Keywords
Trigonella foenum graecum, Cyperus rotundus, alloxan-stimulated diabetes rats, hyperglycemia effects
Trigonella foenum graecum, Cyperus rotundus, alloxan-stimulated diabetes rats, hyperglycemia effects
Diabetes mellitus (DM) is a group of syndromes characterized by high blood sugar; modulated metabolism of lipids, carbohydrate and proteins; and an elevated risk from vascular diseases. It is associated with a metabolic defect with relative or absolute insulin deficiency and/or insulin resistance. Most patients can be classified clinically as either insulin-dependent DM or insulin-independent DM1. Chronic complications include cardiovascular disorders, central nervous system damage, chronic kidney diseases, complication of the retina, and difficulties in wounds healing2. These complications have a pronounced effect on the quality of life of the patients by increasing morbidity and mortality2. Due to the multifactorial pathological nature of DM, it requires several therapeutic agents for optimal management. Most research on DM focuses on early diagnosis and preventive measure in highly at risk people.
Trigonella foenum graecum (fenugreek) has an extended range of applications (medical and non-medical), e.g. a flavoring agent of food3, use as a diuretic agent, ability to reduce blood pressure4 and blood cholesterol, cardiotonic and hypoglycemic properties, ability to enhance insulin production, antidiabetic effects, and antinociceptive and anti-inflammatory effects5. Fenugreek has been widely used as an ingredient of many blood glucose lowering substances, from different plant parts, including seeds and leaves and extracts in diverse solvents6. Its chemical component consists of different categories like saponins, sapogenins, alkaloids, and flavonoids. The hypoglycemic activity of fenugreek has been studied in different research; with the seeds of the plant as the main focus since its control over diabetes7. Cyperus rotundus (known as saed in Iraq) develops from a system of underground dark green tubers8. The tubers of the saed are utilized in many therapeutic indications, including as an antipyretic, anthelmintic, hypotensive, antihistaminic, and smooth muscle relaxant9,10.
The current work aimed to study the phytochemical properties, and the hypoglycemic effect of the ethanol extract of C. rotundus and fenugreek seeds in alloxan-stimulated diabetes rats. This is a preclinical study to assess these new compounds.
The study conducted at the College of Pharmacy, Al-Nahrain University, Iraq, during the period from November 2019 to January 2020.
All procedures performed in the study were approved by the Institutional Research Committee at the College of Pharmacy, Al-Nahrain University (Ref# 2019/0078) and in accordance with the animals Act 1986 (ASPA) for animals used in scientific research.
All their efforts were made to reduce animal suffering by provision of water and food regularly, changing the beds every other day, letting animals sleep during the night offering a calming and dark place, and checking for any inconvenience to the animals throughout the study.
A total of 40 Wistar male rats (175 – 230 g weight) were used in the study. The animals were obtained from animal house at the Iraqi National Drug Quality Control Center and Drug Research (Baghdad, Iraq). The animals were individually caged and allowed free access to water and standard rodent chow diet (18% Protein, 5% fat, Envigo Teklad Global Rat Food Pellets 2018), food was provided in a standard stainless-steel hopper. The animals were maintained at room temperature (23 – 25°C), 30–40% humidity, and lights on from 7 AM to 5 PM. The rats were maintained in polypropylene cages with husk as the bedding material. The animals were kept for 7 days after transfer from their original housekeeping to allow accommodation before performing the study.
A resource equation approach was used (since it is not possible to assume the standard deviation and the effect size for this study) and the expected statistical analysis was ANOVA, based on the following equation:
DF: degree of freedom (range between 10 – 2011), k: number of groups, n = number of subjects per group. Sample size between 4 – 6 was calculated, in order to increase the accuracy of the results a sample size of 10 was chosen for each of the four groups12.Animals were injected intraperitoneally with a single dose of alloxan monohydrate (Sigma Aldrich, USA) (120 mg/kg) in saline (0.9% NaCl) to induce diabetes13. The rats were fasted overnight for 12-13 hr but allowed access to water before blood glucose level was measured. After 72 h, glucose levels were recorded using a glucometer. Rats with blood glucose levels of 200 mg/dL and above were considered as diabetic and were selected for the study14.
The alloxan-induced diabetic animals were subdivided into four groups using a randomized block design (see below): group A, group A, alloxan-stimulated diabetic rats with administered with distilled water (10mL/kg; control); group B–D, alloxan-stimulated diabetic rats administered with ethanol extract of C. rotundus (10 mg/kg; group B), glibenclamide (10 mg/kg; group C), and ethanol extract of fenugreek seeds (15mg/kg; group D) (Table 1).
Randomized block design was selected as the experimental module. The rats were divided into four blocks: block one (group A) given distilled water at first week, block two (group B) started treatment the next week, block three (group C) started treatment the third week, and block four (group D) started treatment the 4th week. All the animals were kept in the same conditions. The previous order established when each block received their treatment, then in each block serum blood glucose was measure at baseline, 1 week, 2 weeks, 4 weeks, and 6 weeks after the initiation of treatment (no rat were sacrificed in the study). The allocation of rats was random in each block with the matching of between block in rats’ weight, age, diets, and other fixed parameters. The treatment was considered a fixed effect.
Each of the groups contained 10 rats (total 40 rats).
All treatments were administered to the rats with 1.0 cc syringe using an oropharyngeal cannula. Doses were decided upon after review of the literature, taking into consideration of trying to provide the lowest dose possible.
Assessment of the change in blood glucose from baseline and after 1 week, 2 weeks, 4 weeks, and 6 weeks after the administration of the treatment.
The glucose level in the blood was measured with a glucometer (ACCU-CHEK Active, Mannheim, Germany), and values > 200 mg/dL were considered diabetic15. The animals were kept on fasting status for 12 – 13 hrs on days of sampling, and blood samples were obtained from tail vein by tail nipping procedure (no anesthesia was required). Blood glucose was measured early in the morning after overnight fasting14.
Experimental diabetes in rats was induced by injecting alloxan monohydrate (Sigma Aldrich, USA) at a single dose of 150 mg/kg, i.p. in ice-cold normal saline. After 72 h, glucose levels were recorded using a glucometer. Rats with blood glucose levels of 200 mg/dL and above were considered as diabetic and were selected for the study.
The Iraqi plants were collected from Baghdad, in November 2019 and the plants were specified and authenticated by the Biology Department, College of the Sciences, Baghdad University, Iraq. The rhizomes of C. rotundus, and seeds of T. foenum graecum were obtained.
The preparation of extract was performed using the method described by Ali et al16. In total, 500 g of coarse powdered C. rotundus (rhizomes were coarsely grounded and sieved) was loaded into a Soxhlet apparatus for the extraction process. The powder was first defatted with petroleum ether solvent (60–80°C) before extraction with ethanol. The ethanolic extract of 500 g T. foenum graecum seeds was obtained from the reflex apparatus that was prepared two days previously (defatted extract). The ethanol extracts were evaporated using a rotatory evaporator and dried in a water bath.
Chemical tests to assess active constituents in the plants were done using plant crude extracts and standard procedures17 (Table 2).
Reproduced with permission from 18.
| Constituent | Test |
|---|---|
| Alkaloids | Mayer’s Test: 10 ml of crude extract was mixed with 5 ml of 1% HCL on a steam bath then added the reagent; the result is white precipitate indicating +ve test for alkaloids. Wagner’s test: 10 ml of ethanol extract was mixed with 5 ml of 1% HCL on a steam bath then add the reagent; the result is reddish-brown color precipitate indicating +ve test for alkaloids. |
| Flavonoids | Lead acetate: 5ml of ethanol extract mix with 1 ml of 10% Lead acetate to yield yellowish-white precipitate indicating the presence of flavonoids. |
| Steroids | Liebermann-Burchard: 3 ml of extract stirred with chloroform, acetic anhydride and few drops of sulphuric acid to yield dark pink or red color indicate the presence of steroids. H2SO4: 2 ml of extract were treated with sulphuric and acetic acids.to develop a greenish color to indicate the presence of steroids |
| Anthraquinones | Borntrager’s test: 3ml of the extract was shaken with 3 ml of benzene. Then, filtration was performed and 5 ml of 10% ammonia solution was added to the filtrate. The mixture was shaken well, the development of a pink, red or violet color in the ammonia (lower) phase indicates the presence of anthraquinones. |
| Tannins | FeCl3 solution (5%w/v): 3 ml of distilled leaves extract (in water) mixed with 3ml of FeCl3 solution (5%w/v) to produce dark green or blue-black precipitate indicate the presence of tannins. |
| Terpenoids | Keller-Kiliani: Ethanol extract (2ml) was dissolved in chloroform (2ml) and evaporated to dryness. Concentrated sulphuric acid (2ml) was then added and heated for about 2 min. A grayish color was considered an indication of the presence of terpenoids. |
| Table 2 has been reproduced with permission from [Khamees AH, 2017. In vitro Analysis of Antioxidant and Antimicrobial Activity of Iraqi Bryonia dioica]18 | |
The phytochemical analysis revealed the presence of many secondary metabolites, including alkaloids, flavonoids, anthraquinones, tannins, and steroids (Table 3).
At baseline, there was no significant difference in blood glucose levels between rats as they had typical blood glucose levels (range, 73.4 ± 5.2 to 78.8 ± 5 mg/dl) at the beginning of the experiment.
At 1 week, group D (fenugreek seeds) showed a similar reduction in glucose levels to animals in group B (C. rotundus) (p-value = 0.99). This reduction was significantly more than group A (negative control) (p-value < 0.001).
At 2 weeks, there were no significant differences in values between groups D and B, and both groups showed no significant differences compared to group C (glibenclamide).
At 4 weeks, the results showed a significant decline in all groups' blood glucose readings from baseline. There was no significant difference between groups C and D.
At 6 weeks, the final readings showed that all groups had a significant decline in blood glucose level compared to the group A. Group C had the lowest value, followed by group D and group B. Figure 1 shows the assessment of glucose levels during the whole study period.
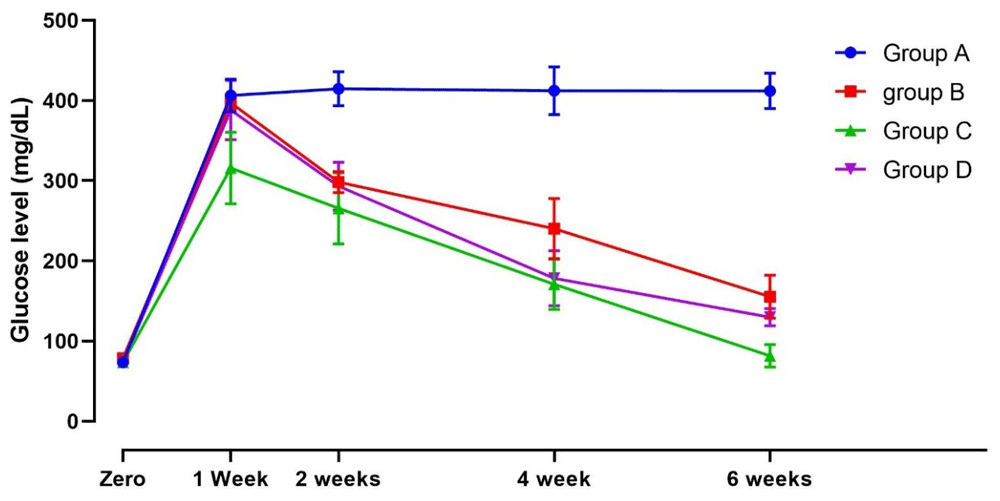
Group A, distilled water (10mL/kg); B, Cyperus rotundus ethanol extract (10mg/kg); Group C, glibenclamide (10mg/kg); Group D, fenugreek seeds ethanol extract (15mg/kg).
No adverse effects were reported during the study period.
The existence of these groups of compounds provides scientific evidence for the previously presumed medical applications of C. rotundus in the treatment of specific disorders. The existence of flavonoids, alkaloids, tannins and terpenoids appears to be considerable. T. foenum graecum also showed positive results for alkaloids, anthraquinones, tannins, and steroids, while the extract exhibited negative results towards flavonoids.
The results indicate that both ethanol extracts of fenugreek seeds and C. rotundus reduced the blood glucose level in diabetic rats. This hypoglycemic effect shows that these extracts have medicinal activity. The bioactive constituents of both extracts may play a role in increasing insulin secretion from β-cells19. Other supposed mechanisms may include this insulin-like effect or other modes of action, such as initiation of peripheral tissue glucose uptake, inhibition of endogenous glucose output, or enhancement of gluconeogenesis process in corresponding tissues, for example the liver and muscle tissue. A similar mode of action has been shown for other plant extracts with hypoglycemic activity20. In addition, it is established that flavonoids control aldose reductase enzyme21. Many researchers report that there are several flavanols that can control diabetes, by bringing about restoration of pancreatic islet cells and increasing insulin production in streptozotocin-induced diabetes. In addition, it may also enhance Ca2+ uptake from isolated islet cells thus giving an indication that is very effective in type II DM22. Further analyses are needed to detect the complete mode of hypoglycemic action of these plants.
The current study observed that fenugreek seeds and C. rotundus ethanol extracts possess considerable hypoglycemic activity in alloxan-stimulated diabetic rats with considerable higher activity for fenugreek seeds in contrast to C. rotundus tuberous roots extract. The hypoglycemic activity of both plant extracts was compared with standard oral hypoglycemic treatment glibenclamide. These plants are considered as a perfect candidate for further investigations for justifying the modes of hypoglycemic effect, as well as for isolation and determination of phytochemicals that possess or are responsible for the hypoglycemic and anti-diabetic activity of the plant. Further extensive pharmaceutical surveys, including long term studies, could be of value to estimate the possible toxic activity of these plants.
Zenodo: Hypoglycemic Activity of Two Herbal Extracts, https://doi.org/10.5281/zenodo.370136223
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Lin Y, Sun Z: Current views on type 2 diabetes.J Endocrinol. 2010; 204 (1): 1-11 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Endocrinology. Reproductive endocrinology, Effects of Phytochemicals on Diabetes.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmaceutical product development and Isolation of active principles from plant materials.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 08 Apr 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)