Keywords
pulmonary rehabilitation, respiratory
pulmonary rehabilitation, respiratory
In the United Kingdom, chronic obstructive pulmonary disease (COPD) affects approximately 3.7 million people1, is responsible for approximately 30,000 deaths per year, and is the fifth most common cause of death2. COPD is an independent risk factor for postoperative complications (odds ratio OR 1.35 (CI 1.30-1.40)) and death (OR 1.29 (CI 1.19-1.39))3–6. Complications include pulmonary and cardiac events, sepsis, renal insufficiency and an increased reoperation rate3. Surgical patients with COPD thus represent a high-risk group in whom there is an unmet need to improve post-operative outcomes.
Pulmonary rehabilitation is “A physical exercise and education programme, tailored for each person. It includes information on looking after the body and lungs, advice on managing symptoms, including feeling short of breath, nutrition and psychological support. People who smoke are given advice on how to stop."7
Pulmonary rehabilitation is usually delivered in an outpatient setting, consisting of one hour of exercise and one hour of education, twice weekly for six weeks. It has profound benefits on breathlessness, exercise capacity and quality of life (number needed to treat (NNT)=2)8, no side effects are reported9. Pulmonary rehabilitation is associated with decreased hospital admissions (NNT=3–4), and mortality (NNT =~6) following COPD exacerbations10–13. Crucially, pulmonary rehabilitation is inexpensive9. Its effect is so powerful that it has a negative cost per quality adjusted life year (QALY), meaning it saves money for the NHS14. The main challenges facing pulmonary rehabilitation are the barriers to its uptake, as attendance and completion of the programme is often poor15,16.
Despite adoption in the National Institute for Health and Care Excellence (NICE) guidelines17 for stable COPD, pulmonary rehabilitation is not regularly offered to pre-surgical patients with COPD18. We believe that pulmonary rehabilitation merits investigation as a potential means to improve postoperative outcome in people with COPD undergoing surgery for the following reasons:
A handful of small surgical studies suggest beneficial effects of pulmonary rehabilitation on the incidence of postoperative pulmonary complications19–23. Differing endpoints, small sample sizes and restriction to specific surgical groups limits conclusive interpretation.
In the National Emphysema Treatment Trial (NETT)24,25, lung volume reduction surgery was compared with medical management of COPD. All patients underwent pre-operative pulmonary rehabilitation. In the thoracic surgical population of NETT similar outcomes (in terms of functional exercise capacity and health related quality of life, assessed prior to surgery) were observed to what would be expected in the treatment of non-surgical patients with COPD. In fact, approximately 10% of participants in NETT decided against lung volume reduction surgery because they felt so much better after pulmonary rehabilitation.
Shortened durations of pulmonary rehabilitation are efficacious8,26. This is important, because an adapted course may be necessary to fit within surgical time frames.
Pulmonary rehabilitation is widely available and standardised across the NHS in over 200 UK centres. This has important implications for scalability.
Pre-operative pulmonary rehabilitation needs sufficient time between the decision to operate and the operation, requires cross specialty working, and involves patients with two conditions (COPD and a surgical condition). A randomised controlled trial is therefore justified, as the current evidence base is either not specific to a surgical population or is case series based and therefore subject to selection bias. Furthermore, it is unclear whether a randomised controlled trial of pulmonary rehabilitation before surgery would be practical. This study investigated the feasibility of running such a large randomised controlled trial. The feasibility study design matched the expected full study design except in scale.
This feasibility study was run as an open, parallel group, randomised trial with an allocation ratio of 1:1. The study was conducted across two research sites (Oxford and Newcastle upon Tyne), chosen as two areas with different demographics and incidence rates of COPD. Ethical approval was granted by the South Yorkshire Research Ethics Committee (approval number 17/YH/0220). Written informed consent was obtained from all participants prior to the start of the study. The primary aim of the study was to determine feasibility for a randomised controlled trial and focused on recruitment rate, barriers to recruitment and uptake of pulmonary rehabilitation. The trial was registered on ISRCTN on 19 August 2017 (ISRCTN29696295).
Inclusion criteria:
Adult patients aged 18 years or older with COPD
Has capacity to take part in this study
Scheduled for elective major (body cavity) surgery OR laparascopic surgery that is anticipated to last longer than 2 hours
People with more than 20 pack years smoking history were approached to take part in the study if spirometry subsequently confirmed COPD.
Exclusion criteria
Inability to give informed consent
Insufficient command of English to understand the study documentation
Unable to participate in pulmonary rehabilitation treatment according to British Thoracic Society guidelines27.
Patients scheduled cardiac, thoracic and orthopaedic surgery and orthopaedic surgery
Patient involvement in study design: As we anticipated that recruitment to this study may be challenging, we discussed the study design with patient groups consisting of people with COPD who had either undergone surgery, or those who had experienced pulmonary rehabilitation. The key messages from these patient representatives were to ensure that transport to and from pulmonary rehabilitation would be provided, and that a flexible approach to scheduling would be necessary so pulmonary rehabilitation could fit with other appointments.
Participant identification and recruitment: To determine the best point in the surgical pathway to recruit participants, research nurses screened for study participants from the following sources.
From the surgical multidisciplinary team (MDT) meetings
In oncology clinics
From the electronic patient record for patients scheduled for surgery
From hospital anaesthetic preoperative assessment clinics
From cardiopulmonary exercise testing clinics
Participants were initially approached by their clinical team, and in those who agreed to take part in the study informed consent was taken at the first research visit.
The study aimed to collect 48 full data sets (24 in each centre, 12 pulmonary rehabilitation, 12 control arm). To achieve this 48 dataset target, based on known drop-out rates from pulmonary rehabilitation18 and potential further data loss due to surgical scheduling, we anticipated that we would need to recruit 72 patients. This sample size was chosen pragmatically, with the aim to test efficacy of recruitment, randomisation, how best deliver a control arm the best way to conduct the study across multiple sites and importantly to enable us to evaluate the performance of the primary outcome measures (e.g. for ceiling and floor effects).
Pulmonary rehabilitation: A pragmatic, exploratory approach was used to explore what is practically deliverable and tolerated by patients, working closely with local pulmonary rehabilitation teams in Oxford and Newcastle upon Tyne. The aim was for patients to be enrolled in 3 pulmonary rehabilitation sessions per week, for 3 or 4 weeks, depending on timing of surgery. Pulmonary rehabilitation of this shortened duration has been shown to be effective26. Patients were to attend standard NHS pulmonary rehabilitation groups run for patients with COPD.
Control arm: Patients randomised to the control arm would receive standard care including advice on smoking cessation, exercise and appropriate referral and education for those with newly diagnosed COPD.
Recruited participants were randomised 1:1 to either pulmonary rehabilitation or treatment as normal, minimised for study site and Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage, using an online randomisation service (Sealed Envelope Limited, London, UK). Participants were assessed prior to pulmonary rehabilitation or control treatment (preoperative assessment) and following surgery during hospital inpatient stay on postoperative days 3, 5, and 8 and again at a 6-week and 6-month follow-up (Figure 1 and below). Other than a 6-minute walk test, the research data consisted of self-report questionnaires on mood, symptoms, and quality of life. Data obtained from the NHS clinical record included various perioperative risk scores, co-morbidity scores, and measures relating to the operation and outcomes.
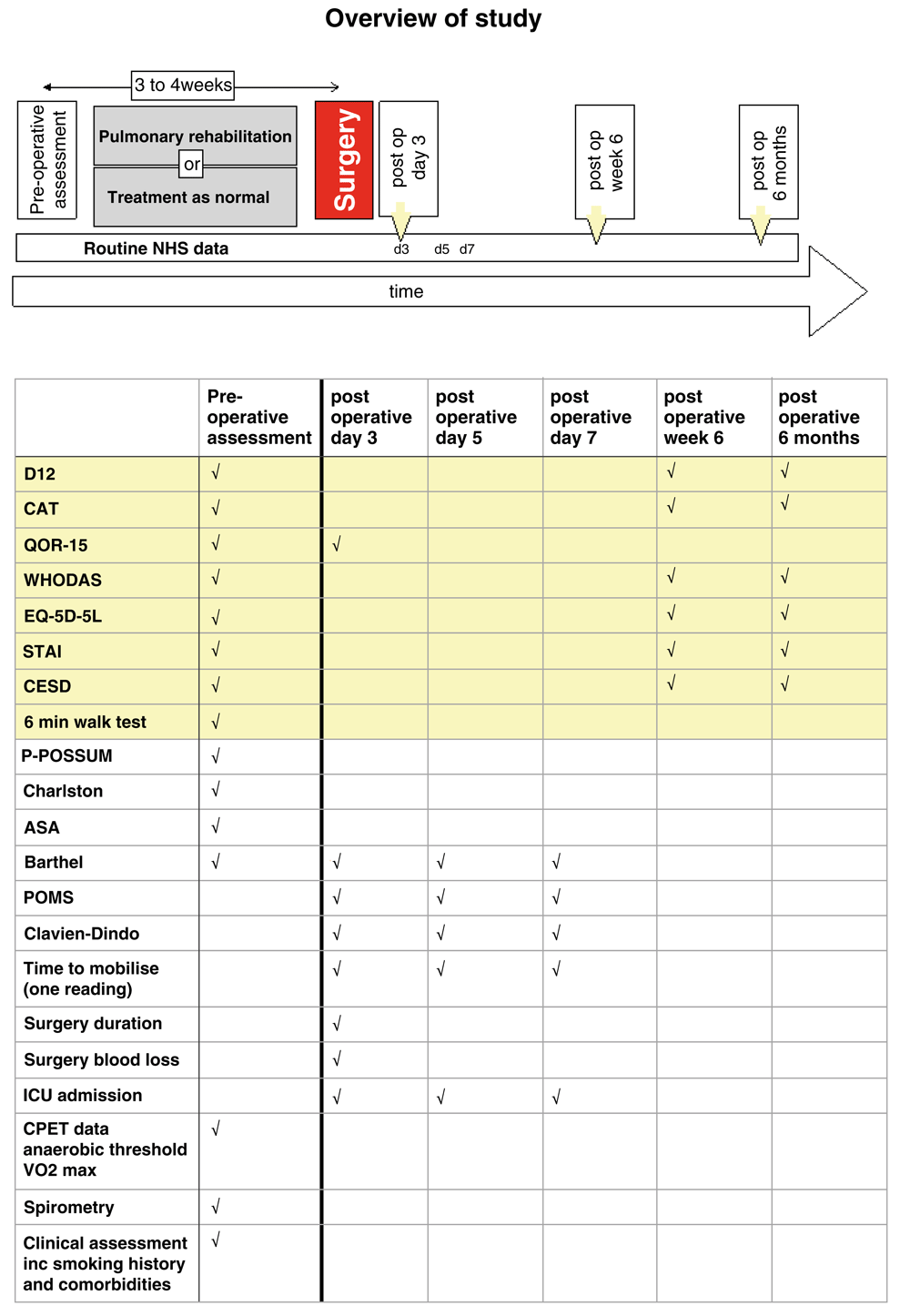
Data in yellow boxes is research data collected from the patient, whereas the data in pink relates to that collected from the patients' clinical record. Abbreviations: NHS; National Health Service, D12; Dyspnoea-12 questionnaire, CAT; COPD assessment test, QOR-15; quality of recovery score, WHODAS; World Health Organisation (WHO) disability score, STAI; Spielberger state and trait anxiety inventory, CESD; Center for epidemiolgic studies depression scale, P-POSSUM; Portsmouth Physiological and Operative Severity Score for the enumeration of Mortality, Charlston; Charlston Morbidity Index, ASA; American Society of Anesthesiologists Physical Status Classification System, Barthel; Barthel scale, Clavien-Dindo; The Clavien-Dindo Classification of surgical complications, ICU; intensive care unit, CPET; cardiopulmonary exercise test.
Medical, surgical, anaesthetic assessment including comorbidities including full detailed smoking histories.
Physiology
Psychology and health-related quality of life
○ Dyspnoea questionnaires (Dyspnea-12 questionnaire)
○ Anxiety (State and Trait Anxiety Inventory), Depression (Center for Epidemiological Studies Depression Scale), Fatigue (Fatigue Severity Scale), COPD Assessment Test (CAT)
○ Health status assessment with EQ-5D-5L and WHO disability assessment schedule.
These measures will be collected on postoperative days 3, 5, and 8 during hospital inpatient stay.
○ Surgical factors (duration of operation, blood loss), measured once only
○ Time to mobilisation
○ Assessment of activities of daily living (Barthel).
○ Intensive care admission, discharge, mortality
○ Patient-related outcome measures, including time to return to normal activities.
○ Morbidity tracking using the postoperative morbidity survey instrument and Clavien-Dindo surgical complication score.
Postoperative measures collected on day 5 post-surgery only
Data collected at discharge from hospital
Postoperative measures collected during 6-week follow-up visit.
Postoperative measures collected 6 months following surgery
Following confirmation that patient remains alive (NHS Spine and communication with general practitioner) we will invite the patient to attend a follow up assessment and collect the following measures.
Can we recruit at a sufficient rate to run an RCT?
What is severity (GOLD/MRC) of the recruited patients and how does this compare with the screened patients
Whether it is feasible to deliver the pulmonary rehabilitation intervention in the time available. This will include assess the impact of changing surgical dates, e.g. earlier (so insufficient rehabilitation delivered), later (so effect of rehabilitation wearing off).
Number of complete data sets collected
Percentage of missing data
Barriers to uptake of pulmonary rehabilitation
Number of patients identified in clinic with spirometry defined COPD, and their MRC and GOLD scores
Number of patients invited to participate in the study, and their MRC and GOLD scores
Number who accept invitation
Number who decline invitation but agree to participate in qualitative study
Number who decline invitation/don't reply
Number of patients who attend research assessment
Number of patients who sign consent form
Number of patients who complete pulmonary rehab or control treatment (i.e. compliance with study intervention)
Number of patients who have surgery in allocated timeframe (3 months following the second research visit)
Number of patients who continue the study during postoperative period
Number of patients in whom we can collect 6-month follow up data.
Scheduling of research appointments within suitable timeframes
Scheduling of pulmonary rehabilitation sessions within the surgical waiting time
Effectiveness of transport to/from pulmonary rehabilitation. Although we plan to contribute transport costs for the study there needs to be consideration for when pulmonary rehabilitation is offered as a treatment.
Factors relating to scheduling, including effect of changes in operation date.
Feasibility of tracking patients postoperatively-either in person and/or via electronic and paper based patient records
Outcome measures being collected to get an estimate of
Check for post randomisation dropouts because of allocation to unfavoured treatment group
Do the "treatment as normal" patients seek exercise sessions elsewhere?
Is the drop-out rate from the study similar in both groups?
Does the randomisation system work?
Health economics. We know there is health economic benefit for pulmonary rehabilitation in the treatment of COPD - does this translate to a surgical population?
Assessors will be blinded to treatment group.
We will trial ways to ensure that the outcomes chosen are as fair as possible and are collected in a way that avoids bias. This will include objective criteria scoring by blinded individuals.
Outcome measures: We anticipated that the primary research outcome measures for a future randomised controlled trial would be morbidity, mortality, length of hospital stay and hospital readmissions so we collected data on this to help with future study design.
The progression criteria to a substantive study were as follows:
a) Recruitment rate greater than 66% of predicted for the present study - this would account for a minimum rate that would result in a practical number of centres for a randomised controlled trial.
b) Screened and recruited patients similar in severity - assessed by clinical judgment
c) Compliance with treatment approximately in line with national COPD audit figures
d) To be confident that there were no insurmountable barriers to the uptake and running of pulmonary rehabilitation for these patients.
Recruitment commenced in Oxford in January 2018 and in Newcastle upon Tyne in May 2018. A total of 266 patients were screened of which 65 met the inclusion criteria. As of January 2019, one participant had been recruited in Oxford and two in Newcastle upon Tyne. At this point it was determined that running a randomised controlled trial of pulmonary rehabilitation would not be feasible and the study was terminated in March 2019. Further details are presented in Figure 2. We have not presented the research data here due to interpretability and confidentiality issues arising from only acquiring two datasets.
We found that the main barrier to study recruitment in both centres was associated with the way the surgical pathway is organised, especially with regards to two specific aspects; surgical timelines and identification COPD.
In Oxford, challenges were faced in identifying patients with COPD soon enough before surgery.
It was challenging to identify patients with COPD at surgical clinics and multidisciplinary meetings as patients had just received a diagnosis of cancer, but a definitive treatment plan had yet to be instituted. At this point the focus is on the surgical condition rather than medical conditions such as COPD. Medical records focused mostly upon surgical condition and respiratory records were often in separate (unavailable) notes and smoking histories were rarely present. This made screening laborious and time inefficient.
The definitive decision on whether to operate would only be made following neoadjuvant treatment. Oncology clinics were assessed as an identification point, but we found that potential participants attended too many different clinics to find a suitable point for screening.
Screening the electronic patient record for patients scheduled for surgery did not successfully identify additional people with COPD. Therefore, COPD was often not formally diagnosed until the following the anaesthetic preoperative assessment clinic which usually occurred 2–3 weeks before surgery, with cardiopulmonary exercise testing (CPET) testing taking place at a similar time before surgery.
The difficulty in identifying potential participants with COPD was somewhat unexpected. As audit data from the pre-operative cardiopulmonary exercise testing clinics in both Oxford and Newcastle-upon-Tyne suggested that COPD was present in 10 to 15% of the 2,000 to 3,000 patients each year passing through those clinics. This meant that our pool of potential participants was around 300 in each centre each year.
Vascular surgery clinics were also assessed; these non-cancer patients have a more clearly defined pre-surgical pathway. However, we found that due to changes in surgical practices, most patients with respiratory disease were treated endovascularly and thus recruiting from this clinic was also deemed low yield.
At anaesthetic pre-assessment clinics, the main challenge was that potential participants with undiagnosed COPD may not have been formally diagnosed after the pre-assessment clinic (when patients were sent for lung function tests); this made confirmation of eligibility difficult, and further lessened time for study inclusion.
In Newcastle upon Tyne, surgical patients attend the anaesthetic pre-assessment clinic about one month before surgery, this is in contrast to Oxford where the time between anaesthetic assessment and surgery is often much shorter. In Newcastle-upon-Tyne we were more successful at identifying patients with COPD, but despite this only two patients were recruited into the study (one of whom subsequently withdrew).
We discussed increasing the number of sites for the study with three other potential UK sites (two teaching hospitals and one large district general hospital) who performed evaluations of their services, taking into account the preliminary findings of this work. This would help us evaluate whether the identification and recruitment issues were generalisable to other centres. However, we found that in all three centres the main point of identifying COPD was found to be at anaesthetic pre-assessment clinics, which occur two to three weeks prior to operation date (similar to Oxford).
CPS, in his role as Royal College of Anaesthetists National Clinical Lead for Perioperative Medicine, surveyed perioperative medicine and preoperative assessment clinics in 110 hospitals in England over the course of 201728. This piece of work found that the usual time interval between anaesthetic preassessment and surgery was often only 2–3 weeks, but with wide variability (unpublished observations). This is equivalent to current practice in Oxford.
Pulmonary rehabilitation is a potentially valuable treatment for improving the health status of people with COPD prior to surgery. We established that a full randomised controlled trial is not feasible. As a result of this study we have identified an unmet need in the early identification of COPD in patients presenting for surgery.
Although the study was only run in two UK centres, further scoping work in three additional centres and a related England-wide survey of anaesthetic services means that we are reasonably confident that similar challenges in identification and recruitment would be found if a randomised controlled trial were to run across the UK, and thus we believe that our findings are generalisable.
Pulmonary rehabilitation is an integral part of the NICE guidelines for the treatment of COPD, and therefore every person with COPD should be offered this treatment (alongside the other components of therapy recommended by NICE). This raises the question about whether a randomised controlled trial is actually the most appropriate methodology for future work. Barriers to pulmonary rehabilitation are well recognised, even when implemented as a clinical treatment15,16. These barriers can be even more pronounced when tested as an optional research intervention29. We provided free transport and offered flexible scheduling for potential participants. These were recommended by our patient liaison group during the study design phase to help overcome barriers to taking part in pulmonary rehabilitation, but clearly were insufficient to enable us to recruit at a sufficient rate.
We therefore speculate that if it we could identify COPD at the beginning of the patient's surgical journey, patients would be much better placed to have appropriate management and optimisation of their COPD. Spirometry is cheap, widely available and reliable; and thus perfect for a simple primary care test which should be offered much more widely, and would allow for early optimisation of drug therapy. Pulmonary rehabilitation could occur in a more timely fashion as this intervention could be integrated and planned alongside chemo- and radio- therapy, rather than in the weeks immediately preceding surgery. Patients with COPD would benefit even if they do not eventually proceed to surgery, with potential cost savings for the NHS14,30. Potential solutions to this are illustrated in Figure 3.
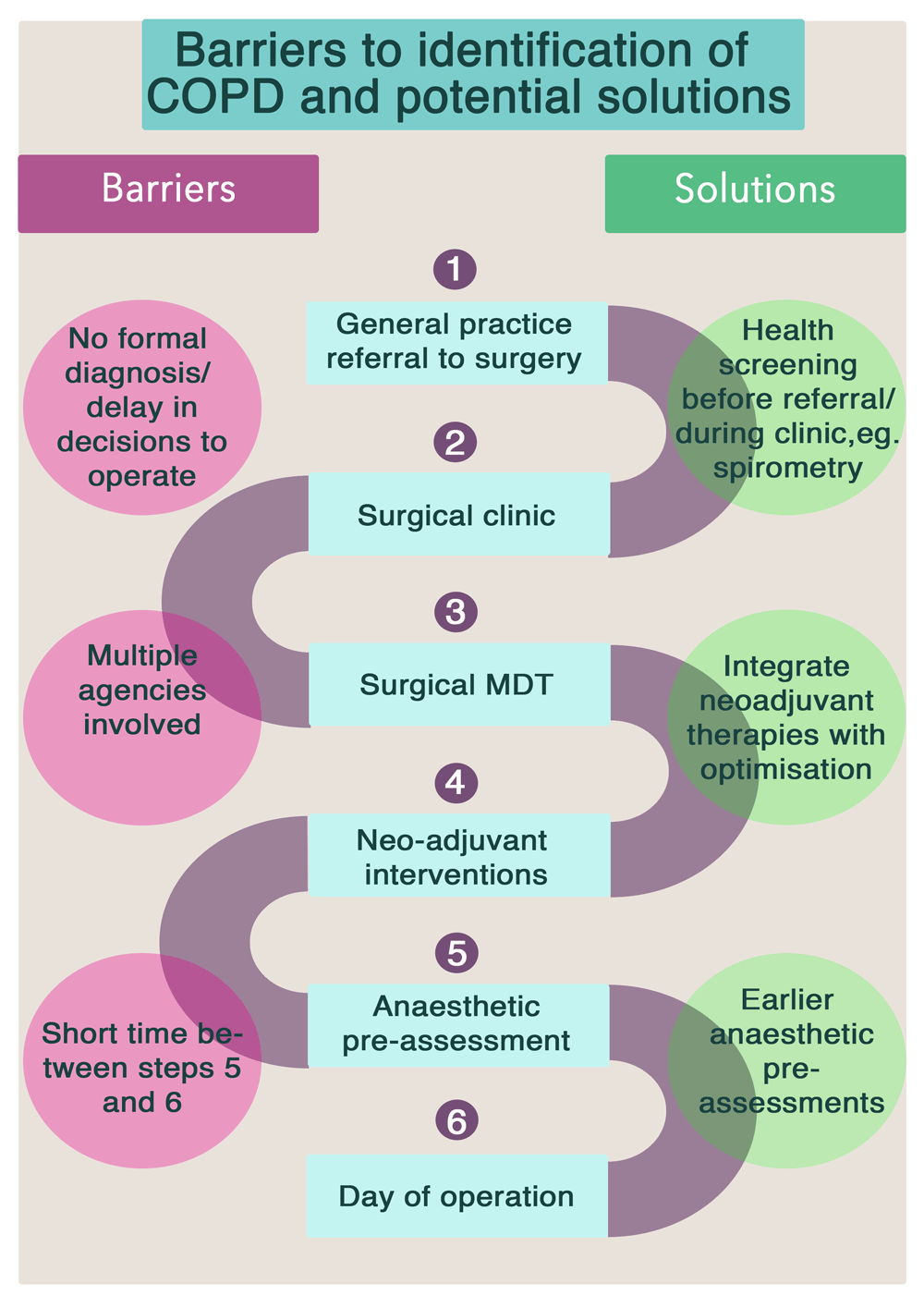
This study has demonstrated the considerable challenge in performing additional interventions in the immediate period before surgery. However, the duration of the patient’s journey from referral to surgery can take several months and remains an ideal period to optimise COPD if appropriate patients are identified earlier in the process. Some UK hospitals have recently implemented initiatives to ‘re-design’ this surgical pathway, which may help overcome this barrier31. The importance of identifying and engaging with patients early after the “moment of contemplation” of surgery is clearly a critical success factor for interventions such as pulmonary rehabilitation; which are known (from other contexts) to require a defined period of time to implement and provide benefit. However, we should take caution from evidence from studies in lung cancer which show that the time of diagnosis is a difficult time to consider pulmonary rehabilitation32. Although there may be an opportunity to provide pulmonary rehabilitation whilst neo-adjuvant therapy is being provided patients often do not want to engage with pulmonary rehabilitation at a time when they are dealing with a new, life changing diagnosis and having burdensome, potentially toxic cancer treatment14. Thus, it might turn out that pulmonary rehabilitation can only really feasibly delivered once cancer treatment has finished. There is emerging evidence that exercise therapies enhance cancer survival33,34 and that a recommendation from the oncologist may be influential in the view patients might take.
Pulmonary rehabilitation represents an important part of the NICE guidelines for the treatment of COPD and is readily available in the NHS. We know that patients will benefit from pulmonary rehabilitation, even if it is consequently shown not to have a specific effect upon postoperative pulmonary complications. Patients who consequently do not require surgery will still benefit. We have shown that a randomised controlled trial is not feasible, so we need to approach this in a different way using alternate methodologies.
All data underlying the results are available as part of the article and no additional source data are required.
Open Science Framework: CONSORT checklist and flow diagram for ‘Findings of a feasibility study of pre-operative pulmonary rehabilitation to reduce post-operative pulmonary complications in people with chronic obstructive pulmonary disease scheduled for major abdominal surgery’. https://doi.org/10.17605/OSF.IO/4ZQNK35
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: perioperative medicine
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Physiotherapy, critical care, perioperative medicine, health economics, clinical trials, postsurgical recovery
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 09 Mar 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)