Keywords
Sodium hypochlorite, Passive ultrasonic irrigation, Enterococcus faecalis, Escherichia coli, Endotoxins.
Sodium hypochlorite, Passive ultrasonic irrigation, Enterococcus faecalis, Escherichia coli, Endotoxins.
Sodium hypochlorite (NaOCl) is the most commonly used irrigant in endodontics (Iqbal, 2012). It has been used since the second half of the 18th century (Sedgley, 2004) because it has antimicrobial action (Luebke, 1967) and dissolves necrotic tissues (Taylor & Austin, 1918).
Enterococcus faecalis is a Gram-positive bacterium found in the root canal system (RCS) and can be disinfected by NaOCl (Siqueira et al., 2000). It may also be found in secondary infections of endodontically treated teeth (Machado et al., 2020). In addition, Escherichia coli, a Gram-negative bacterium, is also found in endodontic infections (Narayanan & Vaishnavi, 2010).
Bacteria have endotoxins in their outer membrane known as lipoteichoic acid (LTA) in Gram-positive bacteria (Ginsburg, 2002) and lipopolysaccharide (LPS) in Gram-negative bacteria (Mergenhagen & Varah, 1963). Endotoxins can be released during the duplication or death of these bacteria in infected RCS and this has a role in developing periapical lesions (Endo et al., 2012). Endodontic treatment using NaOCl can detoxify endotoxins, but not completely (Cavalli et al., 2017).
NaOCl is a cytotoxic substance (Salazar-Mercado et al., 2019); overflow during endodontic treatment can cause diverse exacerbations (Goswami et al., 2014). Thus, NaOCl gel may be safer due to its minor apical extrusion tendency (Nesser & Bshara, 2019). Passive ultrasonic irrigation (PUI) improves RCS disinfection (Plotino et al., 2007), removing the smear layer and vapor lock during endodontic treatment (Bueno et al., 2019; Dioguardi et al., 2019), and permits greater penetration of irrigants to the dentinal tubules (Sáinz-Pardo et al., 2014).
The purpose of this study was to evaluate the effect of NaOCl solution (2.5%) and gel (3%) with/without PUI on E. faecalis, E. coli, and their endotoxins, LTA and LPS, respectively.
This study was approved by the research ethics committee of São Paulo State University, Institute of Science and Technology (no1.504.995). The teeth used in this study were obtained from clinics where teeth are donated during routine procedures and following authorization of the patients. The research team presented the terms of donation by the clinics from which the teeth where obtained to the research ethics committee when submitting the study methodology. A total of 40 human lower premolars were collected (based on dimensional and morphological similarities).
To standardize root canal diameter, the teeth were initially instrumented with a #30 K-file (Maillefer, Ballaigues, Switzerland) and irrigated with 5 mL of NaOCl 1% for each file used. The canals were dried with sterile paper points (Dentsply Ind Com LTDA, RJ, Petrópolis, Brazil) and the apical region was sealed with light-cured resin composites (3M Dental Products, St Paul, MN). The outer surfaces of the specimens were covered with two layers of epoxy adhesive (Araldite - Brascola, Sao Paulo, Brazil), except the cervical opening (de Oliveira et al., 2007). Then they were fixed with chemically activated acrylic resin in 24-well plates and sterilized by gamma radiation with cobalt 60 (20 KGy for 6 hours) (Csako et al., 1983).
Specimens were contaminated with E. coli (ATCC 25922) for 28 days and E. faecalis (ATCC 29212) for 21 days and incubated at 37°±1°C, following the protocol of Maekawa et al. (2013).
Specimens were divided into four experimental groups (n=10/group) as follows: (1) 2.5% NaOCl (Asfer, São Caetano do Sul, São Paulo, Brazil) irrigating the canals without PUI activation; (2) 2.5% NaOCl irrigating the canals with PUI; (3) 3% NaOCl gel (Ultradent, South Jordan, UT, USA) irrigating the canals without PUI; and (4) 3% NaOCl gel irrigating the canals with PUI. All specimens were instrumented as a part of the biomechanical preparation by Reciproc R40 (VDW, Munich, Germany) following the protocol of each experimental group (Table 1).
NaOCL, sodium hypochlorite; PUI, passive ultrasonic irrigation.
PUI activation was performed using an E1-Irrisonic stainless-steel tip (Helse, Santa Rosa de Viterbo, Brazil) (0.10mm in diameter) at the working length using CVDente 100 ultrasound activator (CVDentus, São José dos Campos, Brazil) at 10% frequency.
After treatment, all specimens were filled with 17% ethylenediaminetetraacetic acid (EDTA) (Biodinâmica, Ibiporã, PR, Brazil) for 3min and then washed with nonpyrogenic saline solution.
Three samples were collected during the experiment, as in (Maekawa et al., 2013): S1, at baseline to confirm biofilm formation; S2, immediately after treatment; and S3, after EDTA application.
Serial dilutions of all samples were performed with sterile saline solution and aliquots of 30µl of each sample were seeded in two different culture medias: Enterococcosel agar (Becton, Dickinson and Company Sparks, MD, USA) for E. faecalis; and MacConkey agar (Himedia Laboratories, Mumbai, India) for E. coli. The plates were kept at 37°C for 24h and then CFU/mL were counted.
LPS levels in each sample was assessed as in Machado et al. (2020) using kinetic chromogenic limulus amebocyte lysate assay (Lonza, Walkersville, MD, USA). The plates were incubated at 37±1°C for 10 min in a KineticQCL reader, which was coupled to a computer with the WinKQCL software (Lonza). As soon as the kinetic test started, absorbance at 405 nm was read in each microplate well and automatically calculated the log/log linear correlation between reaction time of each standard solution and corresponding endotoxin concentration.
LTA was assessed using enzyme-linked immunosorbent assay using ELISA 96-well plates (Nunc Thermo Scientific, Waltham, MA, USA) sensitized with anti-LTA monoclonal antibody (manufacturer) and kept overnight at relative humidity. Next day, the plates were washed with a wash buffer (PBS with 0.05% Tween 20) and incubated with a blocking buffer (PBS with 2% bovine serum albumin, BSA) for 1 h at room temperature. Then, they were washed with a wash buffer and received 100 μL of the samples collected and 100 μL of the LTA standard followed by serial 2-fold dilutions (standard curve) and maintained for 2 hours at room temperature. Afterwards, the plates were washed again and 100 µL of anti-LTA antibody was added for 1 hour at room temperature. The plates were washed again and 100 μl of horseradish peroxidase HRP conjugated rabbit IgG antibody was added for 1 hour at room temperature. Lastly, the plates were washed, and the reaction was developed using tetramethylbenzidine (TMB). After 20 min under the light, 50 μL of stop solution (2 N sulfuric acid) was added to each well of the plate and optical densities were read in the microtiter plate reader (BioTek Instruments, Inc., Winooski, VT, USA) at 450 nm absorbance (Machado et al., 2020).
All experimental groups were effective in reducing the microbial load (CFU/mL) of E. coli (Figure 1) and E. faecalis (Figure 2) in S2 (from S1 levels). There was no significant difference among the experimental groups for S2 or S3 (Table 2).

CFU, colony forming units; NaOCL, sodium hypochlorite; PUI, passive ultrasonic irrigation.
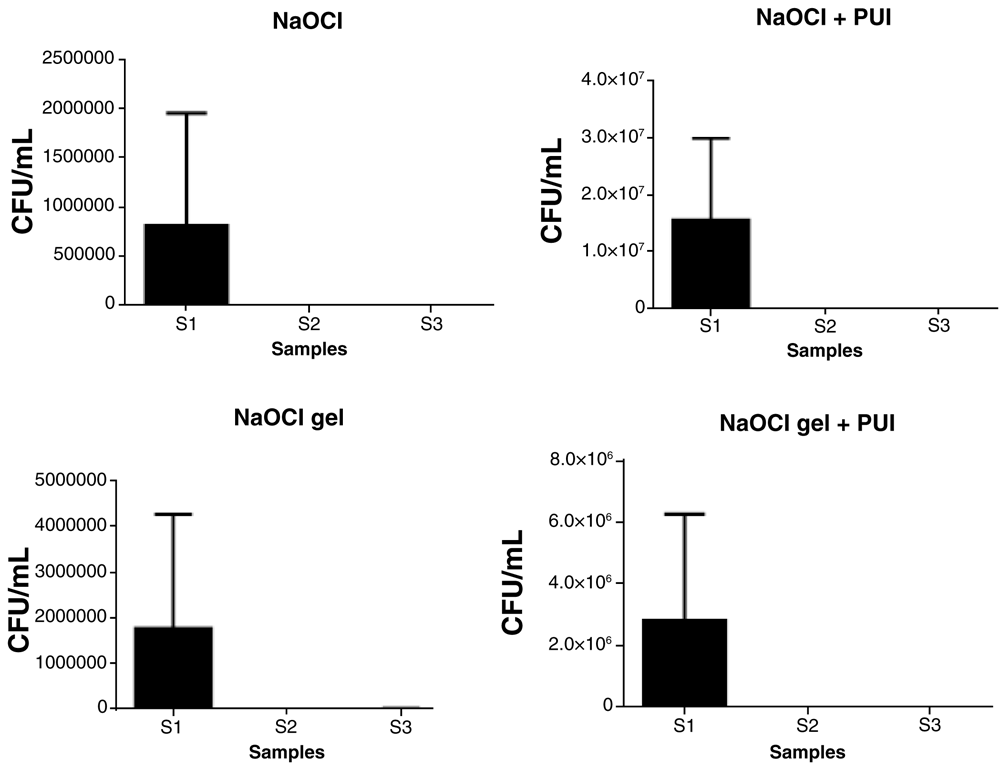
CFU, colony forming units; NaOCL, sodium hypochlorite; PUI, passive ultrasonic irrigation.
NaOCl and NaOCl gel groups had no significant difference in reducing LPS and LTA at S2. Groups with PUI showed a statistically increased reduction in LPS and LTA compared with groups without PUI (Figure 3). As a limitation of this study, the LPS and LTA levels were not assessed in S3.
NaOCL, sodium hypochlorite; PUI, passive ultrasonic irrigation.
Well endodontically treated teeth fail mainly because of secondary intraradicular infection (Siqueira, 2001). E. faecalis and E. coli, among other bacteria, are the most detected microorganisms in periapical lesions (Geibel et al., 2005).
The use of NaOCl as an irrigant cannot be over-emphasized. In this study, it was effective in disinfecting E. faecalis and E. coli (Table 2). These results agree with the results of Siqueira et al. (2000), who showed that NaOCl was an effective irrigant over E. faecalis at different concentrations (1%, 2.5%, and 5.25%). In addition, Valera et al. (2009) showed that 1% NaOCl is effective in disinfecting E. faecalis inoculated in the RCS. There have also been more recent studies that have the same results (Carvalho et al., 2020; Plutzer et al., 2018; Valera et al., 2014). Similarly for E. coli, NaOCl has been reported in the literature as an effective irrigant in disinfecting microorganisms in the RCS lumen at 2.5% concentration (Maekawa et al., 2011) and in preventing E. coli regrowth (Valera et al., 2015).
In the present study, NaOCl was not effective in detoxifying LPS and LTA completely (Figure 3). In support of this, the literature has shown that NaOCl is not effective in reducing endotoxin levels in the RCS (de Oliveira et al., 2007), i.e. it reduces the endotoxin level but does not completely eliminate them (Neelakantan et al., 2019). However, adding chloride alkali electrolyte-stable anionic surfactant has been shown to improve NaOCl effectivity because it reduces superficial tension (Valera et al., 2015).
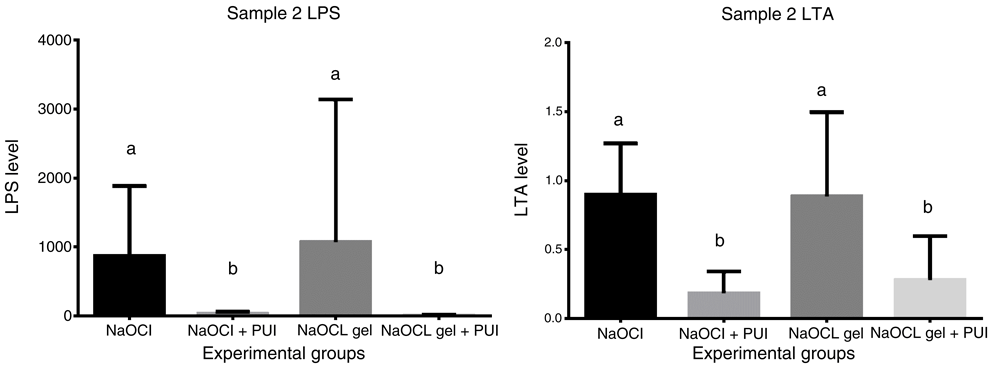
NaOCL, sodium hypochlorite; PUI, passive ultrasonic irrigation; LPS, lipopolysaccharide; LTA, lipoteichoic acid.
NaOCl gel has been suggested as an alternative endodontic irrigant because theoretically it has the same antimicrobial action of NaOCl solution, but with less apical extrusion and could thus be safer (Nesser & Bshara, 2019). It is effective in reducing the microbial load, but has been shown to be less effective when compared to NaOCl solution of a lower concentration (Poggio et al., 2010; Zand et al., 2016). In the present study, NaOCl gel was shown to be just as effective as NaOCl solution in reducing microbial load.
To the best of our knowledge, there are no studies in the literature evaluating the effect of NaOCl gel over endotoxins. In this study it was statistically as effective as the solution. But both were more effective when combined with PUI as it increases NaOCl penetration into dentinal tubules (Faria et al., 2019). The present study is novel as there are no studies evaluating how PUI can affect NaOCl solution or gel action on endotoxins.
PUI is still being studied due to divergence of results in the literature. For example, Paiva et al. (2013) used PUI after instrumentation and showed it was ineffective in reducing microbial load; however, Mohmmed et al. (2018) showed that PUI is effective in biofilm removal from lateral canals. However, this activity may be influenced by the irrigation protocol (irrigation time; irrigant volume; instrument shape and material; and the irrigation frequency and intensity) (van der Sluis et al., 2007).
In conclusion, our study showed that NaOCl gel has the same antimicrobial action of NaOCl solution and can partially detoxify endotoxins. PUI improves NaOCl (gel or solution) action over E. faecalis and E. coli formation and their endotoxins (LPS and LTA).
Harvard Dataverse: Raw Data of NaOCl solution and gel, https://doi.org/10.7910/DVN/JNK3TH (Abu Hasna, 2020).
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Endodontics
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Wang J, Du L, Fu Y, Jiang P, et al.: ZnO nanoparticles inhibit the activity of Porphyromonas gingivalis and Actinomyces naeslundii and promote the mineralization of the cementum. BMC Oral Health. 2019; 19 (1). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Endodontics and operative dentistry
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 24 Jun 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)