Keywords
autoimmune pancreatitis, obstructive jaundice, IgG4-related disease, endoscopic ultrasound, corticosteroids.
autoimmune pancreatitis, obstructive jaundice, IgG4-related disease, endoscopic ultrasound, corticosteroids.
Autoimmune pancreatitis (AIP) is a rare disease, meaning that clinicians have little experience in diagnosis1. Clinical suspicion is the first step for correct diagnosis, arising from the patient’s demographics and clinical presentation. This case presented here emphasizes the major role of endoscopic ultrasound (EUS) and biopsy in the final diagnosis of AIP, when other diagnostic procedures fail to offer an accurate answer.
A female patient, 60-years-old, was admitted to the emergency department with painless jaundice. The patient did not have any history of alcohol consumption, drug abuse, or previous liver and biliary or hematologic diseases.
On clinical examination, the patient’s eyes and skin were yellow. Murphy and Giordano signs were negative. Blood tests showed serum lipase and amylase within normal limits, total bilirubin at 11.2mg/dl [normal range: 0.3–1.5mg/dL] (direct fraction 5.9 mg/dL, indirect fraction 5.3 mg/dL), hematocrit within normal limits, white blood cell count of 5.5 K/μL [normal range: 4.5–11 K/μL], and a normal fasting blood glucose level. Patient’s liver enzymes were elevated (aspartate aminotransferase 109 U/L [normal range 7–40U/L]; alkaline aminotransferase 230U/L [normal range: 5–45U/L]; alkaline phosphatase 238U/L [normal range: 40–150U/L]). Initial ultrasound examination revealed enlargement of the pancreas, with low echogenicity and dilatation of the biliary tree. The diameter of the pancreatic duct was normal (Figure 1).
Dynamic computed tomography revealed free peripancreatic fat with no other signs, indicating acute pancreatitis and mild enlargement of the pancreas with homogeneous density and enhancement. There were no signs indicating neoplasm.
For better evaluation of the pancreaticobiliary tree, an magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MPCP) was performed, showing mild pancreatic enlargement. There was no obvious dilatation of pancreatic duct, with stenosis of the final part of common bile duct (Figure 2).
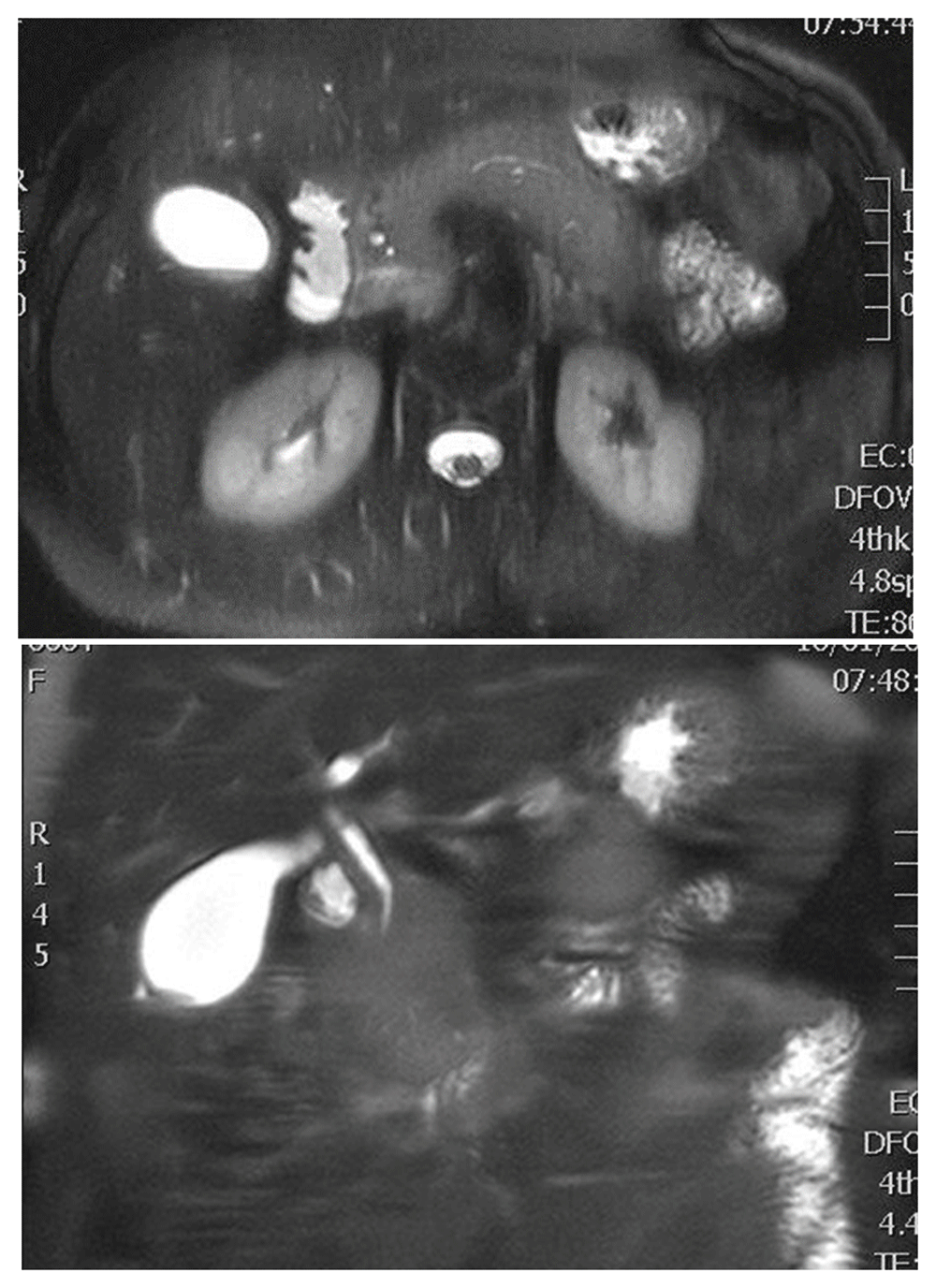
Since MRCP has inferior resolution in the imaging of pancreatic duct, an endoscopic retrograde cholangiopancreatography (ERCP) was performed, which confirmed the MRCP findings, showing no significant prestenotic dilatation of the common bile duct. During the procedure, a dilatation of common bile duct with a balloon-catheter and a plastic stent placement was performed to alleviate the patient from jaundice. Furthermore, tissue-sampling was conducted from the stenosis, with results indicating inflammatory process of the duct wall. The patient was transferred to a tertiary centre, where, in order to obtain a more precise and direct imaging of the pancreas, EUS and EUS elastography was performed, showing a diffusely enlarged gland with hypoechoic, patchy, heterogeneous appearing parenchyma, with regions of stiffness, suggesting AIP. In addition, the presence of a hypoechoic mass in the final part of the intrapancreatic portion of common bile duct was found, which increased the suspicion of cholangiocarcinoma, deteriorating the diagnostic procedure (Figure 3). EUS with fine needle aspiration of the mass, using a 25-gauge needle, was performed. Several inflammatory cells, fibroblasts and traces of fibrous tissue were found in the samples. lgG4 levels were elevated, measuring 250mg/dl [normal range: <140mg/dL]. The combination of clinical, imaging and cytological findings pointed to the diagnosis of AIP.
Prednisolone 40mg daily for a month was given as therapy, leading to clinical and radiological disease remission. MRl one month following treatment revealed almost a normal pancreas (Figure 4), with subsequent normalization of lgG4 levels in serum. Nine months later, during dosage reduction with a taper of 5mg/week, the patient attended the emergency room once again, presenting with painless jaundice. Ultrasound indicated pancreas enlargement, with elevation of IgG4 in serum and [200mg/dL] the relapse of the disease. “Rebound phenomenon” was the most possible scenario. Re-induction/increased dose of steroid therapy to 40mg/daily for a two month period caused the remission of the disease. Α dose reduction rate of 5mg every 1–2 weeks followed, until prednisolone dose reached 15 mg/day. Then the reduction rate was decreased to 2.5mg every 2 weeks, until a dose of 3.0mg/day was reached.
Maintenance therapy for three years was decided to prevent disease relapse, since most relapses occur in the first three years after diagnosis2. At the moment, three years later, the patient stays in remission, receiving 3.0mg/day of prednisolone.
Autoimmune pancreatitis (AIP) consists of an uncommon type of chronic pancreatitis, accounting for 2–11% of all cases of chronic pancreatitis3. Two subtypes of the disease, defined by their histopathology, are clearly recognized since 20104: lymphoplasmacytic sclerosing pancreatitis, known as Type 1; and idiopathic duct-centric pancreatitis (ICDC), Type 2. Both types are steroid responsive. Type 1 is the pancreatic manifestation of lgG4-related disease. There are many extrapancreatic organs that may be involved, such as the biliary tree and gallbladder, kidneys, retro-peritoneum, prostate, the mesentery, blood vessels, gastrointestinal tract, thyroid, lacrimal glands and orbits, salivary glands, lymph nodes, and lungs5. The biliary tract is the most commonly involved extrapancreatic site, hence the painless jaundice in AIP patients, as in the present case5. The clinical features of AIP depend on the phase,acute or subacute. In the acute phase, the most common clinical presentation for both subtypes of AIP is obstructive jaundice, usually painless. In the subacute phase, AIP imitates chronic pancreatitis due to pancreatic atrophy, leading to steatorrhea. The incidence of diabetes mellitus in patients with AIP is up to 50%6. There are three established patterns of autoimmune pancreatitis depending on pancreatic appearance macroscopically: diffuse, as described in the current case, focal, and multifocal.
Diagnosis of AIP is challenging, and many clinical, imaging and biochemical features overlap with other conditions, such as idiopathic pancreatitis, primary sclerosing cholangitis and malignancies, mainly pancreatic cancer and cholangiocarcinoma. A plethora of diagnostic criteria have been proposed by different groups around the world. The first was proposed by the Japan Pancreas Society in 20027, combining cardinal features, concerning histology, imaging, serology, other organ involvement, and steroid effect3 (Table 1). ICDC is the first universally accepted criterion of AIP because it considers national differences and establishes two types8.
MPD, main duct dilation; This table was adapted from Cai and Tan9 under a Creative Commons Attribution License (CC-BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Exclusion of malignancy is crucial for the clinician to choose either conservative or surgical treatment. Focal pattern is most difficult to distinguish from cancer, especially ductal adenocarcinoma (PDAC)10, for which there is no pathognomonic biomarker, including lgG4 levels and CA 19-92,11. The mistaken diagnosis of AIP as PDAC, or vice versa, can result in unnecessary or life-threatening surgery or delayed treatment. Among diagnostic procedures (Table 2), EUS-FNA consists of a sensitive method for detecting/excluding pancreatic cancer, as in our case12.
This table was adapted from Cai and Tan9 under a Creative Commons Attribution License (CC-BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The prognosis of AIP is generally good and complications rare. Our patient remains free from symptoms three years after diagnosis with a successful treatment of relapse. However, the role of AIP as a predisposing factor for pancreatic cancer needs to be investigated. Internists should keep in mind AIP as a rare entity in the diagnostic spectrum of pancreatic diseases and when high clinical suspicion exists, based on clinical presentation, demographics and patient’s history, should consider this diagnosis. Diagnosis can be made according to ICDC 20118. Clinicians, pathologists and radiologists have to be aware of this recently recognized entity13, to suspect and timely diagnose it, to give patient the appropriate steroid therapy, avoiding inappropriate pancreatic resections, which lead to increased morbidity and mortality.
Although diagnosis of AIP is challenging, respect to the established diagnostic criteria helps to have a definite and patient-safe result. When a clinical/radiological or other suspicion exists, clinicians should follow diagnostic algorithms to rule out other pathologies, especially pancreatic and billiard cancer. In our case, based on clinical presentation, the preferable diagnosis was malignancy, but imaging features of CT, ERCP and MRI/MRCP were not indicating this. EUS imaging deteriorated the diagnostic procedure, as it showed a hypoechoic mass, which is highly suspicious for malignancy, specifically cholangiocarcinoma. Finally, the combination of histological results, obtained after EUS biopsy, and elevated IgG4 levels established AIP diagnosis. In the current literature, the important role of EUS in the diagnosis of AIP has been highlighted. Our case emphasizes the role of EUS/elastography and EUS biopsy in diagnosis of the disease.
Exclusion of malignancy is crucial for the clinician to choose either conservative or surgical treatment. The misdiagnosis of AIP, especially focal pattern, as pancreatic duct adenocarcinoma, or the reverse, can result in medical malpractice. Sometimes biopsy is necessary, as in the present case and EUS is an ideal method for tissue sampling.
Glucocorticoids are the cornerstone in the therapeutic approach of AIP and effectiveness of steroid treatment consists of a major diagnostic criterion. Therapeutic goal of AIP is to achieve clinical, serologic and radiological remission of the disease.
Written informed consent for publication of clinical details and clinical images was obtained from the patient.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pancreatitis (acute and chronic), EUS, drainage of pancreatic collections, Third space endoscopy
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the case presented with sufficient detail to be useful for other practitioners?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: My specialty is biliary pancreatic disease. I also have a lot of clinical experience with autoimmune pancreatitis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 18 Nov 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)