Keywords
flavohaemoglobin, nitric oxide, microbiology
flavohaemoglobin, nitric oxide, microbiology
The importance of the small gas nitric oxide (NO) can hardly have escaped the attention of most scientists in the fields of clinical medicine, physiology, biochemistry, microbiology and environmental science. “Popular” textbooks describe this science and medicine1,2. Total citations of “nitric” AND “oxide” in the Web of Science Core Collection approach 300,000. Until the past few decades, the literature was dominated by chemistry but now the largest category is biochemistry and molecular biology (>42,000 citations, representing more than 14% of the total; Figure 1). This clearly demonstrates the pervasive impact of NO in biology. This short review aims to link these impacts with one of the several enzymes known to destroy this invaluable yet potentially toxic molecule.
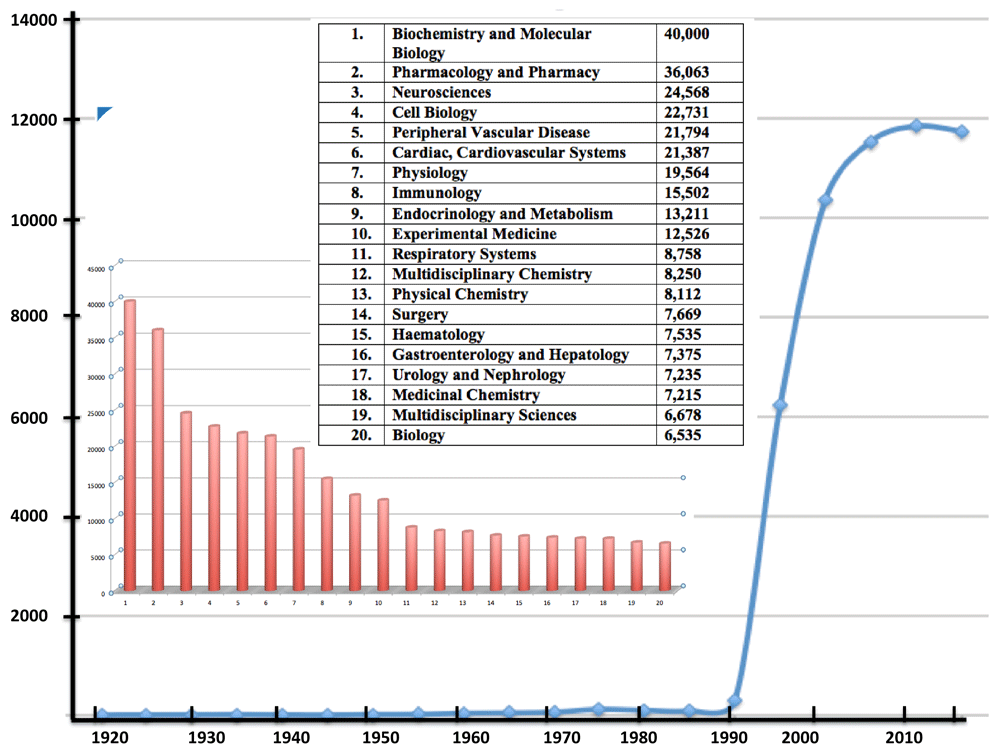
The inset shows the number of citations in the search categorised by subject area.
NO is a free radical species; it may therefore be written formally as NO. (NO-dot) but conventionally simply as NO. The chemistry of NO is complex and, as a result, there are numerous intricacies, misunderstandings and sometimes errors in the literature. It is soluble in water (approximately 1.6 mM at 37 °C and 1.94 mM at 25 °C3) but does not react with it. The difficulties stem from its short lifetime in cellular environments: if generated at 10−7 M (for example, from NO synthases, or NOSs), NO has a lifetime of 30 min if its fate is oxidation to NO2 but may be as low as 1 s on reaction with biological targets, especially haems, thiols and superoxide anion4. A multitude of redox-related species may be generated from NO in biological situations and each may have different targets. This chemistry is not detailed here except where it is required for clarity, but excellent reviews exist5–7. In brief, nitrosonium cation (NO+), nitroxyl anion (NO−, although HNO is dominant at pH 7), nitrogen dioxide (NO2), and dinitrogen trioxide (N2O3, the product of NO reacting with O2) and peroxynitrite (ONOO−, the product of NO reacting with superoxide radical) are not “forms of NO” but products of NO reactions. There is only one NO4,8!
The major source of NO in vivo is via the activity of NOSs. The complex biology, chemistry and medical significance of NOS are outside the scope of this commentary, but excellent reviews and articles cover mammalian9, microbial10 and the elusive plant NOS-like11 activities. The roles of NO in signalling and “gasotransmitters” in higher organisms are also beyond the scope of this article, but see 7. In higher organisms, NO plays a key role in cellular immunity, where the gas, generated primarily by inducible NOS, attacks diverse macromolecules in invading microbes6. The only organisms in which flavohaemoglobins are found are microbes, including pathogens. Flavohaemoglobins are arguably the most important, but not the only, mechanism by which the microbe strikes back.
Why then do higher organisms not also possess flavohaemoglobins? The answer may be that the high concentrations of other (non-flavo) globins protect animal cells from excessive NO; the combination of methaemoglobin reductase and very high haemoglobin concentrations in red blood cells provides an effective NO removal mechanism, functionally equivalent to the NO dioxygenase activities of flavohaemoglobin12.
The term “nitrosative stress” appears to have been introduced to this field in 199613 to describe specifically the reaction of S-nitrosothiols (RSNOs, such as S-nitrosocysteine) with intracellular thiols via S-nitrosation (that is, the transfer of the nitrosonium group NO+ to biomolecules)4. It is important to note that the term should not be used, as it sometimes appears to be, to describe all NO chemistry in biology: “NO cannot act as a nitrosating agent, unless there are oxidizing agents present, such as a transition metal species or oxygen. Thus, NO cannot nitrosate thiols. Reports to the contrary result from the presence, sometimes adventitiously, of an oxidizing agent or from an imprecise description of the reaction”4. Thus, for example, the Escherichia coli flavohaemoglobin (Hmp), the subject of this article, cannot directly protect against nitrosating agents since only NO reacts in a physiologically useful way with this globin. “Nitrosative” is not an adjectival form of NO!
The broad reactivity of NO in biology implies that certain cellular components will be more susceptible to NO damage than others. A comprehensive kinetic model that encompasses this reactivity in E. coli that incorporates spontaneous and enzymatic reactions as well as damage and repair of biomolecules has been developed14. This model, informed by experimental measurements of NO dynamics, allows a detailed analysis of how NO distributes in E. coli cultures and identification of the control parameters of the NO distribution. The simulation predicted that Hmp functions as a dominant NO consumption pathway at O2 concentrations as low as 35 μM (that is, microaerobic conditions): virtually all (99.85%) of the NO consumed by the cells was predicted to be through Hmp detoxification, and most of the remainder through oxidation by O2 and reaction with superoxide anion. Surprisingly, Hmp loses utility as the NO delivery rate increases, as a result of substrate inhibition15. Such models are valuable for rigorously investigating NO stress in microbes and may identify novel strategies to potentiate the effects of NO14.
Hmp was discovered in bacteria in 199116, only a year after key articles from Furgott, Ignarro and Murad identified the endothelium-derived relaxing factor (EDRF) as a gas with a molecular mass of only 30 and a year before the recognition of NO as “Molecule of the Year” by Science in 1992. Between 1989 and 1998, when the Nobel Prize for Physiology or Medicine was awarded to Furgott, Ignarro and Murad17–19, the citation count per annum increased almost 70-fold. In fact, the discovery that EDRF was NO was also made by Salvador Moncada, then at the Wellcome Research Laboratories in the UK, but, astonishingly, the Nobel award did not include Moncada. At the time, those involved in this work might not have foreseen how NO research would be so sustained (currently running at about 12,000 citations per annum) and all-encompassing in biology (Figure 1), nor could we have known that the flavohaemoglobin Hmp would assume the role of the pre-eminent NO-detoxifying enzyme in microbes. However, other contenders exist (see below).
Hmp was not the first bacterial globin to be identified and sequenced. Rather, the first was the haemoglobin of Vitreoscilla, which is an obscure bacterium whose soluble haemoprotein (Vgb) is dramatically increased in concentration under the microaerobic conditions that the organism encounters20. The function or functions of this protein are still unknown: despite evidence that its expression in heterologous hosts can confer some protection from nitrosative stress21 and perhaps acts as an oxidase (although this is disputed22), the generally accepted view is that Vgb facilitates oxygen utilisation; numerous articles have claimed biotechnological applications for this effect (see below). Vgb is unlike flavohaemoglobin: in a recent attempt to classify and logically name the globin family23,24, we proposed that one family should be the 3/3 myoglobin-like proteins. One sub-family comprises the two-domain flavohaemoglobins (having haem and reductase modules), and the second comprises the single-domain globins, of which the Vitreoscilla protein is one example that comprises only the haem domain.
Hmp was the first microbial globin for which a gene sequence was obtained, for which modes of regulation were established and, most importantly, for which a function was unequivocally demonstrated. The serendipitous discovery of the hmp gene by the author and colleagues16 showed it to be a 44-kDa monomer with a haem domain that was almost half (46%) identical to Vgb. The C-terminal domain closely resembles ferredoxin-NADP+ reductase in that both have highly conserved binding sites for NAD(P)H and FAD25. Purified Hmp possesses haem B and FAD26,27, the presence of which was confirmed by the crystal structures of bacterial flavohaemoglobins28–30. This reductase domain, which transfers electrons from NAD(P)H to haem-bound ligands (and soluble molecules25,31), is essential for the function of Hmp32,33.
Here, the original names Hmp (intentionally and cautiously suggesting only a haemoprotein nature and not rashly assuming a function) and hmp are used for the protein and gene, respectively, in enterobacteria16. Yhb in yeasts34 and the more general abbreviation Fhb (flavohaemoglobin) are used elsewhere.
The first clue to function came from our discovery that solutions of NO gas (not a nitrosating agent) were potent inducers of hmp gene transcription35. Shortly after, Gardner and colleagues demonstrated an enzymic function for Hmp, named NO dioxygenase (that is, the conversion of NO and O2 to innocuous NO3 −)36. An alternative interpretation of this critical inducible reaction is that Hmp is not a dioxygenase (that is, in which the two O atoms are used to oxygenate NO36,37) but a denitrosylase38,39; here, the haem-bound NO (FeIIINO−) reacts with an oxygen molecule to produce nitrate. However, later evaluation of the reaction mechanism confirmed the NO dioxygenase mechanism40: analysis of the stoichiometric product (nitrate) showed more than 99% double O-atom incorporation from Hmp18O2. The NO dioxygenation mechanism involves (1) rapid reaction of NO with a FeIII−O2. intermediate (the product of the facile reaction of O2 with Hmp FeII to form FeIII−OONO) and (2) rapid isomerization of this intermediate to form nitrate. The O–O bond homolyzes to form a protein-caged [FeIV = O .NO2] intermediate, and ferryl oxygen attacks .NO2 to form nitrate. This mechanism appears common to all higher haemoglobins and myoglobin that have been examined40,41 (Figure 2).

A typical (Gram-negative) envelope is shown that allows ready access of extracellular NO to intracellular Hmp. A small contribution to the cellular NO pool from intracellular sources is indicated. Hmp comprises a haem domain (red), an NAD(P)H-oxidizing domain (green) and an FAD domain (cyan). The redox centres are shown. The primary reaction catalysed is the conversion, by a dioxygenase mechanism, of O2 and NO to form nitrate. Minor reactions also reported are shown below in the blue box. Transcriptional regulators identified thus far are shown at the right (green box), and the numerous consequences of Hmp activity are indicated at the right (orange box).
Therefore, NO detoxification by Hmp is optimal when oxygen is abundant. If oxygen is low (0–50 μM), NO defences are severely compromised, exhibiting a roughly 30-fold increase in NO clearance time compared with anaerobic and aerobic conditions for the same addition of an NO donor compound (50 μM DPTA NONOate)42. Modelling suggested that a steep drop in anoxic activity of NorV, a flavorubredoxin with NO removal activity, as [O2] fell, combined with impaired translational and Hmp activities at low [O2], results in suboptimal overlap of these two detoxification systems, resulting in up to a roughly 60% loss in their combined NO detoxification activities. In addition, at low [O2] conditions, the concentrations of NO and O2 oscillated, arising from kinetic competition for O2 between the aerobic respiratory oxidases and Hmp42. Other candidates for NO detoxification are described later.
Although the NO dioxygenase activity of Hmp is the key mechanism for NO removal aerobically, an anoxic lower activity has been described43. This exhibits a rate that is orders of magnitude slower than the O2-dependent reaction, and the physiological relevance is a matter of debate44. Nevertheless, NO binds Fe(III) Hmp to generate a nitrosyl adduct that is stable anoxically but decays in air to reform the Fe(III) protein43. NO displaces CO bound to Fe(II) Hmp but CO recombines after only 2 s at room temperature, indicative of NO reduction and dissociation from the haem. Direct demonstration by membrane-inlet mass spectrometry of NO consumption and nitrous oxide production during anoxic incubation of NADH-reduced Hmp confirm the reaction in vitro43 (Figure 2).
NO at nanomolar levels induces biofilm dispersal in numerous bacteria (for example,45,46). Consequently, NO-charged catheters have been investigated to prevent bacterial colonization47. Thus, production of Fhb in Pseudomonas aeruginosa inhibits dispersal while imidazoles (see below) attenuate the prevention of dispersal48.
Interestingly, rendering Hmp inactive in Salmonella enterica Gallinarum, in which RpoS and the SsrA/B regulator were also mutated, generated a hyper-susceptible strain that caused no mortality on injection into chickens. Vaccination of chickens with this strain conferred complete protection against challenge with virulent bacteria comparable to that achieved with a conventional vaccine strain49. Disabling NO defences as a strategic utility is also suggested by the finding that elimination of ClpP (a major ATP-depended protease) largely eliminated NO detoxification by E. coli50. The effect is due to deficient transcript levels of hmp and widespread perturbations in other NO-responsive genes.
Recently, a new function was proposed (Figure 2): scavenging of the mild oxidant sulfur trioxide anion radical (STAR), a product in cells of (bi)sulfite oxidation51. The reaction of STAR with ferrous globins is rapid, and STAR reacts 260- and 1000-fold faster with Ngb (neuroglobin) and Fhb, respectively, than with glutathione, suggesting a detoxification function. The Fhbs of yeast and bacteria exhibit this activity, and a flavohaemoglobin mutant of Saccharomyces cerevisiae was slow-growing in the presence of sulfide attributed to mitochondrial damage51.
Although much of what we have learned about these globins has come from bacteria, predominantly E. coli (sequence, function, gene regulation, and three-dimensional structure), the first reports of a microbial globin were in yeast52–55. No involvement in NO chemistry was then suspected. Sequences for Fhbs are among the most numerous globin genes in bacteria of diverse taxons (533 sequences reported), exceeded only by class 2 truncated globins (622 sequences)24. A newer survey identified 3318 Fhb sequences56, comprising 2363 in bacteria and 204 in eukaryotes. Fhbs appear to be absent from Archaea57. The bacterial sequences were distributed across 10 bacterial divisions with the highest number in the Proteobacteria. Interestingly, other divisions appear devoid of these proteins or they are uncommon, as in Bacteroidetes and Cyanobacteria.
Eukaryotic flavohaemoglobins are also found in protozoa, other fungi and two trypanosomes of insects. The protozoan parasite Giardia intestinalis possesses only five known haem proteins, one of which is flavohaemoglobin; this protein is expressed when trophozoites are exposed to NO or nitrite stresses and acts as an NO dioxygenase58,59. The key roles of flavohaemoglobins in NO homeostasis in filamentous fungi and yeast are now widely recognised60–62.
Both eukaryotic and bacterial flavohaemoglobins are generally considered soluble enzymes. Although bacterial flavohaemoglobins are normally recovered for purification from cytoplasmic fractions, around 30% are periplasmic in E. coli on the basis of Western immunoblotting63, but the haem holoenzyme appears to be uniquely cytoplasmic. In yeast, Yhb is located in the cytosol, mitochondrial matrix and the intermembrane space but also in the inner membrane64; however, the CO-binding fraction of Yhb is not present in inner membrane vesicles.
The manner as to how this important protein has become so widely distributed has recently been addressed23,24,56. We proposed that the flavohaemoglobin gene family arose from an ancestral globin and later spread to eukaryotes via horizontal gene transfer23,24. Such transfers between the domains of life are infrequent in biology, but “single-protein metabolic modules” (for example, Fhb and its self-contained NO detoxification function) are prone to gene duplication (see below) and such horizontal gene transfer during evolution. A striking example of gene transfer from bacteria is afforded by a study of the acquisition by the eczema-causing fungus Malassezia of an Fhb from Corynebacterium and a concomitant increase in NO resistance56.
It is now accepted that Hmp detoxifies NO, primarily aerobically, supported by the following key observations, many of which are from the older literature; illustrative examples are given.
Null hmp mutants of Salmonella and E. coli are hyper-sensitive to the antimicrobial activity of NO or S-nitrosoglutathione (GSNO)65–69.
Hmp catalyses redox chemistry with NO and O2 at the haem, and the haem of Hmp is readily reducible by physiological substrate (NAD(P)H) by means of electron transfer from FAD31–33,70.
The level of Hmp correlates with the level of NO resistance of respiration in E. coli. Respiration of an hmp mutant is highly sensitive to sub-micromolar NO, whereas respiration in cells pre-induced by treatment with sodium nitroprusside (SNP) is resistant to NO concentrations up to 50 μM71.
Null hmp mutants of Salmonella and E. coli are hyper-sensitive to killing by human macrophages66,72,73, and hmp mutants of Yersinia pestis are attenuated for virulence in the NO-rich infection bubo74.
Bacteria growing on the exterior of spleen microcolonies respond to soluble signals and induce synthesis of Hmp, thus eliminating inward NO diffusion and protection of interior bacterial population from NO-derived inducing signals75.
The Fhb of the plant pathogen Erwinia chrysanthemi confers NO tolerance on the fungus but also, by intercepting plant-derived NO, attenuates the hyper-sensitive response76.
Salmonellae experiencing nitrosative stress generate a burst of the alarmone nucleotide guanosine tetraphosphate (ppGpp). This activates transcription of valine biosynthetic genes, thereby re-establishing branched-chain amino acid biosynthesis that enables the translation of Hmp77.
The genome of the yeast Candida albicans contains three genes encoding flavohaemoglobin-related proteins but, based on studies of mutants lacking each of these genes, only one, CaYHB1, is responsible for NO consumption and detoxification78. Loss of CaYHB1 increases the sensitivity of C. albicans to NO-mediated growth inhibition and decreases virulence in mice compared with that in wild-type strains.
Duplicate flavohaemoglobins may have distinctive functions, and one has the established NO dioxygenase function. For example, a gene duplication event in the Actinobacteria is suggested to have given rise to a second clade of type II flavohaemoglobins with unusual structural and functional properties, including D-lactate metabolism79,80.
Similarly, gene duplication seems to have generated fungal Fhb clades with different locations. In Aspergillus oryzae81, Fhb1 is located in the cytosol and the clade 4 Fhb2 in mitochondria, so that mechanisms for NO depletion in each cellular compartment are effected.
NO or nitrosating agents up-regulate the hmp gene. In fact, hmp is consistently among the most highly up-regulated genes seen in genome-wide transcription profiling of E. coli, Bacillus subtilis and Salmonella cultures exposed to NO and nitrosating agents82–84, in Salmonella following infection and induction of NO synthesis in J774 cells73, and in the plant symbiont Sinorhizobium meliloti85. Recently, NO2, an air pollutant, was also reported to up-regulate hmp expression in Pseudomonas strains86.
Although our understanding of hmp regulation is incomplete, several mechanisms have been identified and studied so far. These include several described in early studies87–90 and, more recently, a sigma-dependent small RNA91. In S. aureus, the two-component regulator SrrAB, generally considered an oxygen sensor, regulates hmp under low-oxygen conditions or on exposure to NO92–94. In Salmonella, DksA recently emerged as an important factor for full expression of hmp transcription following NO exposure95.
A major mechanism is undoubtedly via NsrR73,96–98, a transcriptional repressor in the Rrf2 family containing an NO-sensitive FeS cluster (probably [4Fe-4S]). Reaction of the cluster with NO decreases its DNA-binding affinity and relieves repression at sensitive promoters that control expression of not only hmp but also poxB (via read-through from the upstream hcp-hcr genes) and the sufABCDSE cluster involved in iron-sulfur biogenesis and repair99,100. Recent work shows that nsrR is expressed from a strong promoter but that translation is inefficient. This is important since target promoters with low affinity for NsrR may partially escape repression99. When H2O2 and NO coexist (as they do in the phagolysosome), NO detoxification is delayed, an effect attributed to inhibition by H2O2 of hmp gene transcription and translation under the control of NsrR101.
In eukaryotic fungi such as Aspergillus, two proteins, FhbA and FhbB, are differentially induced to catabolise NO61,102. NO is produced endogenously by a nitrate reductase early in the transition from vegetative growth to development. NO homeostasis is critical since NO levels influence the balance between conidiation and sexual reproduction.
Since flavohaemoglobins confer a degree of pathogen resistance to NO generated by the immune system, including within the macrophage and its cocktail of reactive species (see above), inhibitors that target the haem prosthetic group of flavohaemoglobins are potential antimicrobial agents. Imidazoles having bulky aromatic substituents fit into the globin haem pocket and coordinate the ferric iron with a Kd of 333 μM30. Structural studies confirm that azole binds the Fhb haem and reveal major conformational reorganisation103,104.
Others (miconazole, econazole, clotrimazole and ketoconazole) have similar activities against Fhbs105 and inhibit NO metabolism in bacteria and yeast. However, they do not achieve the NO-induced stasis seen in flavohaemoglobin-null mutants. One of these agents, miconazole, is the most effective azole against Staphylococcus and ligates to both ferric and ferrous globin106. Over 20 years ago107,108, we reported that, in the absence of NO, Hmp generates superoxide anion by single electron transfer from NAD(P)H to haem-bound oxygen. Interestingly, miconazole enhanced superoxide production by the S. aureus enzyme, so that, in macrophages, bacteria possessing flavohaemoglobin are compromised in survival compared with flavohaemoglobin-deficient bacteria106. This presumably is attributed to the inhibitor binding to haem and diverting electrons to oxygen107,108. Other acceptors31 may also be reduced when haem function is blocked, as occurs with CO31.
Alternative inhibitors might be found among quinones and nitroaromatic compounds. S. aureus flavohaemoglobin rapidly reduces these compounds, which may act as subversive substrates, diverting electron flux from FAD and enhancing the toxicity of NO by formation of superoxide109.
There have been countless reports of the ability of the Vitreoscilla globin, when expressed in heterologous hosts, to enhance aerobic cell yields or product formation110–112. The mechanistic basis of these diverse effects remains unclear. However, a recent report113 claims that both an exogenously introduced Vitreoscilla globin and the native Fhb enhance pullulan production in the yeast Aureobasidium. Fhb gene expression in the yeast was elevated 3.5-fold over native levels, based on reverse transcription polymerase chain reaction (RT-PCR) data, but CO difference spectra show only a modest increase in a pigment with some of the characteristics of globins or other haem proteins; the broad absorbance spectra and noisy traces should not be interpreted as unequivocal evidence of high globin expression. Expression of either globin increased oxygen uptake, but the data are not quantified and no mechanism for enhanced pullulan yields is presented.
E. coli Hmp, anchored to electrodes, electrolytically interconverts NADH and NAD+ by transfer of electrons to the FAD moiety where NADH/NAD+ is transformed. It is suggested that this might be employed in NAD-dependent bioelectrodes for biosyntheses, biosensors and biofuel cells114.
Many other globins convert NO to NO3− via an NO dioxygenase activity. These include the truncated globin of Mycobacterium tuberculosis, trHbN115, the single-domain globin Cgb of Campylobacter jejuni116, M. tuberculosis HbN115, mammalian cytoglobin117 and Arabidopsis cytoglobin 3118.
When oxygen is available, the catalytic efficiency of the Fhb reaction, kcat/Km, is very high: up to 2400 × 106 M−1 s−1119. However, under anoxic or low-oxygen conditions, where the activity of Hmp is dramatically reduced, NO tolerance in Salmonella is affected additionally by a combination of three enzymes, flavorubredoxin (NorV), and cytochrome c nitrite reductase (NrfA). A study of the effects of all eight possible combinations of norV, hmp and nrfA single, double and triple mutations suggested an important additive role for both NorV and NrfA120,121. None of the NO detoxification systems—Hmp, NorV and NrfA—is solely responsible for nitrosative stress tolerance of S. typhimurium in raw sausages where sodium nitrite is used as a curing agent122. Somewhat different conclusions were reached in a study of a uropathopathogenic strain of E. coli (UPEC) that induces a variety of defence mechanisms in response to NO, including direct NO detoxification (Hmp, NorVW, NrfA), iron-sulfur cluster repair (YtfE), and the expression of the NO-tolerant cytochrome bd-I respiratory oxidase (CydAB)123,124. During UPEC growth and survival during infection, loss of the flavohaemoglobin Hmp and cytochrome bd-I elicited the greatest sensitivity to NO-mediated growth inhibition, whereas all but the periplasmic nitrite reductase NrfA provided protection against neutrophil killing and promoted survival within activated macrophages. Intriguingly, cytochrome bd-I was the only system that augmented UPEC survival in a mouse model, suggesting that maintaining aerobic respiration under conditions of nitrosative stress is a key factor for host colonisation. In Salmonella enterica also, cytochrome bd augments defences against NO in systemic tissues125. Thus, cytochrome bd emerges as a major contributor to bacterial NO tolerance and host colonisation under microaerobic conditions. The hybrid cluster protein Hcp and its NADH-dependent cognate reductase Hcr were not tested in this study, but it is striking that a role for this system as a high-affinity NO reductase could be demonstrated only when the Hcp reductase was introduced into a strain deleted for the nirBD, nrfAB, norVW, hmp and hcp genes126. Other non-globin contenders include the flavorubredoxin and nitrite reductase NrfA of E. coli127, various flavodiiron proteins, Paracoccus denitrificans NO reductase NorBC, and the NO reductase of S. aureus128.
The flavohaemoglobins of numerous bacterial species and groups, yeasts, fungi and protozoa continue to fascinate those devoted to understanding globin functions, NO homeostasis in biology and clinical medicine. Our knowledge has exploded since their discovery in 1991 (at least at a molecular level) and has revealed new paradigms of enzyme mechanisms, gene regulatory mechanisms and physiological significance. Undoubtedly, much remains to be learned. It is striking, though, that throughout this period (almost 30 years), no clear evidence has emerged for a flavohaemoglobin in higher organisms. This continues to offer the hope that such a protein, a “single protein metabolic module”, might represent a useful target for antimicrobial therapies. Imidazoles with great efficacy have been identified as inhibitors and these efforts, in concert with increasing understanding of protein function, ligand and electron migration within such flavoproteins, may yet give us new antimicrobial weapons.
I am indebted to the many talented and industrious postdoctoral scientists, PhD students and overseas collaborators with whom I have enjoyed working over the years.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
|
Version 1 08 Jan 20 |
read | read | read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)