Keywords
Dosage compensation; chromatin; X inactivation; Xist; non-coding RNA; SPEN; Polycomb; gene repression
Dosage compensation; chromatin; X inactivation; Xist; non-coding RNA; SPEN; Polycomb; gene repression
The repression of one entire X chromosome in female cells achieves dosage compensation for X-linked genes between both sexes and has become a paradigm for studying epigenetic silencing. The mechanism by which hundreds of genes are repressed in a coordinated manner has captured the interest of researchers for over half a century but is by no means the only aspect of the mammalian dosage compensation system that remains a puzzling wonder of biology1. The choice of the X chromosome to switch off, either maternal or paternal, is widely accepted to be random in placental mammals, and recent evidence in mice suggests a stochastic process for which a few components have been identified2,3. A mechanism for generating randomness is difficult to establish on a molecular basis but ultimately may inspire technology as well as cell biology. Recent progress in chromatin biology has, however, led to considerable insight into the pathways that accompany the silent fate of the inactive X chromosome (Xi). It is important to state from the outset that once the Xi has been chosen and repressed, its silent state is faithfully maintained and inherited in all progeny of every embryonic cell. This observation requires an epigenetic memory that is established at the Xi but has not been fully deciphered yet1,4,5. The process of X chromosome inactivation (XCI) has been dissected into two characteristic phases: 1) a reversible X inactivation-specific transcript (Xist)-dependent initiation step, when X linked genes become repressed, and 2) a maintenance step when gene silencing becomes irreversible and independent of Xist (reviewed in 6). Notwithstanding, a faulty initiation of the silencing process has consequences for the completion of the latter events, which demonstrates the exquisite functional coordination that is set in motion by Xist.
The 17 kb long noncoding RNA Xist is expressed from and accumulates over the X chromosome, triggering a cascade of events that eventually will result in the formation of the heterochromatic Barr body7–11. Within the Xist sequence, a number of repeat modules, named A to F, have been identified. These modules consist of unique multimeric short sequence stretches that play a functional role in the inactivation process (Figure 1)7,9,12–14. Repeats A to D and F are encoded in Xist exon 1, whereas repeat E resides in exon 7 (Figure 1A). In the last few years, a number of Xist partners have been identified, and their specific interaction with one of the repeat modules in Xist has been characterized6. This has led to the establishment of a preliminary but highly interesting repeat-to-function correlation through which Xist is beginning to be better understood15. Here, we review the latest advances in understanding mouse Xist, highlighting important insights and raising questions that remain unanswered.
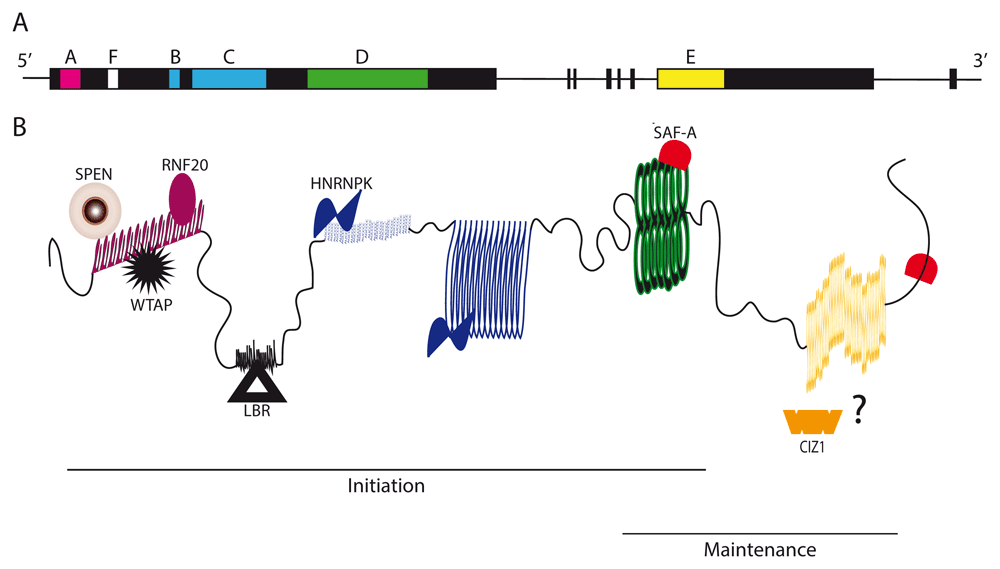
A) Representation of the mouse Xist gene. Exon1 codes for repeats A–D and F. Exon 7 codes for repeat E. B) Mouse Xist RNA with its functional A–F repeats and their corresponding direct interactors. Lines indicate the repeats predicted to contribute to the initiation and maintenance of XCI. The binding of CIZ1 to Xist has not been fully established and is indicated by a question mark. CIZ1, CDKN1A-interacting protein; HNRNPK, heterogeneous nuclear ribonucleoprotein K; LBR, lamin B receptor; SAF-A, scaffold attachment factor A; SPEN, Split Ends; RNF20, ring finger protein 20; WTAP, WT1-associated protein.
Close to the 5' end of Xist RNA, multiple repetitions of a short, conserved sequence are encountered that have been named repeat A9. Each of the 24-nucleotide-long motifs contains small sequence inversions that possibly could fold into a double stem loop structure. The full mouse repeat A module would then comprise eight first-stem and seven second-stem loops13. However, double-stem loop structures might not form within cells, and more complex conformations involving interactions between different 24-nucleotide-long core motifs have been proposed. Jones and Sattler provide an excellent review of the structure of Xist repeat A16. Initial evidence for a role in gene silencing stems from a deletion analysis of mouse Xist using a transgenic system in embryonic stem cells (ESCs). These studies revealed the requirement of repeat A for the repression of X-linked genes but not for localization or spreading of Xist over the X chromosome13.
Recently, the identities of some of the Xist partners responsible for repressing transcriptional activity were unveiled. Forward genetic screening and biochemical purification of Xist partners, in differentiated and undifferentiated cells, identified SPEN as a top candidate responsible for gene repression17–20. SPEN is a 400 kDa protein that belongs to the Split Ends family of transcriptional repressors. Its mutation in mice causes an embryonic lethal phenotype at E12.521. SPEN contains four N-terminal RNA recognition motifs (RRMs) and a C-terminal conserved Spen paralog and ortholog domain (SPOC) that mediates the recruitment of co-repressors and the activation of histone deacetylases (HDACs). Different approaches have confirmed the direct interaction between SPEN and repeat A of Xist17,19,20,22,23. Biochemical purification has shown that SPEN interacts with the full-length but not the repeat A mutant form of Xist19. Deletion of exons encoding the RRM domains of SPEN abrogates the silencing capacity of Xist20. In addition to a loss of gene silencing, Xist appears to lose the ability to recruit chromatin-modifying complexes of the Polycomb group17,20. A pathway for gene repression has been proposed based on the known interaction of SPEN with the SMRT co-repressor that activates HDAC317. HDAC3-mutant ESCs show partial gene silencing upon Xist expression, suggesting that not all gene repression is mediated by HDAC324. Taken together, these findings make SPEN a well-established silencing factor of Xist, though details of the mechanism in XCI remain to be uncovered.
Additional silencing factors for the initiation of XCI might be inferred from biochemical experiments that also indicate the interaction of RNF20 and WTAP with Xist in a repeat A-dependent manner19. WTAP is a component of the RNA N-6 adenosine methylation (m6A) complex that also contains RBM15, its paralogue RBM15B, and the methyltransferase METTL325. RBM15 has been identified independently as an Xist-binding partner by genetic screening and biochemical purification17–19. Methylation individual nucleotide resolution crosslinking and immunoprecipitation (methylation-iCLIP) experiments in HEK293T cells revealed 78 m6A residues in the human XIST RNA, some of which are localized in the vicinity of, and two of which are located within, repeat A26. These sites also show prominent binding of the RNA m6A reader YTHDC1. In ESCs, synthetic tethering of YTHDC1 to Xist was sufficient to complement the depletion of other WTAP complex members, which impairs gene repression by Xist. These findings indicate that m6A acts as a recruitment signal for silencing factors26. However, not all m6A sites are localized at repeat A. In addition, a recent study has investigated the deletion of most m6A sites at the 5' end of Xist and observed only subtle or no effects on X chromosome-wide gene silencing in mouse ESCs27. This suggests that m6A sites in the remaining Xist sequence may act redundantly for the recruitment of additional silencing factors. In mice, mutations in WTAP, RBM15, and YTHDC1 result in embryonic lethality28–30. Although these observations are consistent with a potential role in XCI, female-specific phenotypic dimorphisms have not yet been described. Further evidence would be desirable to establish the precise role of the WTAP complex at the initiation of X inactivation.
RNF20 is a third factor that has been identified to associate with Xist RNA in a repeat A-dependent manner19. The RNF20/RNF40 family of E3 ubiquitin ligases can mediate mono-ubiquitylation of histone H2B lysine 120 (H2BK120ub1), which is required for mouse preimplantation development and ESC differentiation31–33. H2BK120ub1 has been correlated with both transcriptional activation34 and repression35 and is proposed to play a role in stabilizing nucleosomes and chromatin compaction36,37. Depletion of either RNF20 or RNF40 did not impair Xist-mediated silencing in mouse ESCs19. Although a combined disruption of both RNF proteins has not yet been reported, it is tempting to speculate that RNF20 could contribute to chromatin modification and compaction in XCI.
Most studies in mouse ESCs have confirmed a key role of repeat A in gene repression. However, studies of imprinted XCI in extraembryonic cells have uncovered the finding that repression of several genes on the Xi is apparently independent of repeat A. Forced expression of a repeat A mutant Xist from a heterologous promoter in trophoblast cells caused repression of genes that are not inactivated by the Xist repeat A mutant in embryonic cells38. These repressed genes appear to cluster in regions of low gene density and are normally expressed at a low level on the active X chromosome (Xa). One potential explanation for the different requirement of repeat A in ESCs and trophoblast cells might come from considering the timing of Xist expression relative to gene activity: in the mouse embryo, Xist is first upregulated at the four-cell stage, concurring with the zygotic genome activation39. At this stage, not all X-linked genes might be fully activated. It is conceivable that Xist without repeat A is able to repress some X-linked genes by chromatin modifications that normally contribute to the maintenance of gene repression in XCI. Therefore, the repeat A mutant Xist would not necessarily use an active silencing pathway but rather could rely on the maintenance pathways. Consistent with this idea is the finding that the expression of Xist containing repeat A has far greater potential for X-linked gene repression in trophoblast cells.
Mouse Xist repeat B consists of 32 copies of the cytidine-rich (A/U)GCCCC motif and functions together with repeat C, which contains 14 copies of a 120-nucleotide unit of high similarity40. Recent studies have discovered key roles for Xist repeats B and C for the recruitment of Polycomb complexes.
Xist repeats B and C are required for the recruitment of canonical and non-canonical Polycomb repressive complex 1 (PRC1) and PRC2 to the Xi at the initiation of XCI. Work by the Brockdorff lab has unraveled a hierarchy for Polycomb complex recruitment by Xist. Initially, Xist repeats B and C recruit a non-canonical PRC1 that catalyzes the ubiquitination of histone H2A lysine 119. H2AK119ub is bound by RYBP, which is a component of a non-canonical PRC1 complex, and leads to an amplification and presumably spreading of the H2AK119ub mark. PRC2 activity is subsequently recruited and mediates H3K27me3. H3K27me3 in turn is recognized by CBX7, which is a component of canonical PRC1 complexes. Therefore, in XCI, PRC1 is recruited before PRC2 and multiple interactions amplify the spreading of both Polycomb complexes41.
Initial evidence for a role of Xist repeat B in Polycomb recruitment was provided by the discovery of a function for JARID2 in XCI42. Da Rocha and colleagues tested the recruitment of JARID2 in differentiating ESCs containing inducible Xist mutant transgenes with different deletions. Xist lacking repeats F, B, and C did not recruit JARID2, leading to a decrease in the recruitment of PRC2. Soon after that, JARID2 was identified as an essential component for PRC2 recruitment to PRC1-marked chromatin43. Using autosomal Xist-inducible transgenes carrying a deletion of repeat B plus a partial deletion of repeat C, defined as the Xist RNA-Polycomb Interacting Domain (XR-PID), researchers reported a strong reduction in the accumulation of Polycomb complexes by Xist44. Reassessment of these experiments in an endogenous inducible Xist system in XY ESCs showed that both repeat B and repeat C can efficiently recruit Polycomb proteins to the Xi and that a double deletion of repeats B and C leads to a complete abrogation of the accumulation of H2AK119ub and H3K27me3 at the Xi45.
To understand this mechanism in detail, it was crucial to identify the non-canonical Polycomb group ring finger (PCGF) proteins PCGF3 and PCGF5, as they are required for the recruitment of Polycomb complexes by Xist46. Fluorescence recovery after photobleaching (FRAP) experiments showed PCGF3/PCGF5 to form stable interactions with Xist RNA domains, and a double Pcgf3/5 deletion resulted in the loss of Xist-dependent H2AK119ub1 and H3K27me3 deposition46. Notably, Pcgf3/5 double mutant mice present a female-specific lethality at E9.5, consistent with their requirement for dosage compensation46. Xist repeats B and C have been linked to PCGF3/5–PRC1 through hnRNPK, which has been independently identified as an Xist interactor19,47 and shown to be required for Polycomb protein recruitment19. hnRNPK is essential for early embryo development48. It presents several KH RNA-binding domains that are predicted to mediate interaction with Xist repeats B and C and a K protein interactive region (KI) required to bind PCGF3/5–PRC149. It has been shown that deletion of repeats B and C abolishes the interaction of Xist RNA with hnRNPK and secondarily with PCGF3/5–PRC145. These experiments establish hnRNPK as the RNA-binding protein that links Xist to non-canonical PRC1, leading to a change of previous views from a direct binding of Polycomb proteins to RNAs50,51. PCGF3 and PCGF5 associate with hnRNPK and a catalytic RING protein for marking entry sites with H2AK119ub44. The requirement of hnRNPK for early embryo development makes studies of its function difficult. However, synthetic tethering of hnRNPK, but not KI mutant hnRNPK, to transgenic Xist that lacks the XR-PID region restores the recruitment of H2AK119ub44. In the future, it might be interesting to more specifically disrupt interactions between hnRNPK and Xist or PCGF3/5–PRC1 to understand if repeats B and C could have additional functions that have not yet been discovered. Taken together, these findings provide strong experimental support for a pathway of Polycomb recruitment by Xist. A modular architecture of Xist is also suggested, with repeats B and C forming a recruitment site for hnRNPK and Polycomb complexes. More research will be needed to understand to what extent Polycomb complexes contribute to gene repression and in particular if they function in maintaining gene silencing on the Xi.
Studies in ESCs indicate that Xist might initially localize in spatial proximity to its genomic locus in regions pre-marked with PcG decoration and low transcriptional activity52. From these initial “entry sites”, Xist triggers the formation of a repressive compartment that is devoid of marks of active transcription including RNA polymerase II53. These initial events are followed by the recruitment of Polycomb proteins to the Xi and a conformational change that subsequently permits spreading and silencing of gene clusters with high transcriptional activity52,53. Notably, the Xist mutant lacking repeat A is able to recruit Polycomb complexes to the Xi, suggesting that gene repression is not required for Xist localization and Polycomb recruitment4. Recent evidence supports and extends this view, showing that repeat A mutant Xist localizes and mediates decoration over most of the Xi except for regions where active genes reside24. Similar observations were made with HDAC3-mutant ESCs showing that spreading of Polycomb complexes is not observed over active gene loci. These results directly compare transcriptional activity with PcG recruitment and suggest that they are mutually exclusive. Zylicz et al. observed that deletion of repeat A causes a reduction in the deposition of H2AK119ub over the Xi. However, this effect was not recapitulated in HDAC3-mutant ESCs where PRC1 recruitment was unperturbed24. These findings might provide a stringent test for the proposed mechanism for Polycomb recruitment and potentially also suggest a contribution from Xist repeat A in Polycomb recruitment.
One of the downstream effects of the recruitment of Polycomb complexes by Xist is the recruitment of SMCHD1 to the Xi. SMCHD1 was initially identified in a genetic screen in mice as a key player in XCI. SMCHD1-null embryos present a female-specific lethality between E10.5 and E13.5, with derepression of genes and hypomethylation of CpG islands on the Xi54–56. The interaction between Xist and SMCHD1 was also described through proteomics screening in MEFs47, although this is likely not direct, as it depends on hnRNPK57. Recruitment of SMCHD1 is dependent on Xist expression57,58. Xist repeats B and C are required for recruiting SMCHD1 to the Xi in ESCs at the transition between the initiation and the stable maintenance phase of XCI57. Studies in female mouse embryonic fibroblasts (MEFs) suggest that SMCHD1 recruitment by Xist is dependent on PRC1 and H2AK119ub57. Notably, the mutation of Smchd1 results in a stronger recruitment of H3K27me3 to the Xi59. At present, it is not clear how SMCHD1 can be recruited by PRC1 but insulate from the effects of PRC2. Furthermore, additional roles for Smchd1 have been proposed in remodeling the 3D architecture of the Xi58–61. SMCHD1 has been postulated to regulate the transient reorganization of the Xa chromatin towards a bipartite Xi structure58. Studies in Smchd1-deficient MEFs have shown that the overall structure of the Xi is largely unaffected in the absence of SMCHD1. However, genes that are reactivated become reorganized in a manner that resembles more the Xa than the Xi. Importantly, the Polycomb dependence of SMCHD1 recruitment to the Xi would suggest a requirement of Polycomb complexes for the establishment of a memory for the maintenance of XCI. Taken together, these data demonstrate that Xist repeats B and C can engage different effectors and they do this through hnRNPK and a non-canonical PRC1.
One important factor for the precise localization of Xist RNA to the Xi is scaffold attachment factor A (SAF-A/Hnrnpu)62. SAF-A binding has been described at the repeat D of Xist63. Repeat D contains multiple copies of a 290-nucleotide-long motif that can mediate interaction with SAF-A. However, repeat D might not be the only element that contributes to Xist localization. Earlier studies indicated that several regions of Xist act in parallel or redundantly to Xist RNA localization13. However, it remains to be shown if repeat D is sufficient for Xist localization or if other interactions are also required22.
Xist repeat E consists of 50 highly variable copies of 20–25-nucleotide-long units that have been implicated in Xist localization in differentiated cells and maintenance of XCI40. Insight into repeat E function comes from the identification of CIZ1 as a protein that is recruited to the Xi in somatic cells. CIZ1 is a nuclear protein that progressively associates with the Xi in a Xist-dependent manner during female ESC differentiation. Although Ciz1 mutant mice do not show developmental defects, a highly penetrant lymphoproliferative disorder has been reported in adult females64. This hematopoietic phenotype is consistent with an earlier report of leukemia development in female mice, when Xist is conditionally mutated in the blood system65. The blood cell diseases have further been linked with dosage compensation defects64,65, suggesting a special context for XCI in blood cells66,67. Systematic deletion experiments over different Xist regions in ESCs and MEFs unveil that CIZ1 recruitment depends on repeat E64,68. Deletion of Xist repeat E in ESCs does not impair Xist localization or the recruitment of Polycomb complexes64,69. However, in Ciz1-mutant female MEFs, Xist is delocalized and Polycomb complexes are not recruited to the Xi. These defects in somatic cells are accompanied by a partial de-repression of X-linked genes64,68. Importantly, delocalization of Xist has also been observed in hematopoietic cells from Ciz1-mutant mice.
A recent study has uncovered an independent function for repeat E in the recruitment of the ASH2L Trithorax group protein69, but the function of ASH2L for gene repression on the Xi remains largely unclear. Recruitment of ASH2L depends on Xist localization but is independent of repeat A5. Taken together, current evidence suggests that repeat E acts as a binding site for factors that contribute to XCI maintenance. The cell-type-specific effects of the Ciz1 mutation are intriguing. It would be interesting to explore if a mutation of repeat E would resemble the Ciz1 mutation in mice. Alternatively, other repeat E binding factors could be involved in either maintenance or transition to maintenance of XCI.
Xist repeat F was not included in the initial description of Xist 9 but has been more recently defined as a short fragment that is located just 3' of repeat A. Repeat F is composed of two copies of a 10-nucleotide-long G/C-rich sequence motif (UGGCGGGCUU)40 and appears to function together with a region near repeat E as a binding site for lamin B receptor (LBR)70. LBR is a 60 kDa integral membrane protein embedded in the inner nuclear membrane that associates with lamin B and tethers heterochromatin to the nuclear periphery71. Mutation of Lbr has been described to result in an embryonic lethal phenotype with incomplete penetrance, but a female-specific phenotype has yet to be described72,73. LBR was identified as a direct binder of Xist through a biochemical screening approach17. RNA interference (RNAi)-mediated depletion of Lbr in ESCs was reported to cause an impairment of gene repression by Xist. These observations were further corroborated by showing that deletion of the LBR-binding sites of Xist resulted in a loss of gene repression70. Tethering of LBR to Xist lacking the LBR interaction site could restore silencing activity. In addition, tethering a mutant form of LBR that lacked the RNA-binding domain to Xist complemented the silencing defect. Notably, LBR does not possess a canonical RNA-binding domain and associates with Xist through a novel surface. LBR might contribute to gene repression by mediating an association with the nuclear lamina. It is conceivable that also an association with a repressive heterochromatic environment at the nuclear lamina or reshaping of the 3D architecture of the Xi might play a role in XCI. However, a recent study has investigated a deletion of the Lbr gene in ESCs and observed only subtle silencing defects27. In addition, after removal of the entire Xist repeat F, only minor defects in gene repression were observed. These observations are conflicting with earlier observations and suggest that additional evidence will be necessary to understand the requirement of Lbr for gene silencing. Nevertheless, repeat F might provide a binding site for Xist partners that engage additional mechanisms that are not yet fully understood.
The understanding of chromosome-wide silencing on the molecular level has seen impressive advances over the past few years. Progress has been driven by the development of new methodology in RNA protein biochemistry and mammalian genetics. A large number of factors have been identified that cannot all be discussed in this review for space reasons. We focus here on factors that have been implicated and shown to be relevant for understanding Xist function. The current understanding suggests that Xist RNA might act as a link between different functional effector complexes. Engaging a number of molecular activities would then bring about the astonishing properties of Xist, namely its ability to specifically localize over the Xi and its ability to trigger chromosome-wide chromatin modifications and gene repression. This poses the following question: is the current knowledge of known Xist effectors sufficient for reconstitution of Xist function? The identification of the binding sites within Xist could make experimental testing possible as of today (Figure 1B). Certainly, one would include some form of repeat A for engaging the gene silencing pathway. An important question here is whether tethering of SPEN would fully substitute for all functions of repeat A or whether WTAP and METTL3 would be required too.
For repeats B and C, the situation appears less clear. Would Polycomb engagement be required for initiating gene repression? There is evidence that XCI could initiate without Polycomb recruitment, and a repeat B and C mutant Xist can initiate gene repression45. However, this view might change during the maintenance phase of XCI. Without repeats B and C, SMCHD1, and likely other factors, would not be recruited and establishing an epigenetic memory could potentially fail. This idea would suggest a role for Polycomb complexes in establishing a repressive memory in XCI, which is consistent with the traditional view of Polycomb function. Notably, interactions of the PRC2 subunits Ezh250 and Suz1251 with Xist repeat A have previously been observed in vitro. However, the relevance of these observations for XCI remains unclear. Although Polycomb proteins might bind RNA directly, the requirement of hnRNPK in XCI suggests that these interactions are not sufficient in cells. Early studies have further proposed a role for repeats B and C in Xist localization. Jeon et al. implicated YY1 as a DNA- and RNA-binding protein that bridges Xist repeats B and C RNA to repeat F DNA, facilitating the localization of Xist74. Consistent with this observation, peptide linked nucleic acids and locked nucleic acids complementary to repeat C sequences also induced delocalization of Xist75,76. These observations are puzzling when one considers that the deletion of repeats B and C does not abrogate Xist localization or spreading at the initiation of XCI13,45. To reconcile these conflicting interpretations, one might consider different cellular contexts or experimental conditions similarly to the cell-type-specific requirement of Ciz1 for Xist localization64.
Repeat D could be handy for engineering RNAs that mimic the localization of Xist77. However, it remains to be shown if repeat D is sufficient for Xist localization or other interactions are also required such as with LBR. A prominent role of Xist repeat E in the maintenance but not in the initiation step of XCI has been uncovered through the identification of Ciz1. We speculate that both repeat D and repeat E could be important for the maintenance of XCI, where they might recruit factors independent of gene silencing but dependent on a chromosomal memory that is imposed by Polycomb complexes. Thus, repeats D and E of Xist could act as docking sequences for factors that preserve the integrity of heterochromatin of the Barr body and facilitate Xist localization in a cell-type-specific manner. Taken together, the current understanding of molecular interactions of Xist appears to provide a detailed guide for reconstitution of Xist function. Performing these experiments will be important for establishing if the main mechanistic components have indeed been identified or key factors are still missing. In the latter case, a number of unstudied proteins from the list of Xist interactors represent a resource for further exploration.
Progress in understanding the molecular functions of Xist has moved the field towards a new frontier: the chromatin. Conceptually, Xist can be seen as a sender of a message. This view would predict a receiver on the chromosome. How X-linked gene promoters are inactivated on the chromatin level remains an open question. Just a few hundred molecules of Xist accumulate over the Xi and are capable of switching active genes and regulatory regions from an open into a closed chromatin configuration along an entire chromosome. This switch implies that the chromatin has the ability to read instructions from Xist and coordinate gene repression. Although some of these instructions come in the form of histone modifications, it remains unclear how silencing is spread. It is enticing to speculate if a domino-like effect might be triggered by Xist that perpetuates changes along the chromatin fiber. Would this make histones domino pieces? Histone exchange can facilitate switching an active into a repressive chromatin configuration through the removal of actively marked histones. Besides, gene silencing is accompanied by compaction of the chromatin fiber and prevents regulatory proteins from binding and triggering transcriptional events. We speculate that nucleosome assembly and chromatin dynamics might be important aspects for silencing and spreading of repressive chromatin modifications (Figure 2). The factors that allow chromatin to respond to the messages of Xist remain to be discovered. Identification of these components can be predicted to have wide implications for the understanding of gene regulation in mammals.
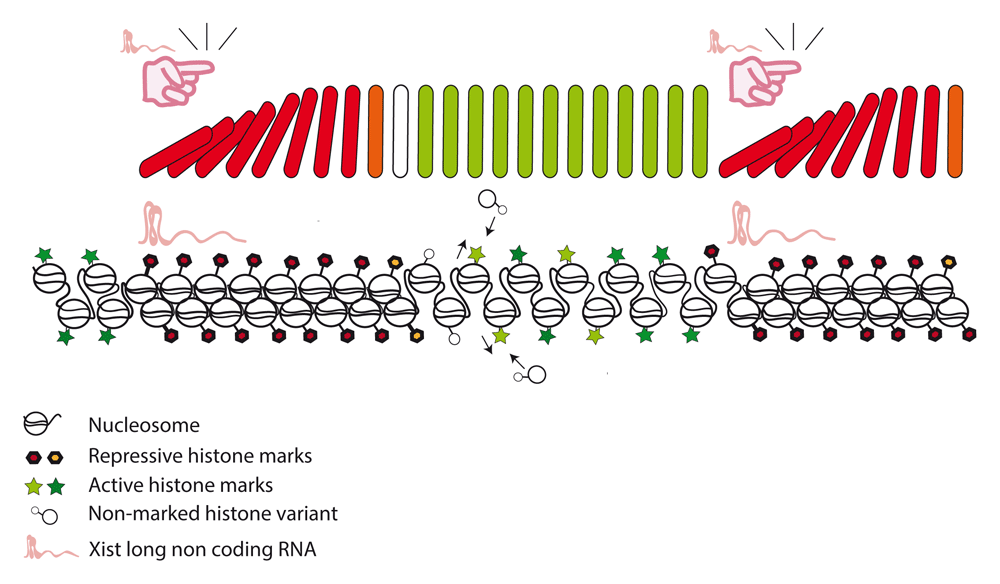
Xist RNA induces local chromatin modifications that spread along the chromosome through an unidentified inter-nucleosomal communication mechanism.
The molecular pathways used by Xist for the generation of a silent and stable Barr body are starting to be elucidated, albeit much remains to be understood. Disrupting X-linked gene silencing leads to female embryonic lethality, showing the importance of this phenomenon for the survival of mammalian species. Functional characterization of the different effectors engaged by Xist can advance our understanding of the striking mechanisms that were selected by nature to warrant gene silencing for survival. This knowledge can be exploited therapeutically for the treatment of genetic disorders where a gene overdose impairs the correct function of cells, such as Down syndrome cell pathogenesis77, X-linked diseases78, or approaches involving cell reprogramming79. Thus, further unraveling the mechanisms used by Xist and its effectors for silencing and heterochromatinization will contribute to advances in science and medicine.
ESC, embryonic stem cell; HDAC, histone deacetylation complex; MEF, mouse embryonic fibroblasts; PCG, Polycomb complex group; PRC, Polycomb repressive complex; RRM, RNA recognition motif; Xa, active X chromosome; XCI, X chromosome inactivation; Xi, inactive X chromosome; Xist, X inactivation-specific transcript.
We thank members of the lab for discussion and reading the manuscript, and the reviewers for their comments.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 28 Jan 20 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)