Keywords
acute kidney injury, AKI, preconditioning, hypoxia, hypoxic preconditioning, RIPC, ischemic preconditioning, diet, dietary restriction, caloric restriction, protein restriction, fasting, contrast induced nephropathy, cardiopulmonary bypass
acute kidney injury, AKI, preconditioning, hypoxia, hypoxic preconditioning, RIPC, ischemic preconditioning, diet, dietary restriction, caloric restriction, protein restriction, fasting, contrast induced nephropathy, cardiopulmonary bypass
Acute kidney injury (AKI) is a highly relevant problem in clinical practice and is associated with an increased risk of mortality1,2, the development of chronic kidney disease, and cardiovascular events3,4. The incidence of AKI has increased in recent years, and, in the context of the demographic change, it is likely that a plateau has not been reached yet5,6.
Imbalances between circulatory demands and perfusion leading to renal ischemia as well as toxic insults are frequent causes of AKI and are often observed as adverse events of medical interventions (for example, major surgery or chemotherapy). In both cases, loss of cell adhesion molecules, cell polarity, and death of tubular epithelial cells lead to cell detachment with subsequent urinary tract dysfunction7. Intensive monitoring and adequate fluid management at the time of diagnosis constitute the standard therapy. However, an effective treatment for established AKI is still missing. Apart from maintaining euvolemia, atraumatic/minimally invasive surgical techniques, and avoidance of potentially nephrotoxic substances, no preventive measures have been proven to exert a protective benefit in clinical practice8.
Animal models have been established for both types of AKI primarily using rodents and employing ischemia-reperfusion injury (IRI) by transient clamping of the renal vessels9–11 or toxic tubular injury induced by the intraperitoneal injection of cisplatin12–14. In both models, various preconditioning algorithms have proven to be very effective. In general, the term “preconditioning” describes the strategy of activating the body’s own stress resistance mechanisms, thereby mitigating consecutive harm15, and commonly exploits the concept of hormesis.
In this review article, we discuss known and effective strategies of preconditioning. Considering promising results from animal models and first results in clinical trials, we will focus on strategies targeting either the cellular response to hypoxia or nutrient availability: ischemic preconditioning (IPC)16, remote IPC (RIPC)17, hypoxic preconditioning (HP)18, prolyl-hydroxylase (PHD) inhibition (PHDi)19, and dietary interventions9.
The oldest known procedure is IPC: repetitive periods of short-term sublethal organ ischemia enhance the resistance against cellular stress and mitigate the damage of subsequent profound ischemic injury16. The underlying mechanisms have not been fully deciphered yet. So far, it has been assumed that the repetitive stimuli lead to a release of various chemical messengers (for example, calcium ions, reactive oxygen species, and hydrogen sulfide), vasoactive substances (for example, adenosine, bradykinin, opioids, and urocortins), neurotransmitters and hormones (for example, acetylcholine and angiotensin) as well as cytokines (for example, tumor necrosis factor-alpha [TNF-α], interleukin-6, and prostaglandins) activating G protein–coupled receptors20,21. A number of pathways have been implicated to be involved downstream of these mediators. First is the activation of nitric oxide (NO) and NO synthase (NOS) by activation of protein kinase C (PKC), phosphoinoside 3 kinase (PI3K/Akt), and the guanylatcyclase leading to the activation of ATP-dependent potassium channels (KATP) and the priming of mitochondrial permeability transition pore (MPTP)20,22–26. Second is the reperfusion injury salvage kinase (RISK) pathway by activation of PI3K/Akt, 70 ribosomal protein S6 kinase (p70S6K), glycogen synthase kinase 3b (GSK3b), and the inhibition of MPTP opening20,21,26–29. Third, the survival activating factor enhancement (SAFE) pathway gets activated by activation of glycoprotein 130 (gp130) or TNF-α receptors, the Janus-activated kinase (JAK) signal transducer, and the stimulation of the activator of transcription (STAT)20,21,26,29–31. Furthermore, a role for SIRT1-mediated NAD+-dependent deacetylation32 and mitochondrial biogenesis33 has recently been shown.
Although IPC shows promising results in animal models and clinical pilot trials, it is obvious that translation to the clinical setting is restricted to surgical settings and therefore is not easily feasible. Consequently, aiming to exploit similar mechanisms, the strategy of RIPC was developed. Through RIPC, an organ (for example, the heart) can be protected from damage by applying repetitive short-term sublethal periods of ischemia to a remote tissue (for example, the kidney)34. This protection goes along with a gene regulatory effect in the target organ35. Several different mediators of this distant effect have been hypothesized in the past: on the one hand, a neuronal effect in which the afferent neurons of the preconditioned organ provide protection of the remote organ has been described36. On the other hand, a humoral effect by various mediators has been described in several publications and the actual key candidates remain elusive. More recently, a very elegant study using the ischemia-reperfusion model of the heart could show that activation of hypoxia signaling in skeletal muscles increases the production of alpha-ketoglutarate (alphaKG). Circulating alphaKG induces kynurenic acid (KYNA) formation in the liver, and KYNA itself was sufficient to protect the heart from ischemic injury37. Furthermore, a humoral activation of various cellular pathways (for example, by NO38, connexin 4339, or hypoxia-inducible factor 1 alpha [HIF-1α]40) has been published. The major advantage of RIPC lies in its simple clinical applicability since the remote effect can also be achieved by repetitive inflation of a blood pressure cuff on the arm or thigh. In a randomized, double-blind, sham-controlled clinical trial investigating the effect of RIPC prior to elective coronary angiography, a significant reduction of the incidence of contrast media–induced acute injury (defined as an increment of serum creatinine of at least 0.5 mg/dL or a relative increase of more than 25% over baseline value within 48 hours) could be shown for the intervention group in a cohort of 100 patients with chronic kidney disease (that is, serum creatinine of more than 1.4 mg/dL or estimated glomerular filtration rate [eGFR] of less than 60 mL/min * 1.73 m2) and at high risk of developing contrast medium–induced kidney injury according to the Mehran risk score41. In another randomized, prospective, multicenter, and double-blind clinical trial investigating 222 patients with a reduced eGFR (that is, <40 mL/min per 1.73 m2 or between 40 and 60 mL/min per 1.73 m2) and two or more risk factors (age ≥75 years, diabetes mellitus, or heart failure New York Heart Association [NYHA] III or IV) undergoing elective coronary angiography or percutaneous transluminal coronary angioplasty (or both), no significant difference for any study group could be shown regarding the incidence of contrast media–induced nephropathy (primary endpoint), change of serum creatinine, or change of eGFR (secondary endpoints)42. The comparability of both studies is limited because the population of the latter trial included fewer patients with diabetes mellitus (~50 to ~62%, respectively) and the Mehran score differed markedly (~8 to 13). Besides, the incidence of contrast media–induced nephropathy in the control group of the trial by Roubille et al.42 was reduced in comparison with their own database (4.5 to 28%) and in comparison with the trial by Er et al.41 (20%). Furthermore, the volume of contrast media used was much smaller in the trial by Roubille et al.42 (~75 mL) in comparison with the trial by Er et al.41 (~120 mL).
In addition, the clinical relevance of contrast-induced nephropathy (CIN) has been debated extensively in recent years, supporting the idea that its incidence is far overrated in clinical practice; this, in turn, limits the value of the used model43. The largest trials examining the protective potential of RIPC were performed in cohorts undergoing surgery on cardiopulmonary bypass. Unfortunately, owing to major differences in both outcome and patient characteristics, these studies, in line with the data on CIN, have not provided conclusive results. Zarbock et al. conducted a prospective randomized, double-blind, and sham-controlled multicenter trial enrolling 240 patients who underwent cardiac surgery with the use of cardiopulmonary bypass44. RIPC led to a lower incidence of AKI (primary endpoint), a reduced need for renal replacement therapy (RRT), and a shorter length of stay in the intensive care unit (ICU). These results were highly promising at first; however, two much larger randomized, double-blind trials published in 2015 dampened the enthusiasm. The RIPHEART trial examined RIPC in 1403 subjects undergoing coronary artery bypass graft (CABG) surgery and did not detect any benefit concerning the primary endpoint (death, myocardial infarction, stroke, AKI, or length of stay in the ICU)45. The same holds true for the ERICCA trial46. Here, RIPC did not result in improved resistance to cardiovascular and cerebrovascular events within 12 months after surgery (primary endpoint) and perioperative myocardial infarction, acute myocardial infarction, AKI, length of stay on the ICU, ejection fraction, and quality of life (secondary endpoints) in 1612 patients after CABG with or without cardiac valve surgery (801 RIPC and 811 sham). An important aspect that has been discussed to explain some of the differences between these trials is the different mode of anesthesia that may interfere with the potential of RIPC. In ERICCA, the anesthetic procedure was not standardized46 whereas it was limited to inhalation anesthesia in Zarbock et al. and intravenous anesthesia using propofol in RIPHEART. Furthermore, Zarbock et al. explicitly enrolled only individuals at a high risk of AKI defined by a Cleveland Clinic Foundation score of 6 or higher. In RIPHEART, there was no stratification for the risk of AKI and the participants showed a moderate risk for death 30 days after surgery (mean Logistic EuroSCORE47,48 for both groups: 4.2). In ERICCA, patients were eligible only with a EuroSCORE of 5 or higher without any specific risk assessment for AKI. In a meta-analysis of RIPC in the prevention of AKI in patients undergoing CABG surgery, a benefit of this pre-treatment could be demonstrated only in the subgroup that received inhalation anesthesia. Here, it is important to note that volatile anesthetics themselves are being discussed as preconditioning agents49,50. Nonetheless, RIPC appeared to have a potential additive effect to this protective potential in this study. With regard to the development of dialysis-dependent kidney injury, there was no advantage in any of the subgroups examined51. From the perspective of the authors, owing to the different risk stratification in the selection of subjects and non-uniform operating procedures, a final assessment regarding the potential of RIPC in the clinical setting is currently not possible.
Although for a couple of years the field focused on driving RIPC toward a potential clinical use, other modes of preconditioning have regained increasing attention more recently. In 1994, a model for HP was published52. Six-day-old rats that were preconditioned by subjecting them to an ambient oxygen content of 8% for 3 hours showed significantly reduced cerebral infarct zones following unilateral occlusion of the carotid artery52. Altered HIF signaling could be identified as a mediator of this effect18. Even though HP, like IPC, cannot be directly transferred to the clinical setting, activation of HIF can be induced pharmacologically, allowing potential future interventions in the patient setting. HIFs are heterodimeric proteins consisting of a hypoxia-regulated HIF-α and a constitutive HIF-β subunit53. Under normoxia, PHDs hydroxylate specific proline residues of the HIF-α subunit, inducing its proteasomal degradation. Hypoxia inhibits PHDs and allows the nuclear accumulation of HIF-α where the assembly of dimers of α- and β-subunit leads to the transcription of numerous target genes that have been hypothesized to be involved in the protective effect (for example, erythropoietin, vascular endothelial growth factor [VEGF], and heme oxygenase-1)54,55. PHD inhibitors are available and have been shown to mediate a protective effect regarding renal IRI comparable to HP using a rat model19. Interestingly, in the meantime, a PHD inhibitor—roxadustat—has been approved for the treatment of anemia in patients with dialysis-dependent and non-dialysis-dependent chronic kidney disease in China56–58 and Japan. More data on roxadustat were recently presented at Kidney Week59–61 aiming at approval for the US and Europe. Additionally, various other PHD-inhibiting compounds are being tested in clinical trials53. A double-blind, randomized, single-center phase II trial (ClinicalTrials.gov Identifier: NCT01920594) investigating a possible reduction in neurological, renal, or cardiac ischemia (or a combination of these) by PHDi prior to elective aortic aneurysm surgery has been completed but not fully published yet.
Apart from targeting the cellular response to hypoxia, dietary interventions have been shown to be one of the most promising strategies in organoprotection. Calorie restriction (CR) has long been known to mediate life-span extension, a finding that was first demonstrated in a rat model in 193562 and confirmed in various different species, including primates63. Importantly, as known for many longevity-inducing interventions, CR leads to a profound and conserved increase in organismal and cellular stress resistance64,65. More recent work could show that changes in nucleolar biology are a shared mechanism of several life span–extending interventions with CR inducing a significant reduction in nucleolar size in Caenorhabditis elegans, fruit flies, mice, and humans66. Importantly, nucleolar size early in life was also predictive of life span in the nematode model. Regarding the kidney, Mitchell et al. could show that a short-term reduction in food intake (to 70%) protected against murine renal IRI9. Improved insulin sensitivity and reduced insulin/IGF-1 signaling and increased expression of antioxidant defense enzymes were hypothesized to be among the key mechanisms for protection against renal and hepatic IRI as well as genotoxic or chemotherapeutic stress9,67–69. Recently, it was shown that the CR-mediated improvement of insulin sensitivity is mediated by mTORC2 signaling. However, disruption of mTORC2 signaling inducing insulin resistance in a Rictor knockout mouse model did not diminish the increase of fitness and life span70, indicating a potential mechanistic difference in longevity and stress resistance. Although the CR-mediated protective effect has been published in several mammals, effectiveness in humans has not yet been clearly demonstrated71,72. Yet there is first evidence of feasibility and potential efficacy in humans. The safety of a preoperative calorie- and protein-restricted diet in healthy kidney donors and obese patients undergoing bariatric surgery was shown by Jongbloed et al.73. Furthermore, feasibility was addressed in living kidney donors74. A large-scale trial investigating permissive underfeeding compared with standard enteral feeding in critically ill patients demonstrated a significantly lower rate of RRT in the group with a calorie-restricted enteral feeding protocol71,72. Recently, we studied the effects of a 7-day preoperative CR on renal function in a randomized controlled clinical trial75. Eighty-two patients at risk for post-surgery AKI were randomly assigned 1:1 to receive either a formula diet containing 60% of their daily energy requirement or ad libitum food for 7 days prior to elective cardiac surgery involving cardiopulmonary bypass. Although CR had no impact on the primary endpoint (the increase of serum creatinine at 24 hours after cardiac surgery), there was a significant between-group difference with a favorable effect of CR on creatinine kinetics at 48 hours and at discharge. Additional subgroup analyses suggested that the positive effect appeared to be most prominent in men and obese individuals with a body mass index of more than 25 kg/m275. In parallel, a second randomized controlled clinical trial for preventing AKI in patients undergoing percutaneous coronary intervention was performed to determine the feasibility and effectiveness of pre-interventional CR. As in the above-mentioned trial, patients were randomly assigned either to receive a formula diet containing 60% of their calculated daily energy expenditure or to ad libitum food intake. Again, beneficial effects were detected only in post-hoc subgroup analyses76. Although the findings of these clinical trials did not reflect the effects of CR seen in animal experiments, the studies could show that the intervention is safe and feasible even in a morbid patient population. The fact that the magnitude of the observed effects is smaller than in the rodent models may be due to several aspects. It is still unknown how long a diet must be applied in humans, how much the caloric content has to be restricted, and how the most potent dietary regimen should be designed75. Given these caveats of implementing CR in the clinical setting, it is extremely important to have a better understanding of both the molecular mechanisms underlying CR-mediated organoprotection and the ideal dietary interventions to obtain these effects. From the authors’ point of view, this is necessary in order to further develop targeted approaches (for example, by drugs or targeted dietary interventions) for improving feasibility and effect size in a clinical setting.
There is ample evidence that CR does not mediate stress resistance through mere reduction of calories, and several other dietary interventions that modulate specific dietary components have shown beneficial effects in organ injury. Here, protein restriction (PR) is an important example, and PR has been demonstrated to confer additive effects to CR77. Interestingly, restriction of any single essential amino acid appears to be sufficient to mediate systemic adaptive responses leading to metabolic benefits78–80. Regarding renal organ protection, an important aspect was highlighted by a study published in 2015 showing that PR (with identical calorie intake in both groups) prior to hepatic IRI caused strong protective effects similar to those of CR and that these positive effects could be reversed by the addition of sulfur-containing amino acids81,82. Mechanistically, restriction of sulfur-containing amino acids caused an increase of hydrogen sulfide (H2S) formation by activation of the transsulfuration pathway81 and addition of H2S induced cellular stress resistance83–85. Hence, both H2S donors (for example, MESNA86) and diets reduced in sulfur-containing amino acid intake87 may be future strategies to transfer the potential of CR to the patient setting. A clinical trial (ClinicalTrials.gov Identifier: NCT03715868) investigating a non-dairy (significantly reduced in sulfur amino acids) formula diet prior to cardiac surgery was recently initiated at our center.
Intermittent or periodic fasting enables the activation of cellular signal transduction similar to that of CR with preserved nourishment88. Fasting-mimicking diets (FMDs) are a tool to reach comparable effects, and their safety and feasibility have been proven in several phase I and phase II studies89–91. Mechanistically, FMD results in cellular and metabolic effects similar to those of CR, including improved glucose homeostasis and insulin sensitivity, as well as improved cellular stress adaptation89,92 (for example, by modulation of the mechanistic target of rapamycin [mTOR] pathway93). It will be interesting to see whether these approaches have a role in organoprotection as well.
mTOR is an evolutionary conserved protein kinase orchestrating growth and metabolism. By inducing autophagy and by the reduction of protein translation leading to decreased proteotoxic and oxidative stress, mTOR inhibition results in cellular stress resistance94,95. mTOR is regulated through nourishment, and dietary inhibition of the mTOR pathway can be achieved either by restriction of the branched chain amino acids (BCAAs) valine, leucine, and isoleucine or by changes in the ratio of macronutrients replacing proteins with carbohydrates96–98. Increased exposure to BCAA is associated with hyperphagia, obesity, insulin resistance, and mortality99–101. Additionally, a protein-to-carbohydrate ratio of 0.07 resulting in low-protein and high-carbohydrate diet leads to improved stress resistance, health, and life span82,98,102. Strikingly, low-sugar diets also revealed beneficial effects on health and life span in C. elegans103. On the other hand, glucose supplementation did not interfere with fasting-induced renal protection in the ischemia-reperfusion mouse model104. Because the optimal diet for humans in the context of organ protection remains unknown, there is much room for improvement. Here, clarification whether reduction of specific amino acids or the changes in ratio of the macronutrients are the key drivers to improve metabolism, fitness and health in humans will be required.
Ketogenic diets are high in fat and very low in carbohydrates and result in synthesis of ketone bodies and exceeding β-oxidation of fatty acids. Similar to CR, ketogenic diets extend the life span in rodents with preserved physiological functions but do not lead to malnourishment105. Ketone bodies, such as β hydroxybutyrate, suppress oxidative stress, resulting in nephroprotection106. Owing to their additional neuroprotective effects, ketogenic diets have been proven to be feasible and safe in medical use in human pharmacoresistant epilepsy107. Given that CR in rodents actually does induce ketogenesis because of the nature of the feeding cycles108, ketogenic diets may be another promising strategy to ameliorate AKI in a clinical setting.
Taken together, recent experiments in animal models have helped to increase our understanding of preconditioning in AKI (see Figure 1), although clear-cut clinical effectiveness in humans has not been proven yet. Consequently, dietary interventions that have been tested to date in humans will probably not solve the problem. Nonetheless, given the tremendous effects in animal models in combination with the unmet clinical need, such research is of high importance. Direct pharmacological or optimized tailored dietary targeting of the molecular players may be the more straightforward approach in humans but will not be possible without detailed knowledge of the molecular mechanisms. Given that different modes of preconditioning are potentially based on similar mechanisms, comparative analyses may have a significant added value. In a recent study on HP and CR in the mouse model of cisplatin-induced kidney injury using an integrative analysis of transcriptomics, proteomics, and N-degradomics, we found that mRNA expression only moderately predicted protein expression. But the more the mRNA and the proteome dissociated, the higher was the serum creatinine in the individual animal. N-degradomic studies revealed extracellular, specific proteolytic complement activation that can be alleviated by these two preconditioning methods12; this is in line with results of other groups showing that protease inhibition is a potential therapeutic approach109,110. Further studies comparing two different modes of preconditioning (HP-CR and HP-PHDi) in murine renal IRI models are being carried out, revealing shared mechanisms reflected by overlapping pathways and common regulation of target genes in association with the clinical outcome by integrative multi-omics approaches111,112. Numerous other basic scientific and clinical studies on these procedures are under way. This improved knowledge of the molecular mechanisms involved will be crucial to translate future protective strategies into the clinical setting (for example, using targeted pharmacological approaches).
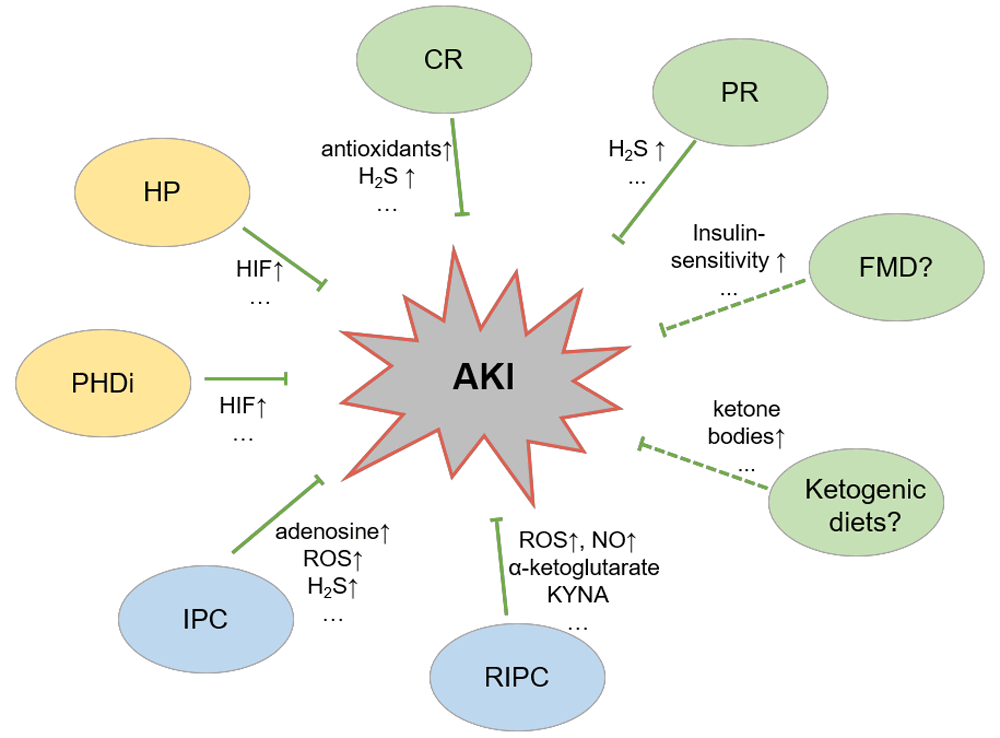
The different modes of preconditioning have been shown, or are supposed, to alleviate acute kidney injury by production of chemokines or metabolites. AKI, acute kidney injury; CR, calorie restriction; FMD, fasting-mimicking diet; H2S, hydrogen sulfide; HIF, hypoxia-inducible factor; HP, hypoxic preconditioning; IPC, ischemic preconditioning; KYNA, kynurenic acid; PHDi, prolyl-hydroxlase inhibition; PR, protein restriction; RIPC, remote ischemic preconditioning; ROS, reactive oxygen species. Bar-headed lines indicate inhibition, dashed lines and question marks indicate hypotheses, “…” indicates that not all mediators are displayed, and ↑ indicates increase.
alphaKG, α-ketoglutarate; AKI, acute kidney injury; BCAA, branched chain amino acid; CABG, coronary artery bypass graft; CIN, contrast-induced nephropathy; CR, calorie restriction; eGFR, estimated glomerular filtration rate; FMD, fasting-mimicking diet; H2S, hydrogen sulfide; HIF, hypoxia-inducible factor; HP, hypoxic preconditioning; ICU, intensive care unit; IPC, ischemic preconditioning, IRI, ischemia-reperfusion injury; KYNA, kynurenic acid; MPTP, mitochondrial permeability transition pore; mTOR, mechanistic target of rapamycin; NO, nitric oxide; PHD, prolyl-hydroxylase; PHDi, prolyl-hydroxylase inhibition; PI3K, phosphoinoside 3 kinase; PR, protein restriction; RIPC, remote ischemic preconditioning; RRT, renal replacement therapy; TNF-α, tumor necrosis factor-alpha
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 03 Apr 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)