Keywords
COVID-19, Test, Trace, Isolate, UK, Health, Economic, Impacts, Mathematical Model
This article is included in the University College London collection.
This article is included in the Coronavirus (COVID-19) collection.
COVID-19, Test, Trace, Isolate, UK, Health, Economic, Impacts, Mathematical Model
In the UK, since the first confirmed case of coronavirus disease 2019 (COVID-19) on January 31, 2020, over 1,538,000 people have tested positive and over 55,838 deaths have been confirmed as of November 24, 20201 with a notable resurgence in the number of cases and deaths from September 2020. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for COVID-19, can be transmitted through inhaling viral droplets from an infectious person2, but infection by fomites3 and aerosols4 may also occur. Reducing contact via testing, tracing and isolation (TTI), restrictions on social contact (i.e. lockdown measures), instigating good hygiene and ventilation, and the use of face coverings (masks) are now widely used non-pharmaceutical interventions (NPIs) to reduce COVID-19 transmission. The magnitude of benefit varies between interventions, with emerging evidence from laboratory experiments5–7, clinical studies8–10, and epidemiological studies11–15 that face masks reduce infection risk.
Following the resurgence in COVID-19 cases since September 2020 in the UK, the UK Government imposed a second lockdown in England from November 5, 2020 to suppress transmission and prevent hospitals being overwhelmed (the devolved governments in Wales and Northern Ireland introduced lockdowns in October and Scotland introduced additional restrictions in November). While rollout of an effective vaccine is awaited, intermittent lockdown may remain the main tool for SARS-CoV-2 suppression. But lockdown strategies have important economic, educational, social and psychological consequences16. Alternative means of SARS-CoV-2 suppression such as via effective TTI and high coverage of face masks are required to balance protecting the public from COVID-19 resurgence with protecting the economy and reopening society. Our study is the first that attempts to shed light on this question by combining epidemiological modelling with economic analysis.
Since the onset of the pandemic, modelling has played an important role in understanding COVID-19 epidemic trends as well as assessing the effectiveness of different intervention strategies17–21. For example, recent modelling work19 highlighted the need for an effective TTI programme that tests sufficient numbers of people with symptoms, traces their contacts effectively and ensures they isolate to prevent COVID-19 resurgence, as schools reopened alongside society from September 2020. However, this study did not include an economic analysis. In fact, to our knowledge, only two models to date have considered the economic consequences of different interventions combined with projecting model outcomes from a model of disease progression, and neither are focused on the UK22,23. This is challenging because uncertainties in data and calibration are magnified when composing models: a combined model can contain more uncertainty than the individual model due to propagation of uncertainties. For example, balancing a detailed model of transmission and a detailed model of cost-effectiveness or net-benefit is very data reliant, but in the context of COVID-19, information on both fronts is scarce, though emerging.
In this study, we handle these limitations by directing our focus on understanding the most important features of the combined model. We use a Susceptible-Exposed-Infectious-Removed (SEIR) model24 and a simplified economic analysis to explore the pattern of infections and deaths from COVID-19 and the economic impact of different NPIs in the UK. To make a wide range of scenarios accessible, we provide a user-friendly web interface that allows policymakers to explore trade-offs between different scenarios. This tool is aimed to facilitate discussion of key proposed high-level policies and their impacts based on an easily comprehensible model. This is opposed to static pronouncements about the optimal reaction, in which a complex model such as Covasim used in Panovska-Griffiths et al.19 must be used to accurately represent the complexity of the projected course of the disease. Importantly, for the first time, we contrast TTI strategies that test only symptomatic people and trace their contacts, with combined TTI and additional universal testing (combined TTI+UT). We also look at the role of face masks in suppressing the epidemic in conjunction with TTI and UT.
On September 09, 2020, the UK government announced that universal testing added to the current TTI strategy was being considered as an option to reopen society. In general, a universal testing programme might include more frequent and mass testing, e.g. at schools, and before concerts and sports matches, as suggested in recent reports from the Scientific Advisory Group for Emergencies (SAGE), which advises the UK Government25. But it can also mean repeated testing of the entire population. In this paper, under our definition, universal testing (UT) means testing everyone routinely, in contrast to testing only people with COVID-19 symptoms (we assume 70% of cases are symptomatic19,26–28). Weekly SARS-CoV-2 testing of the entire population was proposed for the UK earlier in the epidemic29, via saliva testing30 that is now being piloted31, and has been followed by the recent initiative of the UK Government to undertake mass-testing in high-prevalence local authority areas32. Similar universal testing strategies have also been proposed for the USA33,34.
This study combines mathematical and economic modelling to contrast the health impact, in terms of mortality burden, the economic outcomes, in terms of economic costs of shutdowns and isolation, and the direct costs of targeted TTI strategy in absence and in presence of additional UT (TTI+UT strategy). For each testing strategy we model reopening of society with the contact rate increasing from December 02, 2020 to that before the lockdown in November 2020. The contact rate is defined as the daily number of contacts per person and assumed to be around 11 contacts per person in the pre-COVID-19 era35. For each scenario we incorporate the use of face masks under assumptions of mean effective coverage (EC), which is the product of the efficacy of face masks and the proportion of contacts in which they are worn (details on methods and sensitivity analysis are in Methods, at the end of the article).
The results of the modelling and economic analysis are shown in Figure 1 and Table 1. The graphs in Figure 1 were generated with Streamlit.
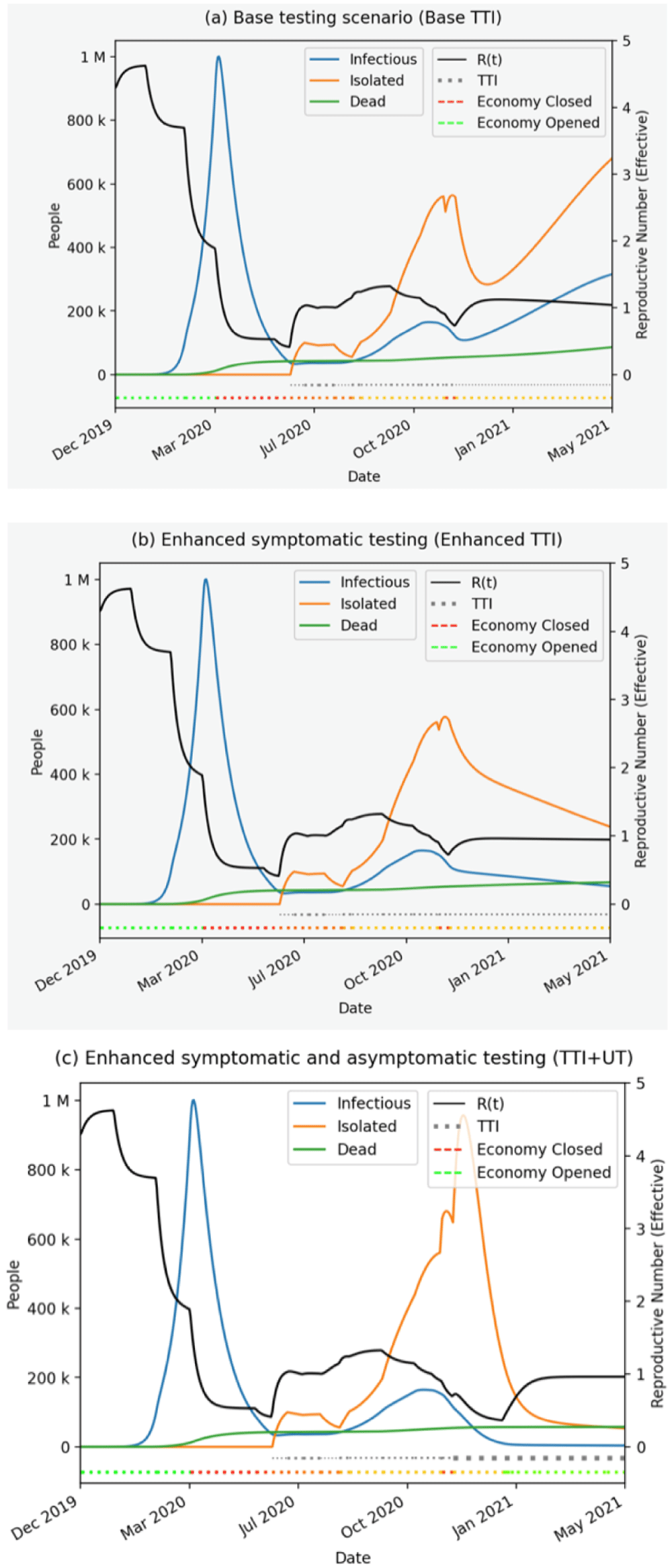
Figure 1A (top): Scenario 1: Base testing scenario (Base Testing, Tracing and Isolation (TTI)), Testing level of 25%, tracing level of 50% and assumed full isolation. Figure 1B: Scenario 2: Enhanced symptomatic testing (Enhanced TTI), Testing level of 50%, tracing level of 50% and assumed full isolation. Figure 1C (bottom): Scenario 3: Enhanced symptomatic and asymptomatic testing (TTI + Universal Testing (UT)) Testing level of 70%, tracing level of 70% and assumed full isolation. Figures were generated under the combination of parameters that define these scenarios as described in the methods and using the Streamlit interface.
The modelling suggests that TTI interventions might prevent future epidemic waves if enough symptomatic people are tested and their contacts effectively traced and all isolated, in combination with at least moderate effective coverage (30% in our case) of masks (Figure 1A).
Specifically, if only 25% of symptomatic people are tested, and 50% of their contacts are traced and all isolated, (Figure 1A), then following the second lockdown in England, an increase in the number of cases is evident. This will push the effective reproduction number R above 1 and the epidemic will persist.
To prevent this, an adequate level of testing of symptomatic people is necessary; in Figure 1B we show the outcomes when the testing level is doubled to 50%, while tracing level remains the same as before (50%) and full isolation of people tested and their contacts is assumed. In this scenario, we see the declining trajectory in the number of cases in January 2021 onwards and the effective reproduction number dropping below 1.
The results for TTI+UT are shown in Figure 1C. In order for the predicted epidemic trajectory to decrease rather than increase after the second lockdown in England and from December 2020, even with nearly universal weekly testing, additional symptomatic testing and tracing is necessary. In Figure 1C we show the combination of 70% testing and 70% tracing that, for this combination of parameters, is necessary to suppress the virus in future and bring R rapidly down.
Overall, increasing TTI testing clearly reduces the future mortality burden: the model projects 110,000 deaths with adequate TTI (50% testing of symptomatic, 50% tracing of their contacts and isolating all) compared to 140,000 deaths with weaker testing (25% testing and same trace-isolate assumptions), by May 31, 2021 (Table 1). Additional UT will reduce this burden even further: 90,000 deaths are predicted in this scenario by the end of May 2021.
Considering the economic impacts, increasing the TTI from testing 25% to 50% of symptomatic people reduces the economic loss (£543 billion vs £541 billion by the end of May 2021; Table 1). Additional UT reduces the economic loss further (£454 billion by the end of May 2021). Across all scenarios the majority of costs incurred are for tracing, whereas UT naturally has much higher testing costs (Table 1).
This work combined epidemiological modelling with economic analysis and developed a user-friendly interface to project our results. We simulated different testing strategies after the lifting of the second lockdown in England in December 2020. Our findings suggest that loosening restrictions accompanied by scaled-up TTI (50% testing of symptomatic people, 50% tracing of their contacts and full isolation of those tested positive and their contacts) and moderate (30%) effective coverage of masks can significantly further limit spread of COVID-19 after the second lockdown, and protect the economy in the UK. Additional weekly universal testing of the entire population from December 2020, can suppress the virus further, save more lives, and avoid more economic losses, though costs more to deliver. To achieve this, UT would have to be combined with additional TTI covering symptomatic cases (70% testing of symptomatic, 70% of their contacts traced and full isolation), and combined TTI+UT is more costly than scaled-up TTI.
The particular value and novelty of our work lies both in combining the epidemiological impact with an economic model and in the comparison between two different testing strategies that are currently being considered by the UK Government. Our epidemiological model, described in detail in a separate paper24 is a version of the classic population-based SEIR model extended to include different testing and tracing strategies. SEIR models are well-established as a tool in modelling infectious diseases and have been widely used in modelling different COVID-19 questions36. Our economic model represents a simple economic analysis developed explicitly for this study to capture public sector costs of testing and tracing and wider economic impacts of shutdown and isolation. Our approach is deliberately simple and our interface is easy to use.
The epidemiological model is readily extendable to include varying degrees of symptomaticity, including the pre-symptomatic period and its duration, varying amounts of viral load correlated with levels of transmission, infectiousness levels, duration of exposure, infectiousness period, and test sensitivity. It can also be easily adapted to include different age and risk stratifications, describe more elaborate representation of the natural history of the disease and progression among different subpopulations, and indeed how contact and transmission varies among and across different cohorts. One aspect that needs to be explored further in future is our modelling assumption of perfect isolation following tracing. Adherence to isolation is an important aspect of a TTI strategy and the balance between testing, tracing and isolation levels needs to be untangled more. But this was beyond the scope of this paper. Finally, uncertainty can be added to the model by setting parameter distributions. But all of these complications add different levels of nonlinearity, which makes the model multidimensional and makes it more difficult to untangle the real contribution of various factors. Our intention was to keep the epidemiological model as simple as possible and combine with economic modelling. For this reason, we also do not consider spatial variation, the difference between cities and rural settings, or epidemics developing in different ways in different regions. For this study, we chose to focus specifically on testing, tracing and isolation, and the trade-offs between different strategies at an aggregate level. An important reason for this is the difficulty of obtaining sufficient good quality data with which to parametrise a more sophisticated model: doing so would have no guarantee of accuracy and risks distracting from our central point. Future work could usefully examine the extent to which the present conclusions hold in more complex models.
In particular, our model does not model specific populations at heightened risk of COVID-19 infection or death, e.g. males, people of black, Asian or mixed ethnicity, or people with co-morbidities37. Future work could look at risk-group and/or geographical stratification within the model, but this was beyond the scope of this study. Recent work has identified additional population cohorts, e.g. people with multiple morbidities or learning disabilities, and people at heightened risk of hospitalisation, intensive care unit admission and death from COVID-1938. Future extension of the work presented in this paper will combine these findings with the modelling and economic framework developed here, to explore the health impact and economic outcomes of different testing interventions on cohorts at higher risk from COVID-19. This will allow us to extend our outcome measures. We limited our examination of health outcomes to direct mortality, but recognise that chronic illness and organ damage from COVID-1939 may have long-term effects not only on the health and well-being of the people affected, but that COVID-19 also creates many second order impacts including but not limited to damage to the economy longer term, impacts on mental health, and social structures. We have not included these outcomes in order to keep the modelling framework as simple as possible, so our conclusions on the potential benefits of different interventions are likely to be conservative.
Our economic model does not explicitly model any costs to enforce isolation, or costs to provide separate accommodation for people to isolate in. We also assume perfect isolation in our model and future work will explore this further27. Policies to support effective isolation, such as lost income reimbursement, community support, childcare, online education, and volunteers to run errands for those isolated, are important. Costs of enforcement may be covered by a combination of using existing policing systems and paying for additional measures with fines gathered from violators.
Despite omission of important details such as detailed modelling of household and community layers, and assuming perfect isolation policies, the model indicates some priorities for immediate piloting with transparently calculated cost estimates. Tracing should focus on how to improve actual isolation rates among all recent contacts, not on targets. An important implication is that with high compliance regular testing might end the need for social distancing and should therefore be properly piloted. To minimise unnecessary isolation and encourage compliance the rapid tests that will be available to NHS and care home staff could be offered daily to anyone in isolation. Automatic payment of (say) £500 initially then £100 per day might achieve high compliance with isolation in those at highest risk including the unemployed and homeless. Average isolation until 2 or 3 days between negative tests would be about 3 days for uninfected contacts and up to 10 days for cases, so the average cost of full furlough would be less than £1,000 per person. Furlough for 10 times the half a million or so adults currently infected in the UK at an average of less than £1,000 would cost less than £5 billion and should be considered.
In summary, we have combined epidemiological modelling, economic analysis and a user-friendly interface to contrast the impact and costs of two levels of testing and tracing within the current UK TTI strategy and when universal testing is also included. Our findings suggest that increased TTI is necessary to suppress the virus after the second national lockdown in England. With reopening of society in December 2020, as before the second lockdown, scaled-up TTI and moderate (30%) effective coverage of masks can prevent further resurgence of the virus and protect the economy in the UK. Additional universal testing from December 2020 can save more lives and avoid more economic losses.
The model is available from GitHub and is archived with Zenodo40. The impact analysis was conducted using the SEIR-TTI compartmental model shown schematically in Figure 2 and with details provided in Sturniolo et al.24 The model is an extended version of the classic SEIR model that incorporates probabilistically the effects of testing, contact tracing, and isolation24.
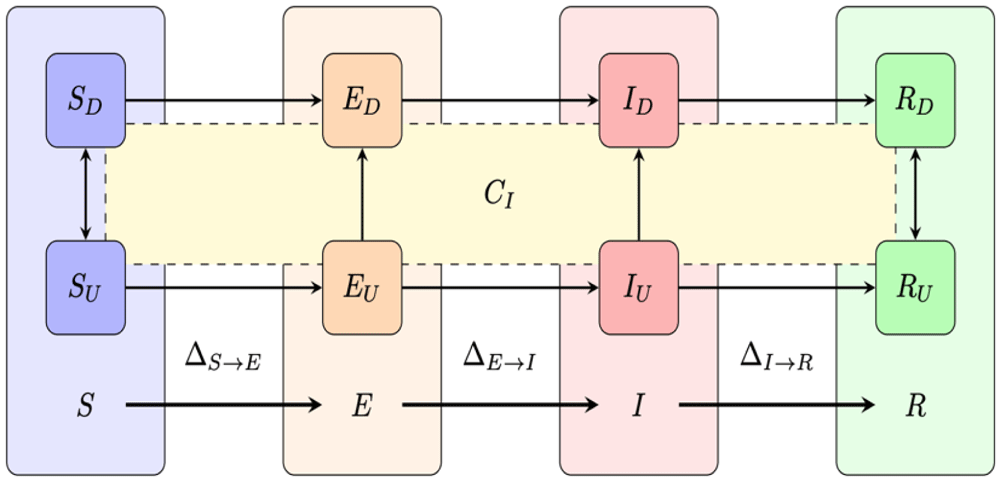
SEIR is a compartmentalised model describing susceptible (S), exposed (E; infected but not infectious), infectious (I) and removed (R) population cohorts. Individuals move between these compartments in sequence as they become exposed, infected and infectious during disease progression until recovery. Each compartment comprises diagnosed (D) and undiagnosed (U) individuals with diagnosis leading to isolation. We assume that diagnosis happens through testing or putatively through tracing. A non-infectious individual that is “diagnosed” has effectively been misdiagnosed and the result is that they are needlessly required to isolate. Individuals transition between compartments X and Y at rates ∆X→Y which we derive in Sturniolo et al.24 from which this figure is reproduced.
Within the model, possible transitions between cohorts are indicated with arrows. Within each of these states, an individual can be unconfined or isolated. Infectious (I) individuals who are unconfined may be tested and become isolated. An individual in any state who is traced is isolated. Once isolated, individuals remain so for 14 days. Susceptible (S) isolated individuals cannot become infected due to their isolation, and return to the unconfined state after a 14-day delay. Exposed (E) and infectious individuals (I) do not return directly to the unconfined state and first progress to removed (R). Removed (R) and isolated individuals return, as with susceptible (S) individuals, to an unconfined state once 14 days has elapsed. Tracing is described by a rate of tracing eta and a probability of success chi.
Model parameters and details of the calibration used are detailed below. Briefly, the model-projected deaths between January 21, 2020 and November 07, 2020 were matched to the publicly-available data on deaths reported within 28 days of positive tests, using the UK dashboard. This allowed us to determine the transmission probability beta, the numbers of contacts, the date of the onset of the epidemic, number of infectious people at the onset of the epidemic, the infection fatality rate (IFR) and the testing and tracing levels to constrain the model to mimic the reality of the COVID-19 epidemic in the UK until November 07, 2020.
We note that within the model an intervention changes the model parameters at a defined time. The principal parameters that are changed are the contact rate (average number of contacts per person per day) representing differing regimes of social distancing or lockdown, and the testing and tracing rates, representing building up capacity of TTI. A trigger changes parameters when a condition is met. The trigger conditions are the number of infections passing a set threshold. We use different thresholds according to whether the number of infections is increasing or decreasing to avoid rapidly oscillating between distancing regimes, which would not be politically or economically feasible. We use a threshold of < 10,000 infections to release lockdown as it approximates what may be a safe level of limited community transmission. We use a threshold of > 40,000 infections for beginning lockdown to reflect time elapsing between opening and closing given exponential growth. Lockdowns are not triggered in the scenarios shown in this paper.
We developed an economic model based on closures and isolation that uses the impacts from the SEIR-TTI model to calculate the cost on the economy41. Specifically, within the economic model we modelled GDP reduction as a function of the reduced contact rate and isolation requirements. That means reduced economic activity was modelled when people stay at home, rather than shop, work, or engage in other economic activities, as during the lockdown period for example. Because there are already estimates about the degree of contraction which occurred during the lockdown (e.g. from the Bank of England), we use reduction in interpersonal contacts as a valid proxy for the proportion of full shutdown effectively continuing to occur. This is more closely related to the epidemic evolution than direct estimates of shocks to supply and demand used in the past42, and has the advantage of using actual estimates of the economic impact of COVID-19 based on mitigation policy choices. Our economic model is further detailed below. Potential health and social costs of lockdown that are not included in our economic model are shown in Table 2 below.
| Sector | Processes affected | Potential adverse health outcome | ||
|---|---|---|---|---|
| NHS | Programmes | Screening across the life course, e.g. neonatal, cancer | Delivery, uptake and action52,53 | Avoidable morbidity and mortality |
| Immunisation | Reduced uptake54 | Reduced herd immunity Increase in vaccine preventable infection | ||
| Child and adolescent health | Health visitor checks and support for parents Adolescent mental health55 Safeguarding | Avoidable morbidity Increased violence against children/child abuse while in lockdown (particularly linked with alcohol, drug use) | ||
| Maternal health | Antenatal care in pregnancy and post- natal follow up56 | Birth experience Anxiety - giving birth alone/impact of self-isolation – reduced peer and family support for new mothers Missed risk factors and antenatal diagnoses | Adverse birth outcomes Postnatal depression | |
| Severe trauma | Still managed but Intensive Care Unit (ICU) availability may be stretched Secondary infection in hospital COVID-19 acquired | Avoidable morbidity and mortality | ||
| Cancer | Potential new cancer Existing cases | Delay diagnosis and treatment Radiotherapy and chemotherapy | Avoidable morbidity and mortality | |
| Acute cardiovascular disease (CVD) | Still diagnosed and treated Secondary acquired in hospital COVID-19 ICU availability | Avoidable morbidity and mortality, including from delayed presentation to hospital for CVD/acute MI | ||
| Other acute care (respiratory, fall, outpatients etc) | Diagnosis and treatment | Avoidable morbidity and mortality | ||
| Chronic disease management | Less monitoring (e.g. hypertension, diabetes, asthma, epilepsy) Poorer control Access to medication Difficulty following healthy lifestyle advice | Avoidable morbidity and mortality57 | ||
| Elective surgery | Delayed, Quality of Life (QoL) may worsen, less operable if condition worsens. Backlog | Avoidable morbidity Poorer Quality of life | ||
| Services for vulnerable groups | Homeless | Temporary housing provision, but often without access to food or basic necessities Lack of access to health services58 Disrupted support services during lockdown Removal of temporary housing at the end of COVID-19 | Poorer health outcomes | |
| Dementia | Isolation, less carer support59 Harms e.g. falls | Poorer quality of life Higher morbidity and mortality | ||
| Patients with disability | Access to services for complex medical needs60 Isolation Anxiety – may not be a ‘priority’ group for ICU Inequity in access to public health messaging | Worse health outcomes | ||
| Severe mental illness (inpatient services) | Deterioration, potential relapse Loss of access to inpatient services (secondment of staff to Covid-related support) Reduced community mental health teams during lockdowns | Suicide Hospital Admission | ||
| Prisoners | Mental health, addiction Higher COVID-19 risk due to poor living conditions61 Isolation (due to loss of visitation rights) Difficulty in isolation Risk of riots (like in Italian prisons) | |||
| Older people | Likely to live alone and have less access to online communication | Health impacts of isolation and loneliness | ||
| Refugees and migrants | Exclusion of migrant populations from health services: in the UK NHS Charging Regulations deter migrants from accessing health services (particularly those undocumented) Culturally or linguistically inappropriate care Increased discrimination/xenophobia during COVID-1962 Difficulty in isolating or applying preventative interventions for those living in overcrowded conditions, intergenerational households, or those held in detention centres Low-wage migrant workers on precarious contracts | Poorer health outcomes Higher COVID-19 mortality for BAME groups63 Higher morbidity and mortality from COVID-19 due to delay in accessing health service/lack of access to health service/ inability to apply preventative interventions Higher exposure to COVID-19 if continuing to work as key worker during lockdown; additional adverse effects of loss of income if precarious employment | ||
| Health and care staff | Post-Traumatic Stress Disorder (PTSD) Generalised Burnout | |||
| Diagnostic services X-Ray, Escopy | Delayed diagnosis and treatment | Poorer long-term outcomes (avoidable morbidity and mortality) - Costly for individuals and the NHS | ||
| Rehab Physio/ Occupational Therapy | Poorer long-term outcomes | Increase in disability or duration of recovery, poorer quality of life – additional individual and societal costs | ||
| Addiction services | Smoking cessation Alcohol Drugs | Some success with quitting Less support for dependent patients | Avoidable morbidity and mortality | |
| Sexual health services | Less access | Avoidable morbidity | ||
| End of life care | Impact on hospices and care for those dying at home - reduced staff and funding Adverse grief reactions for bereaved relatives of COVID-19 patients - evidence suggests that there will be increased rates of PTSD and depression for those affected by COVID-19 related loss, as it is essentially a form of traumatic loss – unexpected and without closure. | |||
| Mental health services (common mental disorders) | Increased rates of suicide and self harm64 Increased rates of depression64 Increased rates of condition related anxiety (COVID patients)64 | Difficulties accessing primary care for early diagnosis and treatment | Avoidable morbidity and mortality | |
| Social isolation and distancing measures | Household isolation | Less physical activity Mental health (stress, insomnia, anxiety, depression) Domestic abuse Family breakdown Elder abuse Safeguarding Loneliness Infection transmission from crowding Increased substance misuse Poorer diet (BMI impact, type 2 diabetes risk) Reduced access to medications Increased experiences of racialised policing (BME groups) Loss of access to public spaces (closure of parks likely to impact communities who live in crowded housing) Lack of access to free school meals for children who need them, and increased use of food banks | Depression Suicide Physical trauma Adverse impact on physical WB Increased falls in the elderly isolated at home Poor reporting of moderate health risks to health professionals (i.e. early signs of cancer, heart disease, etc) | |
| Access to food | Especially if vulnerable and isolating | Hunger, poor nutrition (both obesity and under-nutrition / vitamin deficiencies) | Adverse impact on mental and physical wellbeing and on child development | |
| Transport | Less travel | Fewer accidents Less air pollution, including greenhouse gases | Less trauma from RTAs and therefore reduced admission to hospital Less cardiovascular, respiratory illness Less morbidity and mortality Increased health risks to those who continue support of essential transport services and their households | |
| Employment /income loss | Household income loss on top of existing poverty especially those made unemployed, reduced hours outside Chancellor’s support initiatives | Vulnerable groups for pre- existing poverty, low pay sectors (accommodation, catering, retail, care) Single mothers with children, People with disability, ethnic minorities | Food insecurity–hunger, nutrition Heating costs, cold related illness Mental health including alcohol and drug misuse (see above) Homelessness/loss of home Gambling Increased uptake of universal credit system due to lack of protection for economic shocks in poor households | Increased vulnerabilities Avoidable mortality and morbidity among already high- risk groups |
| School closure | Education | Loss of free school meals if not attending school Loss of regular physical activity Impact on social development and education (widening inequalities) Safeguarding | ||
| Higher education closure | ||||
| Longer term wider inequality post COVID-1962 | ||||
Estimated costs for TTI and UT are shown in Table 3 below. There are three principal components, which we also explain in detailed narratives below: (1) contact tracing using a network of public health community officers, mobile phone apps, and supervisors; (2) home-based saliva testing for active SARS-CoV-2 infection; and (3) follow-up and isolation of infected individuals and households. As per the economic model, total costs are variable depending on policy scenario and case numbers.
Shown are unit/daily costs. Total costs are variable dependent on policy scenario and case numbers. *Costs of testing are based on a pilot study in Southampton of mass home-based saliva testing31.
| 1. Contact tracing | |||||
|---|---|---|---|---|---|
| Staff | Function | Number | Rationale for number | Salary per day | Notes |
| Public Health Community Officer | Trace contacts via apps and in person - follow-up to check isolation and re-testing | 81463 | 1 per 1000 population (like community health workers in many countries) + 20% for sickness cover and absence | £80 | These workers can be people who have lost their employment as a result of the pandemic, they will need minimum qualifications though no prior experience of public health work as can be trained |
| Public Health COVID- 19 supervisor | Supervisor / manager for PHCOs - ~1 per 50, or ~4 per each of the 343 local authority areas | 1629 | these team leads will work full time answering queries from PCHO and helping resolve problems + 20% for sickness cover and absence | £160 | These supervisors could be recent graduates of public health or related Masters courses, or local authority Environmental Health Officers. |
| Local authority team lead | One for each of the 343 Local authorities | 412 | 1 for overall control of contact tracing effort for each local authority area + 20% for sickness cover and absence | £300 | These team leads should be public health specialists with at least 5 years experience |
| Online training for all staff | 1 | Three training courses (including refreshers) one for each staff cadre. Assume repeated every 3 months | generously funded at £20,000 per online training course developed (can do on phones which will be used for contact tracing too) + £500 per month for running servers for online training | ||
| Unit cost | |||||
| Recruitment costs | Recruitment costs for all contact tracing staff, including for replacements and cover (per 3-month period - conservative assumption is repeating this every 3 months even though the same tracers may be in post) | 83504 | £200 per recruitment for advertisements, phone interviews, salary of recruiters | £200 | |
| Equipment | Function | Number | Rationale for number | Cost per day | Notes |
| Phone pay as you go credit | for calls and data for all staff including for online training | 83,504 | all staff above | £5 | |
| Unit cost | |||||
| Smart phones | only for ~10% of staff who don't have one | 8,350 | most people have smartphones in the UK | £200 | |
| START-UP COSTS: Mobile phone app development | for rapid contact tracing given rapid spread | 1 | one app needs to be developed (or chosen from many already made?) | £10,000,000 | ballpark estimate of developing, maintenance and running the app over a year |
| 3 MONTH PERIOD COST: Mobile phone app maintenance and running costs | for rapid contact tracing given rapid spread | £1m per month estimate means £3m per 3 month period | £3,000,000 | ||
| Travel | Function | Number | Rationale for number | Cost per day | Notes |
| For supervisors and managers | to check work of PCHOs in person if needed | 2,041 | number of supervisors and managers | £10 | Travel will be in local areas so costs per day for driving or public transport should not be high |
| For PCHO in rural areas | to get around to their whole catchment population of 1000 people | 13,849 | 17% of UK population is rural so have this travel allowance for 17% of PCHO | £10 | Travel will be in local areas so costs per day for driving or public transport should not be high |
| 3 MONTH PERIOD COST: Communications | To advertise the contact tracing scheme and keep people informed of it | 1 | Estimated budget of £100,000 per day for advertising and communications. Advertising campaigns assumed to last for a minimum of 3 months | £9,125,000 | This will be additional to national COVID-19 advertising budgets given current on-going COVID-19 advertising campaigns funded by the government |
| 2. Testing - SARS-Cov-2 viral RNA RT LAMP tests, home saliva samples* | |||||
| Staff | Function | Number | Rationale for number | Salary per day | Notes |
| Lab technicians | running SARS-Cov-2 viral RNA RT LAMP tests | 11,574 | 18 hrs per day, two 9 hrs shifts: 1 technician running one machine, and 1 filling the wells per machine. So 4 shifts per day. Automated reporting into LMIS system - electronic connection into health records automatically. | £200 | |
| Lab supervisors | supervising lab | 579 | two one for each lab (one for each 9hr shift) - average 10 RT LAMP machines per lab | £300 | |
| Lab staff training | training on running RT LAMP tests | 12,153 | Initial 2-day training, 1 day refresher every 3 months | £200 | 5 days training per year |
| unit cost | |||||
| Recruitment costs | Recruitment costs for all lab staff, including for replacements and cover | 12153 | £200 per recruitment for advertisements, phone interviews, salary of recruiters | £200 | |
| Overheads | |||||
| Lab overheads | Overhead (space) costs for ordinary laboratory with category 2 hood (no biosecurity) | 579 | Estimated cost of £500 per day per lab for 289 labs with 10 RT LAMP machines in each | £500 | |
| Machines | Function | Number | Rationale for number | RT LAMP machine cost per day | Notes |
| START-UP COSTS: RT LAMP Machines | SARS-Cov-2 viral RNA RT LAMP testing. Also automatically uploads data to online health records | 2,894 | Enough RT LAMP machines for 10 million tests a day if running 6 days a week 18 hrs a day, one 96 well plate per 30 minutes (20 min start to finish, and 10 min turn around per run). One RT LAMP machine costs £27,000. Having this as an annual cost assumes all machines will be replaced after 12 months on average | £214,041 | Total cost per year based on daily cost. If extending time beyond one year can use this as it is based on daily cost i.e. assumes RT LAMP machine lasts for 1 year or average and will then be replaced |
| RT LAMP Machine maintenance | maintain working order of the 2894 RT LAMP machines used | 2,894 | assume maintenance costs averaging £10 per day | £28,935 | |
| Equipment | Unit cost | ||||
| Test kits, including reagents | viral RNA RT LAMP tests, home saliva samples. RT LAMP is at room temperature and doesn't require RNA extraction, so less reagents needed | 3,120,000,000 | 10 million tests per day | £3.50 | Reagents and materials per test - commercially sensitive source - used for pilot study* costing |
| Home collection of saliva samples | To collect saliva samples by courier to the lab for testing | 3,120,000,000 | 10 million tests per day29 | £0.50 | Home collection by couriers - used for costing for pilot study* |
| Tests Per Day | 10,000,000 | ||||
| 3. Isolation encouragement | |||||
| These costs are all covered under 1. Contact tracing. | Number | Unit cost | Notes | ||
| There may be additional policing costs estimated at £500 for every infringement requiring police action - estimated at 2000 such infringements per day nationally based on France and Italy | 624,000 | £500 | These costs should all be (more than) covered by the fines levied and received for infringements, so are not included in total costs below | ||
We contrasted three testing strategies from December 02, 2020 when the second lockdown in the England is lifted. Specifically, we compared the impact and costs of
A) Baseline targeted test-trace-isolate (TTI) strategy from December 02, 2020 resembling the current strategy that tests only symptomatic people, traces their contacts and isolates those testing positive and their contacts. We model 25% testing, 50% tracing and full isolation.
B) Enhanced TTI strategy from December 02, 2020 modelled by increased testing level compared to the baseline TTI. We model 50% testing, 50% tracing and full isolation.
B) Universal testing in addition to the targeted TTI strategy from December 02, 2020. We model 70% testing across symptomatic and asymptomatic, 70% tracing of their contacts and full isolation.
For each of the strategies we include wearing masks and that the contact rate c increases to a value of 3.4 as the average c of the values between 2.8 and 4 from the CoMix study43 representing the values during the first lockdown and after the relaxing of it. While in this paper we present the results for a moderate effective coverage of masks, lower and higher effective coverage can be explored further on our interface.
On the interface that we have developed, we modelled three different levels of effective coverage of masks (defined as the size of the reduction in COVID-19 transmission in the population as a whole due to the use of face masks): 15%, 30% and 50%. These were estimated as a product of contact coverage (the proportion of infectious–susceptible contacts in which at least one person is wearing a mask), and per-contact effectiveness (the size of the risk reduction when at least one person is masked). Surveys and media reports suggest mask prevalence outside the home is around 40–80%44–47. Assuming no mask usage within the home, and 3–8 daily contacts of which 1–3 are at home34, population-wide prevalence is 30–60%. In a well-mixed population, this translates to contact coverage of 45–85%, because in many contacts involving unmasked individuals the other party will be wearing a mask. Reviews of mask effectiveness suggest a benefit of approximately 45% for uninfected wearers in non-healthcare settings, with a plausible range of about 20–70%8–10. Since these were mostly case-control studies, we adjust downwards for biases that may have inflated the effect size, and for mask type: most people in the UK use cloth masks48, which are probably less effective than the medical masks used by some participants in the reviewed studies7,8,49,50. However, we then adjust upwards for source control, which is hard to quantify but has been clearly demonstrated in the laboratory5–7. Taking all of this into account, and weighting by the proportion of single- and double-masked contacts, we end up with an effect size of 20–60%. Multiplying that by the contact coverage, we obtain an effective coverage estimate of 30%, with a range of 15–50%.
In the paper we present the results for this moderate 30% EC of masks, but the other scenarios can be explored on the interface described below.
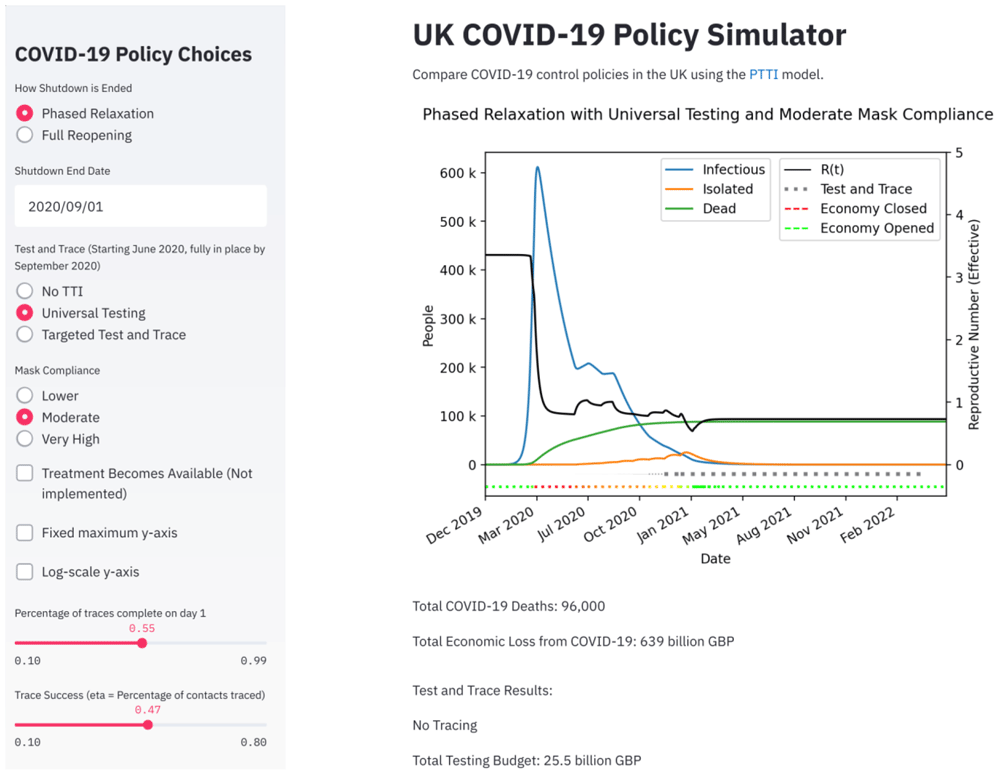
The epidemiological model and the economic framework were combined into a user-friendly interface that we developed for the purpose of this analysis. The interface is available on Streamlit and the figures in this manuscript were directly imported from the interface plots. Within the interface there are clickable options that allow the user to explore different permutations of the scenarios we have considered in this study; a snapshot is shown in Figure 3.
Details of the compartmental SEIR-TTI model we used for the impact analysis are in Sturniolo et al.24 In summary, it is an extended version of the classic SEIR model that incorporates probabilistically the effects of testing, contact tracing, and isolation. For the purposes of the analysis here, we fixed the majority of the model parameters to the values from the literature as per Table 4. We fitted the four parameters: transmission probability β, the rate of contacts, the date of the onset of the epidemic, number of infectious people at the onset of the epidemic and the infection fatality rate (IFR) to match the model projected deaths to the publicly available mortality data from the UK government. To match the UK epidemic, we consider a single infectious individual introduced into the UK in late December 2019. This is simply a mathematical convenience and not a claim about the seeding of the actual epidemic in the UK. It is not our purpose to investigate the origins of the epidemic in this article. In reality it is likely that multiple infectious individuals were introduced into the UK at a later date. This distinction is immaterial to the functioning of the model. By calibrating to the mortality data, we obtain a transmission probability β of 0.0435 which translates to a basic reproduction number R0 of 3.3 when c is 11 contacts per day and under no interventions. The results of the calibration are shown in Figure 4 and further details are provided here.
| Parameter | Description | Default Value | Reference |
|---|---|---|---|
| N | Population size (UK population mid-year 2020) | 67,886,011 | 65 |
| c | Average contacts per day pre COVID-19 | 11 | 35 |
| β (beta) | Transmission rate per contact | 0.0435 on average but fitted across different time points in the epidemic | Estimated from fit to mortality data66 |
| α -1 (alpha) | Incubation period (time from exposed to infectious) | 5 days | 67–70 |
| γ-1 (gamma) | Recovery period (time from infection to recovery or hospitalisation) | 7 days | 71,72 |
| κ-1(kappa) | Isolation period (symptom free days) | 14 days | 73 |
| θ (theta) | Testing rate of infectious individuals | Varied across scenarios | - |
| χ (chi) | Contact tracing rate | Varied across scenarios | - |
| η (eta) | Efficiency or success rate of contact tracing and isolation | Varied across scenarios | - |
We calculate reduction in GDP due to the pandemic and lockdown measures by relating GDP to the model parameter c (contacts per day) as a proxy for economic activity, for every day of the model scenario trajectory. GDP of £186 billion per month is taken as the pre-pandemic level74, when c = 11, whereas during lockdown GDP is 25% lower, when c = 3. For intermediate values of lockdown or distancing, GDP loss is scaled accordingly. The pandemic itself results in GDP loss, as c = 80% of baseline even when lockdown is fully released, i.e. the country is not back to c = 11 (100%) normal economic activity.
Intervention costs are calculated by dividing the budget items shown in Table 3 by start-up costs and on-going costs: for tracing, and for testing. Costs to notify, enforce, and otherwise manage isolation are assumed to be covered by fines levied for breaches of isolation. Overall start-up costs for contact tracing are £10m for the app that supplements human contact tracing efforts, as well as a recruitment campaign to hire the number of needed contact tracers, supervisors, and managers. Start-up costs include recruitment and training costs for personnel, and app maintenance costs, for which we have made several assumptions detailed in Table 3 and below, though these are small enough not to significantly alter overall costs. On-going costs are scaled according to the numbers required by the intervention by estimating the cost per contact traced and the cost per test, as follows.
Contact tracing costs. Using our assumptions around number of contacts before lockdown (c0=11), during lockdown (c=0.3*c0), and after the lockdown is lifted (c=0.8*c0), we determine that over a period of seven days a total of 77 contacts need to be traced before lockdown, while during lockdown only 23 contacts will need to be traced.
As a policy design assumption for the model, we stipulate that contact tracers and supervisors are hired for a minimum of three months (90 days) for the system to function professionally, while team leads are hired for the entire term of contact tracing. Contact tracing costs are therefore blocked into three-month periods based on the anticipated maximum number of tracers needed in the subsequent three-month period. Recruitment and training costs for any additional tracers needed in the subsequent three-month period are added to the cost for that three-month period.
The recurring tracing costs can be used to determine a (marginal) cost per hour of tracing, which can then be used to determine the cost per trace given our estimate of 1.26 hours work per contact traced (Table 5). We estimate the cost per contact traced is approximately £18 (calculations as per ‘Tracing costs per case traced’ sheet here).
| Contact tracing resources required for each new case | Public Health Community Officer (PCHO) hours |
|---|---|
| Interview new case and create list of contacts (45 min - 1hr) | 0.85 |
| Interview 14 high-risk* contacts (20 min each) | 4.6 |
| Interview 16 low-risk† contacts (10 min each) | 2.7 |
| Monitor 14 high-risk contacts daily for 10 days (10 min per call) | 23.3 |
| Monitor 16 low-risk contacts for 10 days (1 min per call) | 2.7 |
| Arrange to test symptomatic contacts (a) (10 minutes) | 0.6 |
| Car service taking 1 hour to test 50% of symptomatic contacts | 3.1 |
| Total hours | 37.8 |
| (a) Assume 3.7 symptomatic contacts per new case (URTI prevalence of 42/100076 and R0 of 2.577) | |
*High-risk exposure contacts are people having had face-to-face contact with a COVID-19 case within two metres for more than 15 minutes; having had physical contact with a COVID-19 case; having had unprotected direct contact with infectious secretions of a COVID-19 case (e.g. being coughed on); having been in a closed environment (e.g. household, classroom, meeting room, hospital waiting room, etc.) with a COVID-19 case for more than 15 minutes; or a healthcare worker or other person providing care to a COVID-19 case, or laboratory workers handling specimens from a COVID-19 case, without recommended PPE or with a possible breach of PPE78.
†Low-risk exposure contacts are people having had face-to-face contact with a COVID-19 case within two metres for less than 15 minutes; having been in a closed environment with a COVID-19 case for less than 15 minutes; having travelled together with a COVID-19 case in any mode of transport; or a healthcare worker or other person providing care to a COVID-19 case, or laboratory workers handling specimens from a COVID-19 case, wearing the recommended PPE78.
Testing costs. We estimate that each test costs £4.79 including start-up and recurring costs. The vast majority of these costs are the £4.50 for each actual test (£3.50 for the test kit, £0.50 for mailing out the test kit, and £0.50 for the courier from the tested person’s address to the local lab). Start-up costs for testing are the cost of the RT-LAMP machines (£27,000 each). Each machine can run 96 tests every 30 minutes75 so if we assume they will be running for 18 hours per day (two 9-hour shifts) they will process 3,456 tests per day. We assume 10 machines per lab on average, each with £500 per day overheads, 40 lab workers (four per machine: two for each shift), and two supervisors (one for each shift).
Testing personnel costs are blocked into six-month periods based on the anticipated numbers of tests per day over the subsequent six-month period. In a six-month period where only 100,000 tests are being done each day, costs per test would still be approximately £4.79, as the number of labs, maintenance costs, and lab workers would be scaled down accordingly, and the RT-LAMP machines would be amortized over the full period of use.
We assume that if people are unable to afford their own face coverings they will be wearing reusable face coverings made from materials to hand in the home, at little or no cost. The UK government has issued advice on how to make and properly use a face covering.
There is emerging evidence that mobile phone contact tracing apps have the potential to facilitate effective COVID-19 epidemic control at scale and at speed18. Nevertheless, personal follow-up on foot will also be required to ensure all contacts, including the most vulnerable, are reached79. The additional costs of such a system are relatively small in the context of the problem we are seeking to address.
For feasibility reasons, we assume that control of COVID-19 would be managed through local authorities by Consultants in Health Protection/Communicable Disease Control and Directors of Public Health. This was the approach used, with success, until the re-organisation in 2002 and it ensured effective control of communicable disease via local knowledge of and relationships with the community, the local politicians and leaders, the laboratory, the hospital and its consultants, and the general practitioners80,81. Legal powers to take such responsibility are available through Schedule 21 (powers relating to potentially infectious persons) of the Coronavirus Act 2020. Regional Health Protection Teams from Public Health England could take on management responsibilities for local authorities in England (public health functions are already devolved in Scotland, Wales, and Northern Ireland) and co-ordinate regionally and centrally through its established infrastructure. This includes regional epidemiologists who have a key role in understanding the epidemic at a regional level, identifying differences between local authorities, and sharing expertise.
Movement of people between local authority areas could be accounted for by data sharing between contact tracing teams. China, while being different in many ways, demonstrates the ability for this hierarchical approach to succeed in identifying contacts82.
Case finding and contact tracing. Contact tracing remains a key control measure for maintaining suppression of case counts83. Table 5 shows the staff needed to handle new cases and control spread through contact tracing and isolation84.
The NHS Test and Tracing Service was launched on 29th May. While information on the structure, duties, and means of collaborating with the contact tracing teams in local authorities has not been published, it is reasonable to assume that this centrally managed service will provide some of the hours required to run the case finding and contact tracing function shown in Table 5. It seems that the service is limited to phone and internet communication with individuals. Because the levels of ascertainment of cases of this approach remains unknown, it will be prudent for local authorities to assume that at least half the manpower shown in Table 5 will be required by them.
Local public health capacity. Each new case will require 38 hours of community health staff and volunteer time to trace an average of 30 contacts and test 3.7 symptomatic contacts, two thirds of whom will have COVID-1984 (these numbers reflect a situation when physical distancing measures are in place). The requirement for staff will vary with time as relaxation of physical distancing increases contact numbers or as subsequent physical distancing reduces contact numbers, and should decline if phone applications as used in South Korea85 are used by sufficient numbers of individuals here and their accuracy increases (though we do not assume any increase in efficiency or success of contact tracing resulting from use of phone apps). On average there will need to be 5.1 full time trained contact tracers (Public Health Community Officers, PHCO; Table 3) to cope with each additional concurrent case, though this will vary by the number of contacts per day. The numbers of contact tracers will need to be adjusted accordingly to accommodate part-time working and to cover all seven days of the week, as all contact tracing should be done within one day for each case.
A fraction of health visitor (HV) and environmental health officer (EHO) staff can be redeployed initially to lead local teams of contact tracers86. Most local authorities have established volunteer registers87 and recently retired HVs and EHOs can also support the contact tracing effort. New staff will also need to be hired, given limited capacity and the existing important duties carried out by HVs and EHOs. The system of contact tracing could be up within weeks with sufficient political will and commitment. We assume that it will be possible for most Directors of Public Health alongside the Public Health Physician secondees from Public Health England to assess if they have control of the spread of the virus in their district a week later. The incidence of new cases will vary between local authorities and regions.
Initially the number of cases can be best estimated from local deaths. As the system gets underway, new cases can be notified in the standard way for notifiable diseases, for which testing is helpful but not necessary. The number of cases will fall as physical distancing succeeds, as in China. An estimated 800 to 1,000 contact tracers would be needed two weeks after peak deaths in the averaged-sized local authority (population ~375,000). We assume this is achievable, given the 750,000 people who have already volunteered to help the NHS tackle the pandemic88. Training is assumed to take one day, as is setting up the administrative arrangements using local authority resources. Testing facilities can be negotiated with the local health laboratory (see Testing section below). The local authority will be assumed to take on the public information function.
Community advisory committees and local health communication strategies
The overall success of this strategy rests on the willingness of citizens to engage with and accept the necessity of contact tracing and isolation for 14 symptom-free days if in contact with a case, and of home testing via spit (saliva) samples. Social psychological literature suggests that health communication messaging and health interventions are most effective when anchored to meaningful dimensions of identity and personal experience89,90, which has been affirmed by evidence from previous epidemics including HIV91,92 and Ebola93. Community-led and co-production approaches in the context of the COVID-19 response have been lacking94, but would be critical in ensuring that local engagement strategies result in significant uptake of testing, tracing and isolation over time. We therefore suggest that each local area develop a community advisory committee, whose role is to advise on the suitability of the national plan in their area, and to support the design of a local public health communications strategy tailored to specific subpopulations. It is critical that this group is composed of individuals from the full range of ethnic and cultural backgrounds within the area, given the importance of identity and context to the promotion of positive health behaviours, and the existing marginalisation of subgroups of the population. A life course approach would also ensure that any and all messaging was targeted to the specific needs and concerns facing individuals across the life course.
At the outset, community advisory committees may need to meet regularly (e.g. weekly to co-develop communication materials); but over time, its role could transition to helping provide an accountability loop between communities and implementers and managers of the TTI programme, which would require less regular contact. In this way, community members are able to feed details of emergent challenges and difficulties that people face in adhering to cycles of lockdown, real-time data on the efficacy of support systems, and ability to adhere to testing requirements over time. These groups could be coordinated by Public Health COVID-19 supervisors (see below).
There are relevant concerns about how much time it would take to set up these groups in each area. However, each local entity will have a range of third and voluntary sector organisations who are already working to support various communities affected by the crisis. Rapid assessments and mapping of existing community networks by public health agencies would allow for a quick deployment of existing and active community groups in each area, to take control of recruiting relevant people from various backgrounds to engage with the committee.
The task of the supervisor will be to create an overarching structure to coordinate their efforts in a unified structure. In times of lockdown where participatory engagement is limited or restricted, evolving frameworks for how to conduct remote participatory research and community engagement could be adapted95. Such a community mechanism will have wide-reaching benefits, including; maintaining local buy-in over time, appropriately tailoring engagement strategies and innovating over time to maintain engagement, and helping citizens to feel as though they are a part of a wider process for promoting collective wellbeing. The latter has been shown as critical in other crisis and recovery focused settings96,97 and can have positive knock on effects for mental health outcomes in the general population, which is a growing concern in the crisis98.
Contact tracing budget. One Public Health Community Officer (PHCO) will need to be recruited per 1,000 population (the exact number needed to be recruited in each three month block depends on the number of infections as explained in the economic model section), with budget for 20% extra posts included to cover sickness and absence to help ensure contact tracing always meets demand. These people should be familiar enough with their community to identify individuals disconnected from government reach and internet apps. They could be unemployed or under-employed lay people, including those made redundant due to the pandemic. No prior public health experience or skills will be required beyond minimal educational attainment and having been resident in their local area for at least a year, though ability to speak appropriate languages will be relevant for some communities. The PHCOs could be trained via a short online course delivered by public health professionals and will undergo online refresher training every month. PHCOs will be paid a living wage of £10 per hour, £80 per day for an 8hr shift.
PHCOs will be supervised by full-time Public Health COVID-19 Supervisors (PHCS), at a ratio of 1 supervisor per 50 PHCOs. These PHCSs could be graduates of master’s degrees in public health or related disciplines and appointed if they can pass a simple test about control of the COVID-19 epidemic in line with this strategy; or, if sufficient numbers are available and they would not be taken away from important existing duties, they could be Environmental Health Officers. They will be based in COVID-19 offices in their local authority area. Given 343 local authorities in the UK, each will have around 3 or 4 PHCS. PHCS will be paid £20 per hour, £160 per day.
Each local authority will need a COVID-19 response team lead overseeing this effort. The team lead will directly manage and supervise the PHCS and have an overview of the COVID-19 situation in their local authority area. They will be public health specialists with at least five years of experience, perhaps already in post in the local authority area. Importantly, their duties will only relate to the COVID-19 contact tracing, testing and isolation strategy. Therefore, if already in post they will be relieved of other public health duties (and an additional public health lead recruited to oversee such duties) – or perhaps less disruptively, individuals without existing duties will be recruited to lead the COVID-19 response in their local area.
The importance of an integrated system with all workers solely focusing on COVID-19 needs to be emphasised. It is likely to be necessary to ensure the consistently high levels of contact tracing, testing and isolation required.
Mobile phone costs and travel costs are included for all cadres as needed.
A population-wide testing programme99 is a core component of population-wide TTI. This would require the following resources, which are either currently available or can be sourced from UK suppliers within a matter of weeks:
1. A register of names, dates of birth, and addresses of all residents registered with a GP, to be updated as necessary with test results, changes of address and addition of unregistered subjects. Anonymous registration with local outlets for sample collection and delivery is needed for those reluctant to give name and address. “Ghost patients”100 can be dealt with using the strategy developed by the ONS.
2. New 96-well machines running direct RT LAMP assays101 18hrs per day processing 96 samples every 30 minutes. Experienced staff to operate them are already in place in large and small academic and commercial labs throughout the UK, including possible demonstration sites. Posts for four 9-hour shifts for lab workers will be needed: 1 technician running each machine and 1 filling the wells with samples.
3. Self-sample spit (saliva) test kits including sample transport tubes individually labelled with name, date of birth, and barcoded ID, LAMPreagents (note RT LAMP does not require the RNA extraction step so needs less reagents), and microtiter plates for 10 million tests per day. Additional production facilities must be commissioned if necessary (Box 1).
4. Arrangements to deliver and collect samples from every household once a week, with delivery to a testing lab within a few hours. Results would be directly uploaded online automatically by the RT LAMP machine into a LIMS system as the sample is diagnosed by the machine, coupled with auto texting of negative results using software already in place. Positive results in those without phone or email would be delivered by courier.
5. This high throughput would depend on various regulatory emergency waivers:
1. Lab staff would wear PPE where necessary but would not be accredited to conduct medical tests.
2. Laboratories would be advised on precautions but not accredited for handling infectious samples.
3. LAMP reagent production with normal non-medical quality control cannot be hampered by patents or regulations on medical test manufacture.
We recommend evaluation of regular COVID-19 saliva testing of the whole population in an entire city as a demonstration site (preferably several towns and cities), with strict household isolation following a positive test. Isolation ends when all residents test negative at the same time. Everyone else can resume normal life if they choose to. This should be assessed for feasibility in one or more cities with populations of 200,000–300,000. This experiment could only be achieved after extensive, transparent public engagement leading to widespread public acceptability across all social and economic groups. Economic and educational measures would need to be provided to ensure equity with the non-quarantined population. Although this is an ambitious proposal, it does need to begin as soon as possible, whilst the infection rate is fairly low but rising. The rate at which it then rises or falls compared with the rest of the UK will be apparent within a few weeks. A decision can then be taken on national roll-out, beginning in high-risk areas.
A local population of 200,000 with 90% compliance will require 26,000 tests per day, plus an excess to offer more regular testing for NHS staff and care workers. Whatever the results, these data will enable policy to be based on real-time evidence (instead of modelling assumptions) on new infection rates in the expanding regularly-tested population and the untested remainder. The latter can be monitored by testing population samples as well as by NHS number linkage to hospital diagnoses and GP records. Complementary aspects of PTTI: contact tracing and phone apps will be critical in the unscreened population and may enable testing to be done less frequently as prevalence falls. Testing would be voluntary, but incentives for staying in isolation following a positive test in a household could be considered in line with those suggested by community advisory committees. Helplines would be provided to support households in isolation with access to income compensation, mental health support and food delivery.
These pilot studies, one of which has started on a smaller scale in Southampton with 14,000 people31, will show whether PTTI is a practicable way of responding to the COVID-19 epidemic. Even if the epidemic is not completely controlled in pilot studies the establishment of far greater testing and tracing capacity will facilitate other initiatives. Different households would return samples on different days, giving a daily sample of each small area. Depending on the proportion of people tested and cases detected a local outbreak could therefore be detected soon after it occurs, as test results would be automatically uploaded online by each LAMP machine.
A register of everyone registered with a GP (suitably amended to deal with unregistered people and “ghost patients”) would be used to deliver and collect saliva (and nasal/throat in a subsample) self-samplers in bar-coded tubes labelled with name and date of birth of all residents to every household once a week. The register would be expanded to include any missing people who are subsequently identified (with unique ID numbers for those with no NHS number) and continuously updated to assign people to the household of their current address. Many “households” would have one resident.
Households would self-isolate on the day that any resident gets a positive test, with earlier self-isolation of a household when anyone in it is thought to have COVID-19 based on a publicised list of diagnostic symptoms, pending the household’s next test results.
Contact tracing (above) could be focused on the “hard to reach” population that the uncontrolled epidemic will then be confined to. Anyone not possessing a negative test result dated in the past week would be required to provide a saliva/nasal/throat sample and their name, address and date of birth. They would be added to the register and sent weekly self-sample kits like everyone else. There will be challenges with this, for example, inclusion of the homeless population, that may need to be overcome.
Samples would be analysed on machines in university and commercial labs, if necessary by continuous (24-hour) operation (with very occasional down-time for maintenance), though we have costed 18hr per day operation. Laboratory and testing regulations would have to be set aside to enable the laboratory staff currently using these machines for other purposes to do the testing supported by additional assistants. Strategic planning to identify essential laboratory work that needs to be continued during the COVID-19 crisis will be required. This should consider the opportunity costs of not doing such work, whilst also considering the opportunities and costs of extra shifts to utilise the same equipment, recruitment and training of extra lab staff and potential efficiency gains to existing processes (including those that could be gained via relaxing regulations, along with the potential costs of relaxing such regulations).
One of the key bottlenecks for ramping up testing to such a large scale is the availability of reagents and test kit supplies for the tests. Creative ways of resolving this issue are urgently needed (Box 1).
TTI and UT are ambitious compared to the number of tests currently conducted each day. However, it is in line with international estimates of the scale of testing required33,34. The UK government’s five-pillar plan for scaling up COVID-19 testing102 reaches out to local manufacturers to ramp up testing capability and pharmaceutical companies are also offering to help103. The extent to which such capacity can be transformed into sustained delivery of the government’s current target of 500,000 swab and antibody tests per day is still unclear.
Studies are underway to confirm that saliva samples collected into simple specimen pots can reliably be used for mass population SARS-CoV-2 testing; if confirmed this would remove the current bottleneck in swab availability. The main testing reagents in short supply are not likely to be the non-biological chemicals used, large enough quantities of which could fairly easily be produced in around three months by industrial chemical companies. Some of these materials are already supplied by large companies such as BASF. The bespoke formulations of the mixtures of bio-based reagents, such as proprietary mastermixes and primers specific to each test kit, are potentially the main bottlenecks104. It will likely be easier and quicker for the existing manufacturers to scale up production than for a new company to attempt to do so, as the new company will require all of the same ingredients in order to exactly match the bespoke formulation of the specific test kit.
Therefore, the UK government probably needs to coordinate industrial consortia of companies with relevant scale-up capabilities and Good Manufacturing Practice approval, such as Robinson brothers105 (based in the midlands), and test kit manufacturers, such as New England Biolabs and OptiGene, to ensure there is adequate supply of key reagents. In this way, test kit manufacturers will be enabled to create the quantities of the bespoke proprietary formulations needed for millions of tests a day in the UK.
To ensure manufacturers have adequate incentive to participate, the government could issue “put options” that allow the companies to recoup most of their losses in the event the kits are never used106. More traditional methods of reducing commercial risk, such as direct purchase orders and public-private partnerships, can also be considered so long as they can be arranged quickly enough.
Initial estimates from an industrial chemist suggest the costs to cover the UK demand, per type of reagent, are on the order of £5-10m. It would require short bespoke use of manufacturing units (equipment) per component, the blending of the final formulation, and finally the development of appropriate logistics. The total cost is estimated to be less than £100m.
Rapid efforts will also be needed to source the swabs required to collect nasal/throat self-samplers and the bar-coded tubes labelled with name and date of birth of all residents, to deliver to every household once a week. Again, option-based guarantees and other de-risking measures could play an important role in ensuring the demand is met106.
TTI: Test-Trace-Isolate UT: Universal Testing
The team of PHCO and PHCS will follow up all those who test SARS-CoV-2 positive and who therefore require isolation. They will ensure that the people requiring isolation understand they need to stay at home for the required period in order to not spread the virus, and steps will be taken to ensure that households have the resources necessary to comply with isolation in the first instance. The costs of policing any infringements will be met by the fines levied for such infringements (likely with surplus funds left over). Therefore, no costs are added for isolation encouragement and enforcement.
For isolation support and enforcement to work without disadvantaging marginalised groups further the following will need to be put in place:
1) financial compensation for time off work to comply with a 14-day isolation order following tracing;
2) clear guidelines on the roles and powers that police and other authorities have in enforcing isolation;
3) a means-based fine system for infringements of isolation, based on household income levels/earnings;
4) development of minimum packages of support that are streamlined to specific vulnerable populations – so support that is provided is bespoke for the needs of each household during an isolation period (i.e. houses where earning levels are not impacted will be offered a different resource package than those where earnings are impacted);
5) assurances that basic resources (heating, water, electricity, internet access) will be guaranteed during the period of isolation, and for a one-month period post isolation.
On rare instances where households still break isolation rules, police officers will be put in touch with households in breach of guidelines. Fines will be levied in line with household income levels (there is precedence for this with speeding fines107).
All data used in this paper is included in the tables of the paper or otherwise publicly available and included in our software, linked below.
Source code available from: https://github.com/ptti/ptti/tree/F1000-final
Archived source code at time of publication: https://zenodo.org/record/4298847
License: GNU GPL Version 3+
This study was developed by TC, JPG, WW and DM with contributions from DF and RR. A previous version of the work was conceptualised by TC, JP, NA, KMG and PR and initially developed with DF, GY, RB, DM, CaB, EP, MO, MS, MG and RR, with early analysis done by GC and TC. The mathematical model used here was developed by WW, SS and JPG with input from TC and DM. The economic model used here was developed by DM and TC with input from WW, SS, JPG, EP, MG and MS. TC, WW, JPG, SS, GC, CaB, KMG, DF, EP, TH, NG, NC, MS, and MG contributed parameter values used in the model or interpretation. The scenarios used in the study were developed by JPG, TC, DM, WW and KG in discussion with all co-authors. WW, DW and JPG ran the modelling analysis with input from TC. DM and WW ran the economic analysis with input from TC, JPG, EP, MG and MS. JPG drafted this version of the paper with substantial contributions from TC, WW, DM, DF and RR. CaB, KMG, JP, RAB, GY, KO, PJR, TH and GC contributed specific sections of the manuscript. Co-authors provided critical feedback across developing iterations of the paper and have read and approved the final manuscript.
The following people have provided helpful comments or suggestions, or have otherwise enabled this work: Katie Brennan, Martin J Buxton, Majid Ezzati, Amy Gimma, Gabriel Goh, Grace Grove, Agnese Iuliano, Christopher Jarvis, Paula Lorgelly, Tara Mangal, Ofir Reich, Justin Shovelain, Mark E Thomas.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
References
1. Petersen I, Phillips A: Three Quarters of People with SARS-CoV-2 Infection are Asymptomatic: Analysis of English Household Survey Data.Clin Epidemiol. 2020; 12: 1039-1043 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Economics of infectious diseases
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 14 Dec 20 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)