight pathogenic germs15. Furthermore, ROS stimulates endothelial cells to form new blood vessels, form an extracellular matrix (including collagen synthesis), and keratinocyte proliferation15, although excessive production leads to oxidative stress. This condition causes adverse effects on the wound healing process, hence the need for antioxidants to achieve a dynamic balance15.
The mean value of total macrophage produced on day 4 – 7 was lower than day 1 – 3. According to previous research, this variation was affiliated with the presence of vitamins, enzymes, proteins, carbohydrates, minerals (calcium, sodium, magnesium, zinc, and iron), and amino acids in Aloe vera, which play a major role in treating inflammation16. Also, various anti-inflammatory agents, including salicylic acid, Indomethacin, Manosa-6-phosphate, B sitosterol, as well as components of Lignin, Saponin, and Anthaquinone consisting of Aloin, Barbaloin, Anhtranol, Anthracene, Aloetic acid, Aloe-emodin, have been identified as essential ingredients of antibiotics and pain killer drugs. Meanwhile, Aloe vera is also rich in vitamins, polysaccharides, and other components with health benefits. The anti-inflammatory constituents can stimulate the immune system, leading to cell regeneration, and the consequent acceleration of healing in the inflammatory phase16.
Based on the results of new epithelialization length measurement, ozone induces a notable effect on the wound healing process. Furthermore, the administration of 1800 mg/ml ozonated Aloe vera oil for seven days had a significant positive mean difference compared to the other groups.
Various studies have been conducted to observe the role of topical Aloe vera on the wound healing process. Oryan et al.17 examined a 2×2 cm wound on the back of rats treated with Aloe vera gel, and the parameters evaluated include wound surface, size shrinkage, and epithelialization. Teplicki et al.8 demonstrated increased healing in terms of proliferation and migration of fibroblasts and keratinocytes after treatment. Aloe vera shows effectiveness through increased cell migration, keratinocyte function, and epithelialization repair8.
The current study results indicate the fastest wound healing rate in the group treated with the highest dose of ozonated Aloe vera oil (1800 mg/ml). This was followed by others, in the order of 600 mg/ml and 1200 mg/ml, the positive control administered gentamicin, and then Aloe vera oil, respectively. Therefore, further research is required to examine the effect of ozonated Aloe vera oil to ascertain the most effective dose for wound healing.
This study has several limitations, including the lack of uniformity in the hygiene level of each rat, which possibly influenced the wound healing process. Also, the wound tissue sampling was not conducted identically, therefore making it impossible to determine the exact phase of wound healing encountered on day seven, as the process in Sprague Dawley rats is perhaps different from humans.
Based on the results and discussion, ozonated Aloe vera oil applied to full-thickness skin defect in Sprague Dawley rats resulted in higher macrophage count and longer new epithelialization length than the control group.
However, uniformity in the hygiene level of each rat is important in order to accelerate wound healing processes and minimize infection. Also, there is a need for further research to investigate the exact component in Aloe vera oil responsible for the treatment progression. Furthermore, subsequent studies are also to determine the effective dose to facilitate implementation is not only experimental animals, but also humans, to observe the antioxidant effect of ozonated Aloe vera oil.
Open Science Framework: The Role of Ozonated Aloe vera Oil in Full-Thickness Skin Defect: Macrophage Count And Epithelization Length Parameter, https://doi.org/10.17605/OSF.IO/7A82V18
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like to express our gratitude to the Center Plasma Research (CPR), Diponegoro University, Semarang.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Wound healing, antioxidant, vitamin E , diabetic and alzheimer's disease
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Skin immunology, skin wound healing, modified herbal therapy, wound infection expert, histopathology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 09 Oct 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)