Keywords
Gynura procumbens, Hypertension, Vasodilation, Cholinergic pathway, Kaempferol, Acetylcholine, Muscarinic receptors
Gynura procumbens, Hypertension, Vasodilation, Cholinergic pathway, Kaempferol, Acetylcholine, Muscarinic receptors
Hypertension persists as high arterial pressure with systolic and diastolic blood pressure (BP) exceeding 140 and 90 mmHg, respectively1 contributing to cardiovascular diseases (CVDs), which are the leading cause of death globally. An estimated 17.9 million people die annually from CVDs, representing 31% of all global deaths2. More than 90% of hypertension is termed as essential or primary hypertension with unknown causes but influenced by environmental, genetic and behavioural factors3,4 including high salt intake, smoking, age, gender, obesity, diabetes, sedentary lifestyles, chronic alcohol consumption and family history of hypertension4,5. The remaining 10% of the cases fall under secondary hypertension caused by potentially reversible biochemical or mechanical pathology associated with renal or adrenal diseases6,7.
A continuous effort has been made to search for a safer and more effective method to control hypertension worldwide. Herbal medicines have been used since the beginning of human civilisation to control hypertension and other cardiovascular disorders including congestive heart failure, atherosclerosis, cerebral and venous insufficiency, arrhythmia and ischemic heart diseases8. Traditional herbs have become significant sources for drug research in pharmaceutical settings as alternatives to conventional synthetic drugs such as thiazide and captopril for hypertension9. Gynura procumbens (longevity spinach or “Sambung Nyawa”) is an evergreen herb that is used traditionally in Africa and Southeast Asia including Brunei, Indonesia, Thailand and Malaysia to treat various diseases such as hypertension, rashes, kidney disease, fever and diabetes10,11. Belonging to the Asteraceae family, this plant is small in size (around 1-3 m in height) with oval-shaped green leaves, fleshy stems and purple tint12, and can thrive in partial, shaded or full sun as long as the soil is moist. The leaves of this plant are traditionally consumed as salad, juice or tea for health benefits.
Various studies have demonstrated the antihypertensive activity of GP both in-vitro and in-vivo8–10,13–16, acting to lower the blood pressure by stimulating vasodilation besides heart stabilisation and diuretic effects. However, its mechanism of inducing vasodilation is not well understood. Activation of the parasympathetic division of autonomic nervous system induces relaxation of the blood vessel17 via the cholinergic pathway by releasing acetylcholine (ACh) from the nerve terminal in blood vessel walls, which in turn stimulates nitric oxide (NO) production that triggers dilation of vascular smooth muscle. The identity of GP’s active ingredient for vasodilation is likely to be the flavonoids, which may be responsible for its antihypertensive effect10,14,18,19. Kaempferol is a flavonoid that is present in the sub-fraction of the ethanolic extract of GP20,21, and kaempferol 3-O-rutinoside is one of the various types of kaempferol that has been identified in GP14. In this present study, we investigated GP’s vasodilatory action and, then, determined its possible activation of the cholinergic pathway mediated by kaempferol 3-O-rutinoside.
All chemicals used for methanol extraction, phytochemical content determination including total phenolic, flavonoid, flavonol and high liquid performance liquid chromatography (HPLC) were obtained from Merck, Germany except for Dragendorff’s reagent, mercuric chloride, potassium iodide, iodine, quercetin and kaempferol 3-O-rutinoside, which were purchased from Sigma Aldrich, USA. All reagents for organ bath studies including those to prepare Kreb’s Henseleit solution (Kreb’s solution), sodium chloride (NaCl), potassium chloride (KCl), potassium dihydrogen phosphate (KH2PO4), glucose, sodium hydrogen carbonate (NaHCO3) and calcium chloride (CaCl2) were purchased either from Sigma Aldrich or Merck.
Kreb’s solution was prepared by dissolving compounds in distilled water and adding calcium chloride last with continuous stirring to eliminate the formation of a salt precipitate. For the organ bath study, drugs used were dissolved following the manufacturer’s instructions and GP extracts were dissolved in Kreb’s solution. For HPLC experiment, kaempferol 3-O-rutinoside was dissolved in methanol.
GP was purchased from Rimba Horticulture Centre, Brunei Darussalam. Leaf samples were then verified by Dr Johan Willem Frederick Slik (Associate Professor in Phylodiversity, Faculty of Science, Universiti Brunei Darussalam). The herbal specimen (dried leaves) was deposited into Universiti Brunei Darussalam Herbarium (UBDH) with a sample number 17H8182.
Fresh leaves of GP were cleaned, weighed, and oven-dried at 60°C for three days. The dried leaves were then crushed using a blender (Waring 800s, USA) to produce fine powder and filter-sieved in dry form using a mechanical sieve (0.3 mm mesh hole size).
Aqueous extract of Gynura procumbens (AEGP) was prepared as described previously22. Briefly, the powdered leaves were macerated twice in distilled water (1:10 ratio of solute to solvent) and kept at 60°C water bath for three days. After filtration, the filtrate was centrifuged at 3000 rpm for 20 min, and the supernatant was recovered, and frozen at -80°C overnight. The sample was then lyophilised by freeze-drying for three days to yield a brown powder of AEGP23 that was stored at 4°C until further use.
MEGP was prepared as described previously24 with slight modifications, using ultrasonic extraction (UE) with 95% methanol in 300 W ultrasonic bath. Briefly, the powdered leaves were placed in a beaker and added with methanol at a 1:20 (solute: solvent) ratio before subjected with ultrasound at 25°C for 30 min. After extraction, the sample was filtered using filter paper, and the powder residues were added again with methanol of 1:20 (solute: solvent) ratio in another cycle of ultrasound. The filtrate obtained was evaporated with a rotary evaporator (Yamato RE301, Japan) at 63°C, and left to dry at 60°C in a drying cabinet for five days yielding a dark greenish-brown sticky solid MEGP that was stored at 4°C until further use. The percentage of yield was calculated using the following equation25:
The extracts were tested using the standard procedures for phytochemical constituents, including alkaloids, glycosides26, flavonoids, saponins, steroids, triterpenoids27 and tannins28. For stock extract solution preparation, AEGP and MEGP (1 g each) were completely dissolved in 100 mL of water and methanol, respectively. The stock solutions obtained were used for phytochemical screening whenever necessary as some of the methods required the solid form of the extracts. Qualitative results were expressed as (+) for the presence and (-) for the absence of phytochemicals. Each test was done by visual observation of colour change or formation of a precipitate after the addition of specific reagents. Hence, the experiments were only carried out once.
Around 15 mg of each extract was separately stirred in 6 mL of 1% HCl at room temperature water bath for 5 min and then filtered using Whatman filter paper (grade 42, 55 mm). The filtrates were divided into two equal parts and used for Dragendorff’s and Mayer’s tests. The orange-red precipitate formation indicated the presence of alkaloids using Dragendorff’s test upon addition of 1 mL of Dragendorff’s reagent (potassium bismuth iodide solution) to the first extract filtrate. Using Mayer’s test, the presence of alkaloids was indicated by the cream-coloured precipitate or turbid solution formation upon addition of 1 mL of Mayer’s reagent (potassium mercuric iodide solution) to the second filtrate.
The presence of flavonoids was detected by the formation of an intense yellow colour solution that turned into colourless upon addition of few drops of 1 M HCl to a mixture of 1 mL of each extract and 0.2 mL of 1 M NaOH.
Using Borntrager’s test, the presence of anthraquinone glycosides was detected by the formation of rose-pink to red colour ammoniacal layer upon addition of diluted (10%) ammonia to the lower layer of chloroform that was recovered from boiling and filtering a mixture of 1 mL of each extract, 1 mL of 5% sulphuric acid (H2SO4) and 2 mL chloroform.
The presence of saponins was detected by the formation of a topmost foamy layer upon thoroughly mixing by hand a dilution of 1 mL of each extract in 20 mL of distilled water for 15 min.
The presence of steroids was detected by the colour change of the upper layer into red and sulphuric acid (H2SO4) layer, and into fluorescent yellow upon addition of 10 mL of chloroform and an equal volume of concentrated H2SO4 into 1 mg of each extract.
The presence of tannins was detected by the development of precipitate upon addition of 1% potassium dichromate solution to an equal volume of each extract.
The formation of reddish-violet colour detected the presence of triterpenoids upon addition of 2 mL of chloroform, 1 mL of acetic anhydride and 1 mL of concentrated H2SO4 to 5 mg of each dry extract.
Total phenolic content. The total phenolic content of each plant extract was determined using Folin–Ciocalteau assay10,22,26. Briefly, 20 μL of extracts, 100 μL of 2 N Folin–Ciocalteu phenol reagent and 1.58 mL of distilled water were mixed thoroughly. After 8 min, 300 μL of Na2CO3 was added into the mixture and allowed to react at room temperature for two hours before measuring the absorbance at 765 nm using a spectrophotometer. A standard calibration curve of gallic acid (0-4 mg/mL) was prepared, and results were expressed as mg of gallic acid equivalent (GAE) per g of extract. Experiments were replicated four times.
Total flavonoid content. Total flavonoid content of each extract was determined using aluminium colourimetric method10,22,26 using quercetin (0-200 μg/mL) as a standard. Briefly, 0.5 mL of extract and 0.5 mL of the standard were placed in separate test tubes with 0.1 mL of 10% aluminium chloride, 0.1 mL of 1 M potassium acetate, 1.5 mL of 80% methanol and 2.8 mL of distilled water, and mixed thoroughly. A blank was prepared in the same manner where 0.5 mL of distilled water was used instead of the sample or standard, and the aluminium chloride replaced by distilled water. All tubes were incubated at room temperature for 30 min, and the absorbance was measured at 415 nm using the spectrophotometer. Experiments were carried out four times. Results were expressed as mg of quercetin equivalent (QE) per gram of extract.
Total flavonol content. Total flavonol content of each extract was analysed using aluminium chloride colourimetric method as described previously26. Briefly, quercetin was used to make a standard calibration curve ranging from 0 to 100 μg/mL. The test tube containing 1 mL of extract or 1 mL of standard solution was added with 1 mL of 2% aluminium chloride and 3 mL of 5% sodium acetate and then mixed well. The mixture was then centrifuged at 3000 rpm for 20 min to get a clear solution. The absorbance of the standard and sample was measured at 440 nm using the spectrophotometer. Experiments were replicated four times. Results were expressed as mg of quercetin equivalent (QE) per gram of extract.
HPLC analysis was performed as previously described29 using Agilent 1200 series HPLC system (Agilent LabX, USA) equipped with an autosampler and coupled to a variable UV wavelength detector. Liquid chromatography was performed on a reversed-phase C18 column (internal diameter 4.6 mm, height 250 mm, and particle size 5 mm). The mobile phase consisted of two solvents ([A] 5% CH3COOH, acetic acid and [B] CH3CN, acetonitrile) using the following elution program: 0–10 min 70% A isocratic, 10–20 min gradient to 40% A and then 20–30 min 40% A isocratic again with a flow rate of 1.0 mL/min at 30 ºC to detect peaks at 367 nm. Standard calibration was done using kaempferol 3-O-rutinoside at a concentration ranging from 0 µg/mL to 100 µg/mL. After the calibration curve was generated, both extracts with or without spiking with kaempferol 3-O-rutinoside (100 µg/mL) were run using the same elution program. Experiments were carried out in triplicates.
A total of 25 adult male Sprague Dawley (SD) rats at age of 16–20 weeks, weighing from 250 to 300 g, were obtained from Universiti Brunei Darussalam animal house. The animals were housed in standard environmental conditions (24°C, 60–70% humidity) on a 12 hour-light-dark cycle feeding. The animals were given standard rat pellets and tap water ad libitum.
The study was conducted following the ethical guidelines of the Laboratory Animal Care (NIH publication #85-23, revised in 1985) and approved by the University Research Ethics Committee (UREC) of PAPRSB Institute of Health Sciences, Universiti Brunei Darussalam.
After sacrificing the rats by cervical dislocation, their descending thoracic aorta was removed and placed in a petri dish on ice containing Kreb’s buffer (118.2 mM of NaCl, 4.7 mM of KCl, 2.5 mM of CaCl2, 25 mM of NaHCO3, 1.2 mM of MgSO4, 1.2 mM of KH2PO4, and 11.7 mM of glucose). The rat aorta was dissected, cleared of fats including connective tissues, and cut into rings of 3 mm long. The denuded endothelium was prepared by inserting fine forceps into the lumen of the aorta with gentle rotation. The aortic rings were mounted using two triangular-shaped platinum wire holders in the vessel lumen and suspended horizontally in an organ bath with 10 mL of Kreb’s buffer, thermoregulated at 37°C, and bubbled with carbogen to a resting tension of 1.0 g (Figure 1A). One of the holders was fixed to the bottom of the chambers, and the other was connected to a force-displacement transducer (Grass® Force Displacement Transducer FT03) for measuring isometric tension. The rings were equilibrated at 1.0 g resting tension for 20 min, with bathing solution replacement every 10 min, and tension readjustment to 1.0 g if necessary. The aortic rings were tested for viability using 40 mM KCl before conducting experiments (Figure 1B). Isometric contraction and relaxation were recorded using a Force Displacement Transducer. The transducer signals were displayed, stored and analysed on a computer using AD Instruments model 4/25T with Power Lab Software version 7. The responses (in grams) were recorded using LabChart Reader 7.

(A) Schematic set-up of the organ bath. The aortic rings were mounted using two-triangle-shaped platinum wire holders, suspended horizontally in an organ bath containing 10 mL of the above solution, thermoregulated at 37°C, and bubbled with carbogen with a resting tension of 1 g. Isometric contraction and relaxation were recorded using Force Displacement Transducer. Transducer signals were displayed and stored on a computer. (B) A summarised protocol of one of the organ bath experiments. Before each experiment, the aortic rings were stimulated with 40 mM KCl to produce a contraction, which represents a reference response, ensuring the viability of the rings just before the start of the experiment. Then the buffer solution was replaced for three times, and baseline was stabilised before adding 50 nM of NA for 10 min to induce stable contractions, and 100 μM of ACh was added for 50 min afterwards to produce the first curve. Then, the aortic rings were washed with Kreb’s buffer three times, allowed to stabilise, then added again with 100 μM ACh for 50 min in pre-contracted rat-aorta before adding extracts cumulatively to obtain the second curve.
The following experiments were conducted using both endothelium-intact and endothelium-denuded aorta, except for the protocol on the addition of noradrenaline (NA) with the extracts or kaempferol, whose effects were previously determined and ruled out as NA-independent regardless of the tissue preparation.
The aortic rings were pre-contracted by adding 50 nM of NA to induce transient and stable contraction for 60 min to generate the first response curve. Then, the aortic rings were washed with Kreb’s buffer three times, allowing the baseline to stabilise for 10 min before pre-contracted again with 50 nM of NA. After the tonic response or contraction became stable for 20 min, increasing concentrations of AEGP or MEGP (0.25, 0.5, 1 and 2 mg/mL) were added cumulatively at 10 min interval to obtain the second response curve.
The aortic rings were pre-contracted with 50 nM of NA for 10 min, followed by addition of 100 µM of ACh for 50 min to obtain the first response curve. Then, the aortic rings were washed with Kreb’s buffer three times, allowing the baseline signal to stabilise for 10 min. The aortic rings were pre-contracted again with 50 nM of NA for 10 min followed by addition of 100 μM of ACh for another 10 min before adding AEGP or MEGP cumulatively in increasing concentrations (0.25, 0.5, 1 and 2 mg/mL) every 10 min to obtain the second response curve.
The aortic rings were pre-contracted with 50 nM of NA for 30 min to obtain the first response curve. Then, the aortic rings were washed with Kreb’s buffer three times, allowing the basal tension signal to stabilise for 10 min. The rings were pre-contracted again with 50 nM of NA for 10 min, followed by addition of 10 μg/mL kaempferol 3-O-rutinoside for 20 min to generate the second response curve. This experiment was to rule out the role of kaempferol in enhancing the ACh-mediated vasodilation.
The aortic rings were pre-contracted with 50 nM of NA for 10 min, followed by addition of 100 μM of ACh for 20 min to obtain the first response curve. Then, the aortic rings were washed with Kreb’s buffer three times, allowing the basal tension signal to stabilise for 10 min. The rings were pre-contracted again with 50 nM of NA followed by addition of 100 μM of ACh before adding 10 μg/mL kaempferol at 10 min interval to generate the second response curve.
Calculations and statistical analysis were performed using GraphPad Prism (Version 6.0) software. Contractile responses were expressed in grams of tension (g). The vasorelaxation due to ACh, the addition of extracts and kaempferol 3-O-rutinoside, was expressed as a percentage relaxation in response to contraction caused by NA. All results were expressed as mean ± standard error of the mean (SEM) per experimental group with n representing the number of aortae used in each experiment. Data were analysed using two-way analysis of variance (ANOVA), which was followed by Dunnett’s test for significant differences between the control and the experimental treatments, except for the data of kaempferol 3-O-rutinoside with the addition of Ach, which was analysed using unpaired Student’s t-test. The line graph for determining the potent vasodilator between the two extracts was constructed using linear regression. P-values of less than 0.05 (P<0.05) were considered statistically significant.
The percentage yield extracted from powdered leaves of GP using aqueous extraction (AEGP) or methanol extraction (MEGP) is shown in Table 1. After drying the extracted fluid, MEGP gave a higher percentage yield of 8.7%, producing a dark greenish-brown sticky solid material, while AEGP yielded 5.5%, generating a light brown fluffy material. The phytochemical analysis is presented in Table 2, which shows that flavonoids and triterpenoids were detected in both AEGP and MEGP while saponins, steroids and tannins were only seen in MEGP. On the other hand, alkaloids were only detected in AEGP but not MEGP. Glycosides were absent in all extracts. Table 3 presents the total phenolic, flavonoid and flavonol contents in both types of GP extracts stated as mg GAE/g extract for total phenolic content and mg QE/g extract for total flavonoid and flavonol contents. AEGP has higher total phenolic content of 28.23±4.68 mg GAE/g extract, which is twice as that of MEGP (13.82 ± 2.42 mg GAE/g extract). In contrast, MEGP has four times more total flavonoid (10.37 ± 0.07 mg QE/g extract) and two times more total flavonol (4.72 ± 0.04 mg QE/g extract) content than AEGP (2.52 ± 0.21 and 2.35 ± 0.03 mg QE/g extract, respectively). The total phenolic content of GP extracts was based on the standard gallic acid curve obtained at y= 0.4332x+0.0339 (r2= 0.993), while the total flavonoid and flavonol contents were calculated from a standard quercetin curve using the linear regression equations y=0.0029x+0.0458 (r2=0.995) and y=0.0045x+0.039 (r2=0.996), respectively.
| Extract type | Description of the solid extract obtained | Yield (%) |
|---|---|---|
| AEGP | A light brown fluffy material | 5.5 |
| MEGP | A dark greenish-brown sticky solid material | 8.7 |
Values are expressed as mean of mg of GAE or QE per gram of extract ±S.E.M; n=4.
| Total phenolic content | Total flavonoid content | Total flavonol content | |
|---|---|---|---|
| AEGP | 28.23±4.68 | 2.52±0.21 | 2.35±0.03 |
| MEGP | 13.82±2.42 | 10.37±0.07 | 4.72±0.04 |
The results of HPLC screening using a reverse-phase C-18 column with a gradient system of acetic acid-acetonitrile combination as the mobile phase for both extracts revealed that when using the spiking technique with the standard reference compound of 100 µg/mL kaempferol 3-O-rutinoside, the height of peaks for both extracts was explicitly increased with the retention time observed at 1.4 min after injection. The amount of kaempferol 3-O-rutinoside calculated based on the difference of the height of peaks at 1.4 min between the standard reference and the extracts were 57.86 µg/mL and 78.35 µg/mL for AEGP and MEGP, respectively. Figure 2 shows the actual HPLC chromatograms for AEGP and MEGP, with and without spiking of 100 µg/mL kaempferol 3-O-rutinoside as the standard reference.
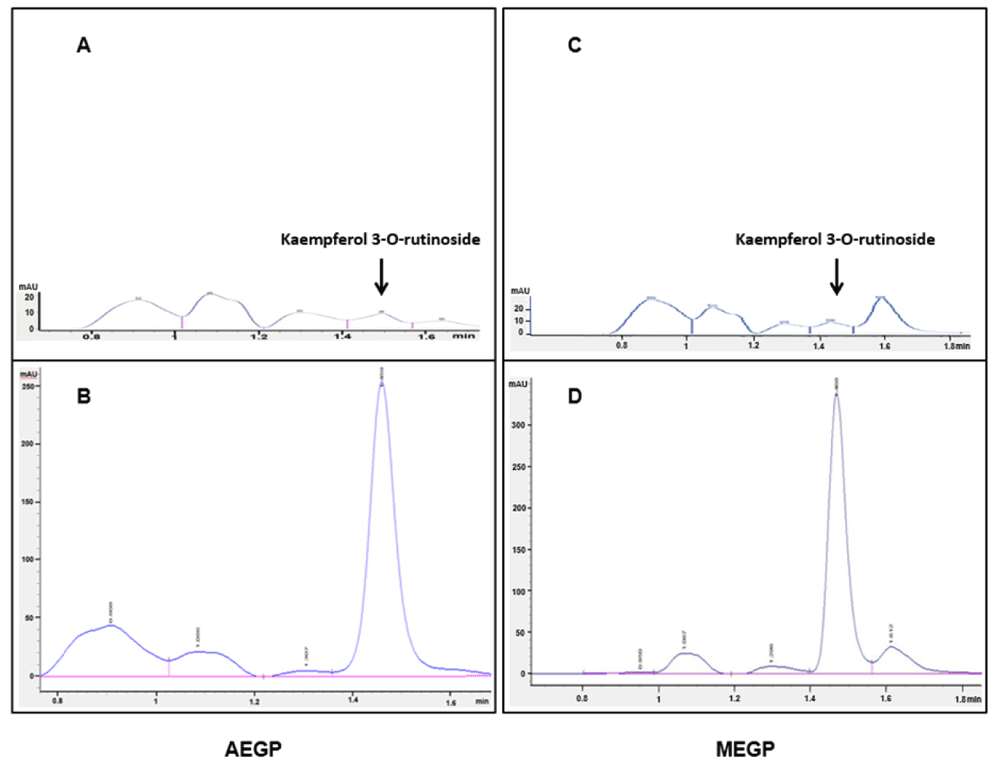
(A) AEGP alone with no spiking. (B) AEGP with spiking using 100 µg/mL of kaempferol-3-O-rutinoside standard reference stock. (C) MEGP alone with no spiking. (D) MEGP with spiking using 100 µg/mL of kaempferol-3-O-rutinoside stock. Detection of kaempferol 3-O-rutinoside was performed at 367 nm; heights of peaks were increased specifically at 1.4 min retention time when spiked with kaempferol 3-O-rutinoside (B and D) as compared with those without spiking (A and C).
Both AEGP and MEGP extracts were cumulatively added at 10 min interval in increasing concentrations (0.25, 0.5, 1 and 2 mg/mL). Addition of these extracts started at 20 min after the aortic rings were pre-contracted with 50 nM of NA. At the lowest concentration, both AEGP and MEGP produced a minimum relaxation of 12 ± 4.46% and 18 ± 3.22%, respectively (Figure 3). At the highest concentration, both AEGP and MEGP produced a maximum relaxation of 95 ± 4.53% and 112 ± 6.07%, respectively (Figure 3). In the pattern of relaxation induced by both extracts, the percentage relaxation of NA-induced contraction in rat aorta was significantly higher when exposed to increasing concentration of GP extracts, especially from 0.5 mg/mL to 2 mg/mL for both extracts, as compared with that of the control NA alone (Figure 3).
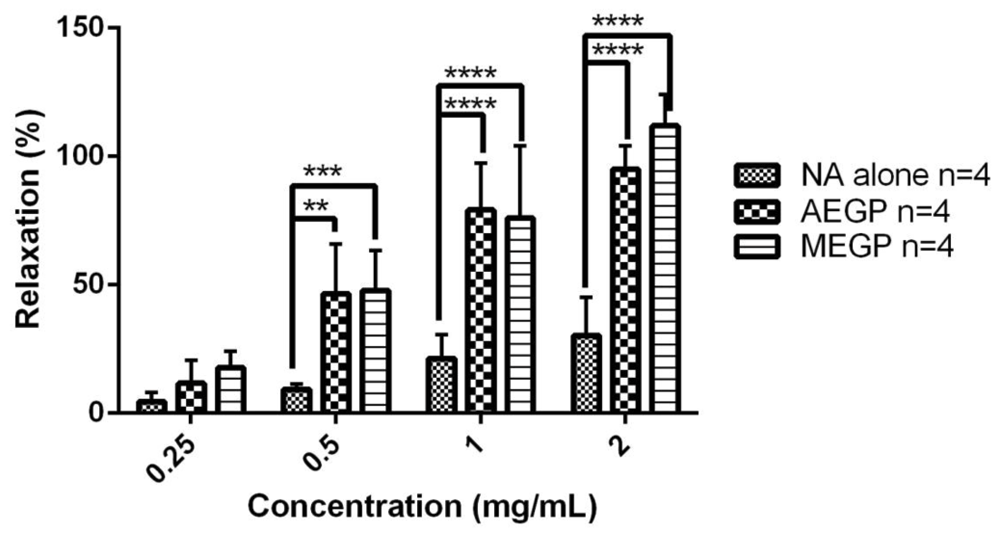
Data presented as mean ± S.E.M. The n values for NA and both extracts are 4. **P<0.05 for 0.5 mg/mL AEGP, ***P<0.05 for 0.5 mg/mL MEGP and **** P<0.05 for both 1 mg/mL and 2 mg/mL of AEGP and MEGP as compared with that of the control, NA alone.
In endothelium-intact rat aorta, both AEGP and MEGP extracts were cumulatively added at 10 min interval in increasing concentrations (0.25, 0.5, 1 and 2 mg/mL) at 10 min interval, starting at 20 min after adding 100 μM of ACh. Addition of these extracts started at 30 min following addition of 100 μM of ACh. At the lowest concentration, both AEGP and MEGP produced the minimum relaxation of 61 ± 13.80% and 72 ± 18.40%, respectively (Figure 4A). At the highest concentration, both AEGP and MEGP produced the maximum relaxation of 87 ± 4.30% and 112 ± 12.3%, respectively (Figure 4A). Relaxation induced by both extracts was significantly different from ACh control alone, especially at 0.5 mg/mL for AEGP and 1 mg/mL for both AEGP and MEGP (Figure 4A).

(A) Endothelium-intact rat aorta (B) Endothelium-denuded rat aorta. Data presented as mean ± S.E.M. The n values for ACh and both extracts are 3 for endothelium-intact rat aorta whereas n values for ACh and both extracts are 6 and 5, respectively, in endothelium-denuded aorta. *P<0.05 for 0.5 mg/mL AEGP and 1 mg/mL both AEGP and MEGP as compared with the non-treated control, ACh alone in endothelium-intact rat aorta whereas *P<0.05 for 0.5 mg/mL MEGP and 1 mg/mL AEGP as compared with non-treated control, ACh alone, and **P<0.05 for 1 mg/mL and 2 mg/mL MEGP vs. control in endothelium-denuded rat aorta.
In endothelium-denuded rat aorta, both AEGP and MEGP extracts were separately added in increasing and cumulative concentrations (0.25, 0.5, 1 and 2 mg/mL) at 10-min intervals. In both cases, the addition of these extracts started at 30 min following addition of 100 μM of ACh. At the lowest concentration, both AEGP and MEGP produced the minimum relaxation of 46 ± 15.70% and 42 ± 13.10%, respectively (Figure 4B). At the highest concentration, both AEGP and MEGP produced the maximum relaxation of 79 ± 5.60% and 114 ± 8.60%, respectively (Figure 4B). Overall, there was a pattern of relaxation induced by both extracts, where AEGP significantly enhanced relaxation at 1 mg/mL, while MEGP enhanced relaxation at a wider range of 0.5 mg/mL to 2 mg/mL (Figure 4B).
Exposure of endothelium-intact rat aorta to either extract when added cumulatively produced a similar vasodilatory effect as a reduction in the percentage of NA peak response. Exposure to increasing concentration of AEGP up to 1 mg/mL reduced the contraction of rat aorta from 39 ± 13.9% to 0 ± 17.1%, and MEGP from 40 ± 18.4% to 5 ± 14.0%. ACh alone without the addition of extracts still showed a 23 ± 4.90% contraction. However, at 2 mg/mL, both extracts had a differential effect, in which MEGP caused further reduction from 5 ± 14.0% to 0 ± 12.3% contraction, whereas AEGP produced a rise of contraction from 0 ± 17.1% to 18 ± 10.2%.
Exposure of endothelium-denuded rat aorta to either extract also produced a similar vasodilatory effect, in which the percentage contraction of NA peak response at concentrations reaching up to 1 mg/mL was reduced from 65 ± 9.35% to 0 ± 6.60% for AEGP and 72 ± 13.1% to 21 ± 9.88% for MEGP, respectively. In comparison, ACh alone without the addition of any extract was only able to reduce the contraction from 80 ± 9.31% to 50 ± 7.58%. However, at 2 mg/mL both extracts had a different effect in which MEGP caused a further reduction of contraction from 21 ± 9.88% to 0 ± 8.64%, whereas AEGP produced a rise of contraction from 0 ± 6.60% to 8 ± 5.61%. Overall, Figure 5 demonstrates the vasodilatory action of both GP extracts, expressed as the normalised data for the effect of both AEGP and MEGP observed as a reduction in the contraction of endothelium-intact and denuded aortic rings that were pre-contracted with 50 nM of NA.

(A) Effect of both AEGP and MEGP in endothelium-intact aortic rings. (B) Effect of both AEGP and MEGP in endothelium-denuded aortic rings. Each extract (AEGP or MEGP) was cumulatively added in increasing concentration of 0.25, 0.5, 1, and 2 mg/mL. The effect of control (ACh alone) was measured at 30, 40, 50 and 60 min, corresponding to the time at which the respective concentration of extracts was added cumulatively. Data presented as mean ± SEM (n=3 for control and both AEGP and MEGP in endothelium-intact rat aortic rings; and, n=6 for control, and n=5 for both AEGP and MEGP, respectively, in endothelium-denuded rat aortic rings).
To elucidate the role of kaempferol-3-O-rutinoside in inducing relaxation, we first determined the vasodilatory effect of kaempferol without the application of ACh in the pre-contracted aorta rings. Kaempferol 3-O-rutinoside was added for 20 min, which started at 10 min after pre-contraction with 50 nM of NA. Addition of 10 µg/mL of kaempferol 3-O-rutinoside caused rapid relaxation (Figure 7), which was significantly increased from 9.8 ± 1.56% to 84.9 ±18.7% at 20 min and from 17.5 ± 5.24% to 94.6 ± 5.59% at 30 min, respectively, as illustrated in Figure 6.

Data presented as mean ± SEM The n values for NA alone and NA with kaempferol 3-O-rutinoside are 5 and 3, respectively. ****P<0.05 for the addition of kaempferol 3-O-rutinoside at 20 and 30 min vs control, NA alone.
The pre-contracted aortic rings with intact and denuded endothelium were supplemented with 10 µg/mL of kaempferol 3-O-rutinoside at 20 min after addition of 100 µM of ACh to determine the involvement of kaempferol 3-O-rutinoside in potentiating the cholinergic pathway. Addition of kaempferol 3-O-rutinoside significantly enhanced the vascular relaxation evoked by ACh in rat aorta with and without the endothelium. Kaempferol 3-O-rutinoside elicited a maximum relaxation of 76.0 ± 4.66% and 70.7 ± 0.73% with or without endothelium, respectively. In comparison, the control produced relaxation of 41.7 ± 6.47% and 19.9 ± 6.69%, with and without endothelium, respectively (Figure 7).

(A) Endothelium-intact rat aorta (B) Endothelium-denuded rat aorta. Data presented as mean ± SEM The n values for ACh alone and kaempferol 3 O-rutinoside are 6 and 4, respectively in endothelium-intact rat aorta. In contrast, the n values for ACh alone and kaempferol 3 O-rutinoside are 5 and 3, respectively in endothelium-denuded rat aorta. **P<0.05 for the addition of kaempferol 3-O-rutinoside at 30 min vs control, ACh alone in both endothelium-intact and –denuded rat aorta.
Raw data for each experiment performed in this study are available, see Underlying data30.
Various studies have been carried out to identify the antihypertensive ability of GP extracts8–10,13–16. The presence of bioactive compounds in GP is believed to contribute to its beneficial properties such as flavonoids, phenolic acids, alkaloids, terpenoids, glycosides and sterols12. In this present study, the antihypertensive property of both crude extracts of AEGP and MEGP was investigated in vitro to determine whether they were able to potentiate the cholinergic mediated vasodilation. Limited studies showed the possible contribution of the cholinergic pathway in enhancing the relaxation effect of GP extracts. Successful extractions with water or methanol from the dried and powdered leaves of GP were achieved, producing the fluffy brown powder and the sticky dark greenish-brown substance as yields for AEGP and MEGP, respectively. The characteristics of AEGP in our study were similar to those reported by Lee et al.23 and Kamaruzaman and Mat Noor31. There was neither an established guideline nor any known protocols for methanol-based extraction for GP plant leaves. However, the characteristics of the dry methanol extracts, yielding a dark brown sticky solid was demonstrated by Korwar et al.32, similar to our findings. The percentage yields for both extracts were different, which might be due to the polarity of the solvents used33. Given an equal initial amount of fresh weight for the plant parts, the aqueous solvent yielded less extract than those extracted with the methanol solvent, suggesting that the plant GP possesses more methanol-soluble components33. Our finding is in concordance with a study done by Njume et al.34 and Ngo et al.35, where absolute methanol or a mixture of 50% (v/v) water with other solvents such as alcohol or acetone was recommended as the solvent of choice to produce high levels of extractable ingredients.
Our results highlighted the observation that both extracts, which were obtained using different solvents, contained phytochemicals, such as flavonoids and triterpenoids. In addition, MEGP responded positively for the saponins, steroids and tannins, while AEGP was detected positive for alkaloids. However, both extracts did not give a positive test for glycosides. The efficiency of methanol in extracting phytochemicals has been reported in the previous studies34,36,37. Our results were consistent with those of others, confirming that methanol is an appropriate solvent for extracting bioactive compounds from GP, giving higher yields than its aqueous counterpart.
Phenolic compounds account for the majority of pharmacological actions. For several decades, phenolic compounds are regarded as having a powerful free radical scavenging activity38,39. As many aspects of CVDs are associated with high oxidative stress, the consumption of polyphenol-rich food that combats oxidative stress can help lower the risk of chronic diseases, including cardiovascular disorders40–42. In this present study, the total phenolic content of AEGP is higher than MEGP. However, the latter was shown to contain more total flavonoids and total flavonol content. The results obtained for the flavonoids are in agreement with the previous report on GP by Afandi et al.22, showing that MEGP contains higher total flavonoid content than AEGP, but the amount of phenolic content was found lowest in AEGP among all the other extract preparations. This variation could be due to differences in the process of extractions and the solvents used. Various factors could contribute to the efficiency of extraction, such as the type and concentration of solvents, pH, time, temperature and solid-liquid ratios43. Besides, the different types and amount of phenolic and non-phenolic compounds extracted are dependent on the extraction solvent used44. The results showing that AEGP contained a lower percentage of total flavonoid and total flavonol compounds than MEGP might be due to flavonoids having low solubility in water20.
The presence of flavonoids in both extracts suggests they have a considerable role in various pharmacological effects, especially the hypotensive action, which might explain the BP- lowering observation and claims in the traditional practice of consuming the GP leaves to treat hypertension19. Experimental evidence has shown that the flavonoids in GP extracts is responsible for an antihypertensive effect10,14. Kaempferol, which is one of the significant flavonoids dominantly present in the sub-fraction of the ethanolic extract of GP14,20,21, was also detected in both extracts in our study. Kaempferol 3-O-rutinoside was detected in a higher amount from MEGP than AEGP. After quantifying this active ingredient, we decided to test its vasodilatory activity in the organ bath system using rat aorta to elucidate its hypotensive contribution. We first added the extracts after NA-induced contraction of aortic rings to confirm the vasodilatory action of both extracts. Our results highlighted the vascular relaxation effect indicating the antihypertensive property of this plant and confirming reports from previous studies that GP extracts might reduce blood pressure by vasodilation8–10,13,14,45. Next, we investigated whether there is a possible contribution of the cholinergic-mediated pathway in the vasodilatory effect of the extracts. Our present finding demonstrated that the vasorelaxation activity of the extracts further enhanced the ACh-mediated vasodilation in the endothelium-intact aorta, primarily upon exposure to 0.5 mg/mL AEGP and 1 mg/mL of both AEGP and MEGP. These results support the findings of Kaur et al.8, which showed that ACh administration to GP-treated rats in vivo significantly reduced the mean arterial pressure (MAP) and diastolic pressure (DP) of spontaneously hypertensive rat (SHR), and that of Kim et al.13, which detected an increased of NO levels in the serum of Gynura procumbens water extract (GPWE)-treated SHR.
Our data further revealed that the extracts significantly exerted their relaxation effect in the endothelium-denuded aorta, suggesting that the vasodilatory action of AEGP and MEGP extracts is also mediated directly through the vascular smooth muscles. The vascular smooth muscle cells are located within the media of the vascular bed, directly modulating the vascular tone primarily via the changes in sarcoplasmic free Ca2+ concentration46. Various signalling mechanisms could trigger the response of the smooth muscles through an ACh endothelium-independent pathway such as the adrenergic pathway. In the adrenergic pathway, α1 and α2 adrenoceptors mediate contraction of the smooth muscles by coupling to Gq and activating phospholipase C (PLC), thereby increasing the concentration of Inositol 1,4,5-triphosphate (IP3), which in turn, causes the rise in intracellular Ca2+ concentration47. A previous study showed that GP extracts could mediate relaxation via α1receptors in-vivo8. PE (an α adrenoceptor agonist) and methoxamine (a selective α1 adrenoceptor agonist) induced a significant decrease in pressor response of GPWE-treated rats8. From our findings, we speculate that the GP extracts might also mediate relaxation via adrenergic pathway by activating α and β adrenoceptors. A more favored explanation for this action, however, is based on the evidence that we obtained so far, which pointed towards the cholinergic pathway. It is known that ACh fails to induce relaxation when the endothelium is denuded and removed48,49, suggesting that vasorelaxation of smooth muscle is inhibited when the formation of NO is impaired due to activation of M2 and M3 receptors, hence causing contraction instead50,51. Thus, we focused more on investigating the cholinergic endothelium-independent pathway via stimulation of M2 and M3 muscarinic receptors that inhibit the contraction. Overall, our follow-up study elucidated and confirmed that the underlying mechanism of action for both extracts was mediated via the cholinergic pathway, with a significant contribution from kaempferol.
Investigation on the vasodilators between these two extracts was carried out in both endothelium-intact and endothelium-denuded rat aorta. Results have shown that upon exposure at the same concentration of 2 mg/mL, MEGP produced a more potent vasodilatory effect than that of AEGP. Although at a lower concentration, both extracts responded in a dose-dependent manner, AEGP seemed to reverse the effect into slight contraction upon reaching the concentration of 2 mg/mL, unlike MEGP, which continued to cause further relaxation. Drug effects are caused by the interaction of the drug molecules and the receptor. At low concentration of the drug, many binding sites are still available; however, further increasing the drug concentration causes more drug-receptor complex formation and a substantial increase in drug effect52 until all of the receptor binding sites are occupied. Further increasing the drug concentration causes receptors to become saturated, leading to no or little effect of the drug52. Thus, the slight reverse effect of contraction by AEGP at 2 mg/mL indicates the possible saturation of all the receptors binding sites for the relaxation effect of the blood vessels, limiting any further vasodilation. Thus, in using AEGP, a possibly saturated concentration exceeding 2 mg/mL would not cause any relaxation effect but a contraction of the blood vessels instead.
Flavonoids rich food, primarily via consumption of botanicals, is often associated with a reduction of cardiovascular diseases53–55. Kaempferol 3-O-rutinoside was detected and quantified as one of the active ingredients in GP20. Thus, we decided to test its hypotensive property in an organ bath system using rat aorta. We first carried out the experiments to confirm the relaxant effect of kaempferol 3-O-rutinoside in rat aortic rings, resulting in vasodilatory activity after NA-induced contraction. This result is in agreement with those of previous studies that demonstrated the vasodilatory effects of kaempferol in different vascular beds55–58. Similar findings have also been reported on the vasorelaxant properties of other flavonoids such as quercetin, myricetin, rutin and apigenin57,59. Kaempferol, however, is considered as the most effective among all flavonoids in potentiating vasorelaxation, thus might have more significant potential in preventing and treating CVDs57. We assessed the involvement of kaempferol 3-O-rutinoside in potentiating ACh-dependent endothelium-mediated vasodilation, and ACh-independent mediated contraction. Our results showed that kaempferol elicited the relaxation response produced by ACh in both endothelium-intact and endothelium-denuded rat aorta. Our observation suggests that kaempferol enhanced the relaxation in rat aortic rings possibly via endothelium-dependent and endothelium-independent cholinergic pathway, following previous reports57,58, which is possibly mediated by kaempferol 3-O-rutinoside, as illustrated in Figure 8.
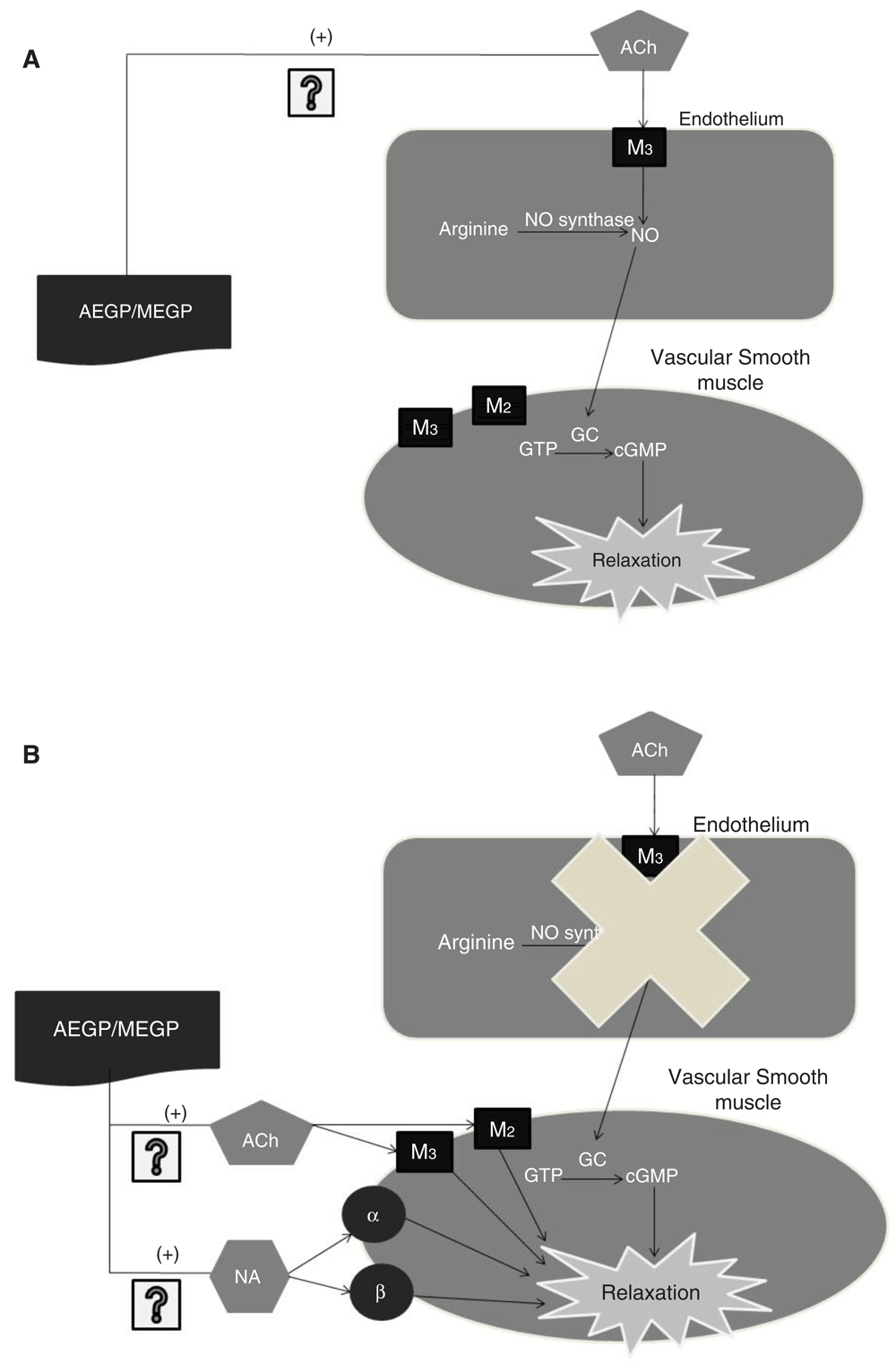
The figure is a simplified representation showing the main component associated with acetylcholine mediated endothelium vasodilation in the isolated rat aorta (IRA) model and showing the involvement of both extracts in inducing relaxation in vascular endothelium or vascular smooth muscle. In the presence of endothelium, both extracts (AEGP/MEGP) mediate vasodilation possibly by acting on the muscarinic ACh receptor M3by producing NO. When the endothelium is disrupted, the extracts mediate relaxation on vascular smooth muscle possibly via endothelium-independent cholinergic pathway by acting on the muscarinic ACh receptor M2 and the muscarinic ACh receptor M3or via adrenergic pathway by acting on α and β adrenoceptors. The vasorelaxant effect produced by the extracts is mediated by kaempferol, particularly, kaempferol 3-O-rutinoside. ACh; Acetylcholine M2: Muscarinic acetylcholine receptor M2; M3: Muscarinic acetylcholine receptor M3; α: alpha adrenoceptors; β: beta-adrenoceptors; NO: Nitric oxide; GC: Guanylyl cyclase; cGMP: cyclic guanosine monophosphate; AEGP: Aqueous extract of G. procumbens; MEGP: Methanol extract of G. procumbens.
The result of this study shows that crude extracts of AEGP and MEGP possessed different characteristics and physical appearance, as well as the percentage yield. MEGP was a dark greenish-brown sticky solid whereas AEGP was a light brown fluffy material. The percentage yield of MEGP was higher than AEGP. The result of this study also reveals the presence of essential phytoconstituents in both AEGP and MEGP, and MEGP was found to contain more active constituents than AEGP. Besides, MEGP also contained higher total flavonoid and flavonol contents than AEGP, although the amount of phenolic content was lower in MEGP. HPLC study has demonstrated the presence of kaempferol 3-O-rutinoside, where it is higher in MEGP than AEGP. Treatment with the pure active ingredient kaempferol 3-O-rutinoside relaxed both tissue preparations. Overall, these results imply the possible involvement of extracts in the cholinergic pathway, which is possibly mediated by kaempferol 3-O-rutinoside, as shown by its vasorelaxation effects in endothelium-intact and endothelium-denuded aorta. The present finding also demonstrates the vasodilatory effect of the two extracts, in thoracic aorta tissues isolated from rats, possibly via the contribution of the cholinergic mediated pathway. It is also concluded that the main active vasodilatory component in both extracts causing such hypotensive effect is the flavonoid particularly kaempferol 3-O-rutinoside. However, further study is necessary to elucidate its mechanism of action.
Open Science Framework: Anti-hypertensive Vasodilatory Action of Gynura procumbens. https://doi.org/10.17605/OSF.IO/W6XJV30
This project contains “Anti-hypertensive Vasodilatory Action of Gynura procumbens.xlsx”, which includes raw data for all experiments performed in this study.
Data are available under the terms of the Creative Commons Attribution 4.0 International licence (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular and safety analysis of food and herb products
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cell biology and drug delivery
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 12 Oct 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)