Keywords
Halogen light, Light-emiting-diode, mouse fibroblasts, temperature increase
Halogen light, Light-emiting-diode, mouse fibroblasts, temperature increase
Dentistry patients are looking for esthetically pleasing smiles and increasingly demanding resin composite restorations and teeth bleaching (Al Otaibi et al., 2020; Sebold et al., 2020). These processes require photopolymerization and photoactivation by halogen bulb (HAL) and light-emitting diode (LED) light curing units (LCUs) (Gallinari et al., 2020; Pieniak et al., 2014). However, these LCUs can increase the temperature and induce thermal transfer, depending on the light source intensity and type (Armellin et al., 2016; Kim et al., 2017).
The elevated temperature resulting from daily clinical procedures can cause an increase in pulp temperature, with the subsequent development of symptoms such as hyperalgesia, dentin hypersensitivity, and spontaneous typical pain of acute pulpitis (Vinagre et al., 2019). The thermal change causes heat-induced bone tissue injury (Eriksson & Albrektsson, 1983) and pulp tissue necrosis, pathology or alteration (Matalon et al., 2010; Nyborg & Brännström, 1968).
Additionally, some thermal injuries may affect the surrounding tissue cells (Baldissara et al., 1997) and form lesions in the odontoblastic layer, which leads to its degeneration, protein coagulation and fluid expansion in the dentinal tubules (Vinagre et al., 2019). Some contributing factors affect the extent of injury, such as the remaining dentin thickness, the type of the LCU, the type of ultrasonic device, or the type of water spray used (Kwon et al., 2013).
Therefore, controlling the temperature increase during the emission of LCUs is an important factor in the use of photopolymerizers. The objective of this study was to compare the temperature increase produced by HAL and LED LCUs by irradiating dentin discs (0.5 mm and 1 mm thickness), and to evaluate their cytotoxic effects on fibroblast culture in the presence of dentin discs.
A total of 20 bovine incisors were used in this study, the crowns were separated from the roots 2 mm below the level of cemento-enamel junction and then embedded in self-curing acrylic resin (TDV, Santa Catarina, Brazil) in a prefabricated PVC mold. Later, longitudinal dentin discs (without enamel) were obtained (10 discs of 0.5 mm and 10 discs of 1 mm) by sectioning the crowns using diamond disc (0.3 mm thickness) and an EXTEC cutting machine (Labpol 8-12, Extec Corp®, Enfield, Connecticut, USA) (Figure 1).
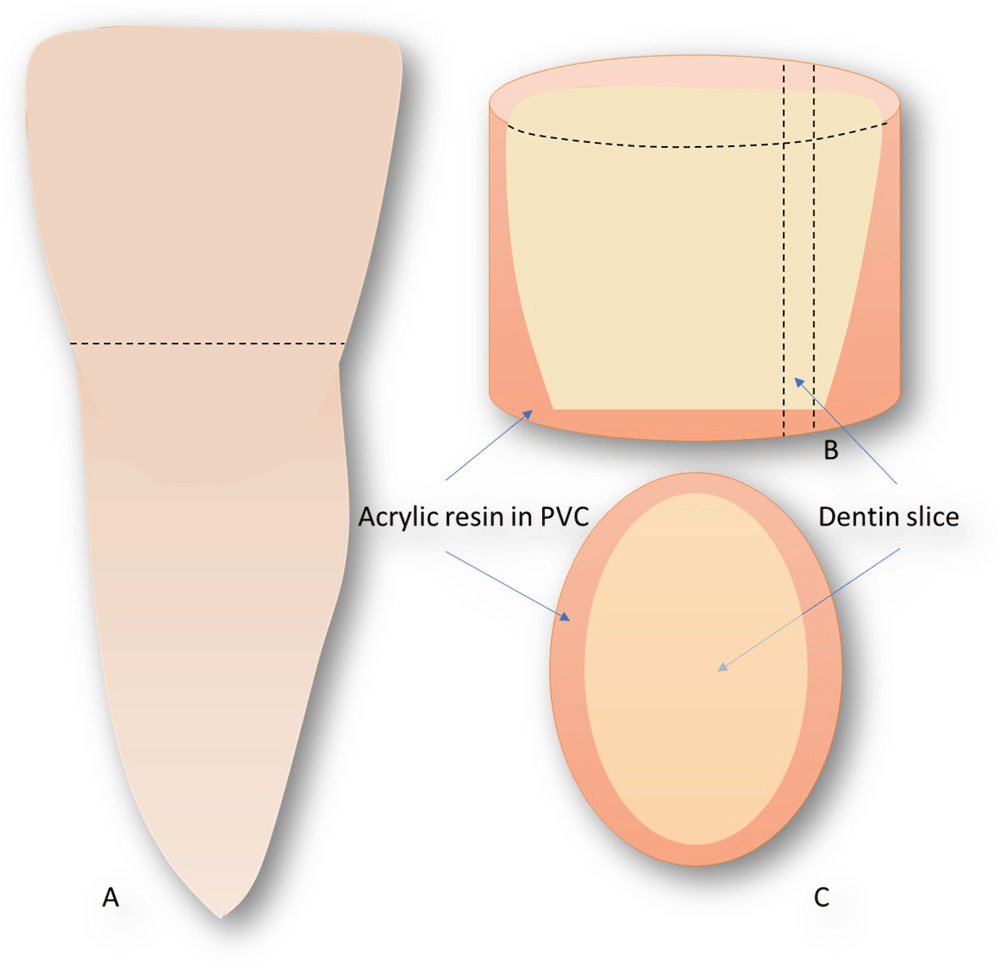
(A) the bovine tooth was cross-cut on the level of cemento-enamel junction of the lateral surface and the enamel was removed totally with sand paper. (B) the crown was fixed in self-curing acrylic resin JET (fabrication) in a PVC prefabricated mold. (C) Longitudinal discs were obtained (10 discs of 0.5 mm and 10 discs of 1 mm).
A digital thermometer (MT-507 Minipa, São Paulo) was used to measure the temperature variation during light irradiation with HAL and LED LCUs (Table 1). The base of the specimen was covered with an insulating thermal paste (Implastec, Votorantim Ind. Brasileira, São Paulo, SP, Brazil), and the tip of the thermocouple surrounded by paste was placed in contact with the lower wall of each dentin disc. The specimens were irradiated for 20 s and the temperature was measured one time for each specimen obtaining 10 measurements for each experimental group (n= 10).
Mouse fibroblast cells (L929) (Rio de Janeiro Cell Bank, APABCAM, RJ, Brazil) were grown in cell culture flasks (TPP, Switzerland) containing Dulbecco’s modified Eagle medium (DMEM) (LGC Biotecnologia, Cotia, Brazil) and supplemented with 10% fetal bovine serum (Invitrogen, New York, USA) at 37°C and 5% CO2 with atmospheric humidity. Next, 2×104 cells/mL were cultivated in 96-well microplates (TPP, Trasadingen, Switzerland) in the same medium for 24 hours for cell adhesion. DMEM was used as a control group (0 mg/mL).
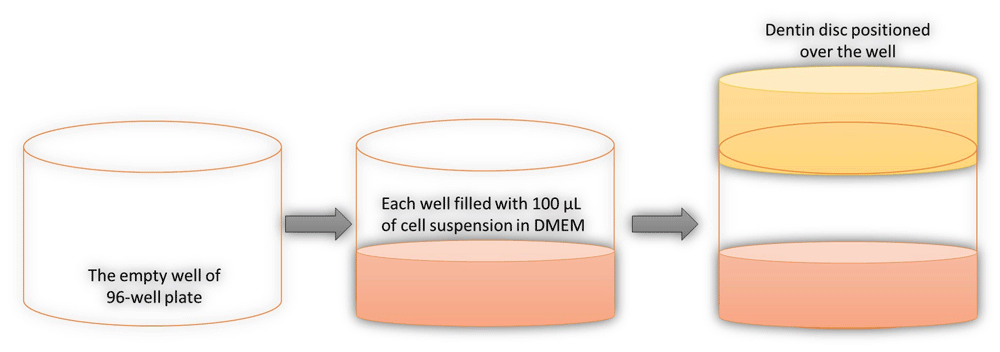
The treatment was carried-out for the groups (n= 10), in which each dentin disc was positioned over a well containing 100 µL of cell suspension in DMEM and light irradiation performed (Table 1). This positioning was to simulate the clinical situation when the LCU irradiates the dentin and this irradiation may affect the fibroblast in the adjacent soft tissues (Figure 2).
Next, MTT solution (100 µL/well) was added to the 96-well plate and the plates were incubated at 37°C with 5% CO2 for 1 h. Then, the MTT solution was discarded and 100 µL/well of dimethylsulfoxide (DMSO; Sigma, Missouri, USA) was added and the plates were incubated again for 10 min and shaken for 10 min. The absorbance of the wells was measured using a spectrophotometer at 570 nm and data generated were converted to cell viability percentage using the formula: = OD of each group × 100 / OD of control group (OD = optical density).
A significant statistical difference was found between the groups HAL0.5 and HAL1 and both were significantly different to LED0.5 and LED1, which presented higher temperatures. However, no significant difference was observed between the two LED groups (Figure 3).
All groups were significantly different to the control group, and promoted reduction of cellular viability. There was no significant difference between the groups HAL0.5 (cell viability 43.5%) and HAL1 (cell viability 41.1%), and between the groups LED0.5 (cell viability 17.1%) and LED1 (cell viability 17.3%). However, both HAL0.5 and LED0.5 were significantly different to HAL1 and LED1 (Figure 4).
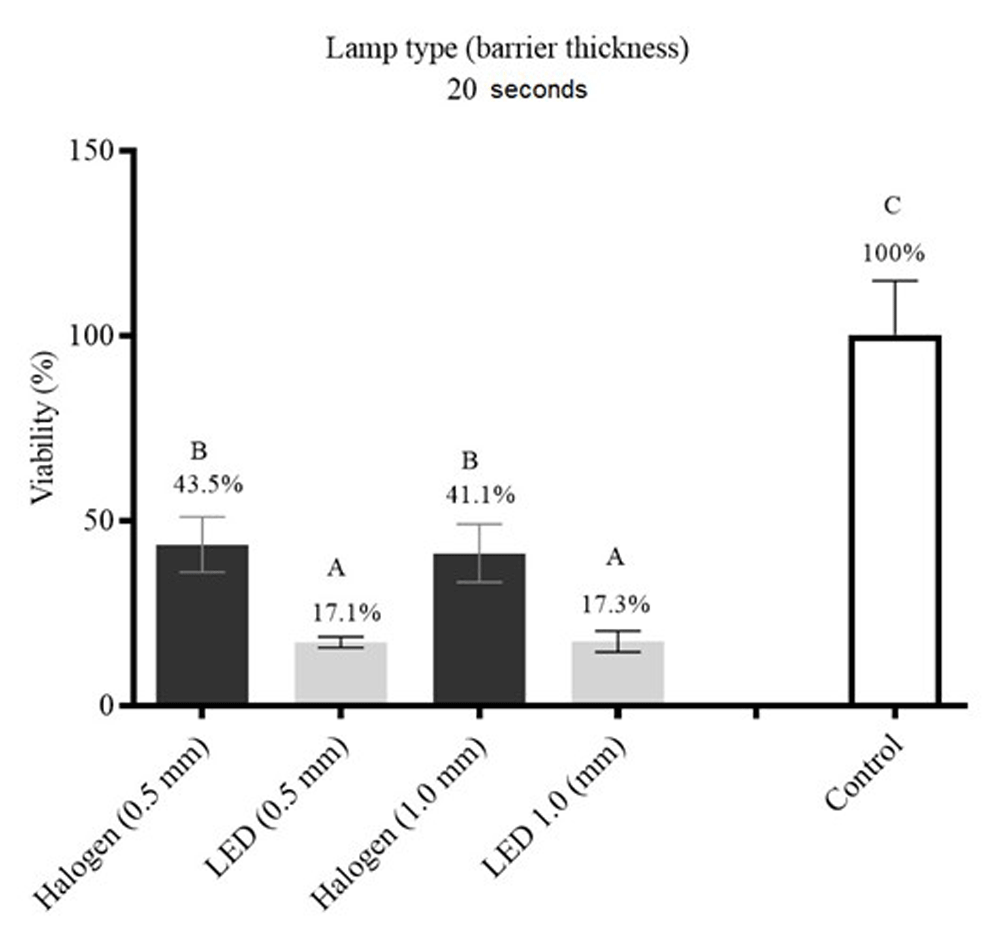
Different letters indicate statistically significant differences among the experimental groups with (P ≤ 0.05).
Raw cytotoxicity and temperature results are available as Underlying data (Paula Ramos, 2020).
This paper investigated the heating generated by HAL and LED when irradiating dentin discs of thickness 0.5 mm and 1 mm of for 20 seconds. Hannig & Bott (1999) obtained different readings were obtained (2.9 to 7.9°C) when they evaluated six LCUs, including HAL, for 40, 10 and 5 s, finding that significantly higher pulp chamber temperatures were obtained when compared to conventional LCUs like Heliolux II. Uhl et al. (2003) evaluated the heating generated after resin composite photopolymeriztion and founded that LED LCUs represent a viable alternative to HAL LCUs for dental composite photopolymerization due lower temperature increases within the composite. Different results were obtained in the present study, as HAL generated lower temperature increases than LED in dentin. Conversely, another study showed no difference between HAL and LED LCUs in generating heat (Drost et al., 2019). These different results in the literature may be related to the kind of temperature sensor and the methodology used (Jiang et al., 2019).
Both LED and HAL LCUs negatively influence cellular viability (Passarelli et al., 2020); however, there is insufficient evidence that they cause pulp inflammation/cytotoxicity (Benetti et al., 2018). In the present study, it was verified that LED was more cytotoxic than HAL LCUs; however, Gonçalves et al. (2016) found that LED had minimal cytotoxicity. This result may be influenced by the dentin thickness, as Daronch et al. (2007) found that the increase in pulp temperature was directly related to the remaining dentin thickness. In the present study, the application of LED light to the thickest dentin disc (1.0 mm) was less cytotoxic than the thinnest dentin disc (0.5 mm).
The divergence observed in the present work in relation to the dentin thickness, light source and the possible greater protection that it can confer to the pulp could be related to the wavelength that the devices emit. The HAL LCU used emits a wavelength of 400–500 nm, and the dental structure is capable of absorbing light in a spectrum from 350–400 nm, meaning it can thus exhibit fluorescence at 410-500 nm. Therefore, the HAL LCU employed herein emits light at an absorbable wavelength for the dentin disc. Thus, the greater the dentin thickness, the greater the absorbance of light and the higher the concentration of photons, thus explaining the increase in the temperature of the disc (Neumann et al., 2005).
This study found that HAL LCU promoted a lower temperature change in the dentin compared to LED, regardless of the dentin thickness (0.5–1 mm). HAL and LED LCUs decreased fibroblast viability; however, LED resulted in greater cytotoxicity.
Harvard Dataverse: Replication Data for: Dataset. https://doi.org/10.7910/DVN/M4FYVV (Paula Ramos, 2020).
File ‘Dataset.tab’ contains raw data for cell viability and temperature generated in the present study.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Platt J, Price R: Light Curing Explored in Halifax. Operative Dentistry. 2014; 39 (6): 561-563 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Dental light-curing units, resin-based composites, dental adhesives.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Mahant RH, Chokshi S, Vaidya R, Patel P, et al.: Comparison of the Amount of Temperature Rise in the Pulp Chamber of Teeth Treated With QTH, Second and Third Generation LED Light Curing Units: An In Vitro Study.J Lasers Med Sci. 2016; 7 (3): 184-191 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Endodontics and Operative Dentistry
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 25 Nov 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)