Keywords
Acromegaly, growth hormone excess, respiratory complication, sleep apnea, quality of life, patients reported outcome
Acromegaly, growth hormone excess, respiratory complication, sleep apnea, quality of life, patients reported outcome
Acromegaly is a disorder almost exclusively caused by a pituitary growth hormone (GH)-secreting adenoma. The high circulating levels of GH and resulting insulin-like growth factor 1 (IGF-1) have pleiotropic effects on many organs1. Ultimately, acromegaly causes an insidious syndrome that severely impacts patient morbidity and quality of life (QoL). Cardiovascular and respiratory complications are frequent comorbidities and are the main causes of mortality among patients2,3. Cancer risk is also increased4 and recently cancer has been shown to surpass cardiovascular causes as a main cause of death compared with the general population5,6. However, with advances in treatment and thus improved biochemical control, mortality has diminished over the past decade7. See Table 1 on multisystem acromegaly complications2,8–12.
Cardiovascular complications and cancer have been extensively reviewed as complications of acromegaly13. The focus here is on the latest developments related to respiratory and QoL effects.
Patients with acromegaly have been reported to have a 1.85 times higher risk of developing respiratory diseases versus the general population14. One of the most common complications of acromegaly is obstructive sleep apnea (OSA); the risk is approximately 10-fold in acromegaly compared with the general population15–17. OSA can exacerbate cardiovascular dysfunction and is an important contributor to impaired QoL and eventually mortality18. Lesser-known facets of acromegaly-induced respiratory complications affect pulmonary function, oximetry, and exercise capacity13,19. Excess GH, on the surface, would seem to be beneficial to physical performance; however, it can also have deleterious effects and contribute to significant fatigue and inability to sustain similar workloads compared with controls19,20. Overall, the mortality rates from respiratory causes are three times higher when compared with that of the general population21–23 and contribute to up to one fifth of acromegaly-related deaths24,25.
Mechanisms by which acromegaly is associated with respiratory complications are not clearly understood. However, there are several proposed mechanisms by which GH can alter lung anatomy; the main hypotheses are as follows:
1. Upper respiratory airways. High GH/IGF-1 can lead to deformities of facial bones, hypertrophy of the pharyngeal and laryngeal cartilages, and soft tissue thickening and macroglossia, which ultimately can lead to inspiratory collapse of the hypopharynx, especially during sleep2,14,26–28. Also, generalized tissue edema, related to high sodium and volume reabsorption by the kidney, which contributes to upper airway narrowing, may be observed in patients with acromegaly29,30. Ultimately, external compression can occur because of goiter or accumulation of fat and contribute to tracheal deviation, tortuosity, and sometimes stenosis31.
2. Lung volumes. GH stimulates lung growth by increasing the number or size of alveoli (or both)14,32,33. Data on histopathology of human lungs in patients with acromegaly are lacking, and clinical data from pulmonary function tests sometimes render conflicting results. In general, increased total lung volumes are measured by total lung capacity, vital capacity, and residual volumes, all of which can be 110 to 160% of a predicted value26,34–39. This is related to the direct effect of GH/IGF-1 inducing alveoli hyperplasia, alveoli hypertrophy, or an increase in alveoli number or a combination of these26,34. Increased lung mass has also been objectively measured by computed tomography (CT) densitovolumetry; active acromegaly compared with controls revealed a 25% mass increase: 885 g versus 696 g (P = 0.017)37.
3. Thoracic musculature. GH and IGF-1 exert proliferative but also degenerative effects on smooth muscle, inducing respiratory muscle dystrophia. Myopathy is evident on pathology as muscle fiber degeneration and abnormal collagen are observed40,41. These findings translate to enhanced muscle fatigue, as demonstrated in a murine model with effects on sternohyodal muscle42.
4. Thoracic bone changes. The axial skeleton is affected in up to 60% of patients, and changes include thickening of soft and cartilaginous tissues, rib cage deformity, formation of osteophytes, and abnormal trabecular bone microarchitecture leading to vertebral fractures and kyphoscoliosis38.
5. Diffusing capacity. Diffusing capacity of the lung for carbon monoxide (DLCO) can also be altered. Some studies reported a lower DLCO27, most showed neutral diffusing capacity26,27,34,35,38,43, and some found an increased DLCO44,45. Higher lung volumes could lead to an increase in DLCO; therefore, diffusing capacity corrected for alveolar volume is likely to be normal in patients with acromegaly46.
6. Lung compliance. Lung elasticity may also change due to excess GH/IGF-1. Decreased lung recoil might be explained by elevated peripheral rib cage resistance caused by hypertrophic muscles and rib cage or caused by obesity26 but could also be related to a higher alveoli size43. Some series did not demonstrate a change (ref 34) in lung elasticity and one (ref 43) showed that patients with acromegaly had higher lung distensibility, which was partially reversible after treatment34,43. Various components of lung compliance may be differently affected in the disease and may explain the heterogeneity of results.
7. Obstructive disease. Small airway obstruction is evident on the basis of a reduced maximum expiratory flow after 75% of forced vital capacity had been exhaled (FEF75). Moreover, more than half of patients had clinically important reductions (<80% of predicted value) in FEF7538,47,48. Up to 60% of air trapping causing ventilation perfusion mismatches was reported38. This small airway obstruction might be due to increased thickness or tortuosity of the bronchial wall, increased lung volumes, or vascular congestion or a combination of these38.
8. Bronchiectasis was also noted on high-resolution CT but might not be more prevalent than what is observed in healthy subjects49.
Sleep apnea in patients with acromegaly. In different series, sleep apnea affects up to 80% of patients and represents a 10-fold increased risk compared with the general population11,13,50. A main symptom of OSA is daytime sleepiness caused by repeated micro-arousal due to hypoxemic events during sleep50. Acromegaly is predominantly associated with OSA, caused by upper airway obstruction2,18,51,52. However, central sleep apnea also occurs, as about 30% of patients with acromegaly have a central sleep apnea component53–56. Pathogenesis remains controversial but might be directly GH-mediated or related to associated cardiomyopathy56,57. Excess GH is associated with central inhibition of the breathing center and may lower the ventilatory response of the respiratory center to carbon dioxide, resulting in a temporary cessation of the respiratory center and respiratory efforts56.
Other factors, notably obesity, concomitant hypothyroidism, and treatment of hypogonadism, also play a role in OSA13. Hypogonadism in male patients with acromegaly and OSA is frequent. Hormonal replacement should be undertaken with caution, especially in cases of severe untreated OSA since there is some evidence that testosterone might deteriorate OSA58.
If sleep apnea is left untreated, it can lead to fatigue, daytime sleepiness, hypogonadism, arterial and pulmonary hypertension, ischemic heart disease, right ventricular failure, cerebrovascular accidents, life-threatening arrhythmias, poor QoL, and cognitive dysfunction14,18,50,52,59,60. Usual predictors such as age, male sex, neck circumference, and body mass index (BMI) are factors associated with the onset and severity of OSA, but finger circumference is an additional predictor specific to acromegaly11,18,61–64. Use of the Epworth Sleepiness Scale or the STOP-Bang (snoring, tiredness, observed apnea, high blood pressure, BMI, age, neck circumference, and male sex) questionnaire can help identify patients, in the general population, but more studies are needed in patients with acromegaly, and polysomnography remains key for diagnosis18,50,65.
It is still a matter of debate whether OSA severity is associated with disease activity or disease duration or both66,67. Some reversibility of OSA may be observed once biochemical control is achieved, but a recent meta-analysis confirms that the prevalence of OSA in patients with controlled acromegaly is similar to that in patients with uncontrolled acromegaly68. Nonetheless, acromegaly patients who underwent treatment have been shown to have improvement in both central and peripheral component of sleep-breathing control, especially by reducing soft tissue swelling and macroglossia14,18,54,61,69. However, changes in bones and cartilaginous structures affecting upper airway anatomy may be irreversible13, and even worsening or de novo OSA, partly related to weight gain after cure, may occur16,18.
In general, prospective studies suggest that more than 50% of patients with controlled acromegaly continue to have persistent OSA13,14,55. Most improvement is attained in the first year after treatment55. Pituitary surgery has been reported to improve sleep apnea and decrease the frequency of apneic and hypopneic episodes by 50 to 60% in a surgically cured cohort39,62. Medical treatment also leads to improvement in apnea–hypopnea index (AHI), as indices of oxygen desaturation, sleep quality, and subjective sleepiness improved after 6 months of octreotide treatment in approximately half of cases70. Intriguingly, AHI changes in patients do not always correlate with GH or IGF-1 levels, and other factors such as age, sex, and smoking also prevail71. Interestingly, one factor influencing AHI is weight, as OSA did not improve and may occur de novo in patients who gained weight after treatment72.
Overall, evaluating patients with acromegaly for OSA at diagnosis is mandatory73. A diagnostic procedure such as polysomnography or home sleep apnea testing should be ordered if clinical suspicion exists. Screening of every patient could be done since the pre-test probability is very high. Follow-up by periodic re-evaluation of OSA is warranted at least 1 year after successful treatment74. Attention to weight gain and physiological hormonal replacement in the post-treatment period may help improve OSA13.
Pulmonary function abnormalities. No definite management concerning respiratory insufficiency exists. Biochemical remission does not seem to improve lung function parameters in most patients39. Pulmonary function tests (spirometry and diffusing capacity) may be ordered in a patient with unexplained shortness of breath or decreased exercise capacity, but cardiovascular dysfunction may also be a cause. No specific treatment has been proposed and tested in this population, but either inhalotherapy or other treatment modalities may be individualized according to findings.
Acromegaly is a chronic disease with multiple physical and psychological complications that carry a significant disease burden. Most patients have joint pain, headaches, low energy and libido, and morphological changes such as craniofacial deformation. These changes may not be completely reversible after treatment and can negatively affect QoL75. The comorbidities and active symptomatology also negatively affect patient productivity and entail high costs related to sick leave, unemployment, or disability76,77. Other factors, such as cognitive dysfunction and restless leg syndrome, are more prevalent in patients with acromegaly and are associated with poor QoL78,79. Moreover, diagnosis delay and acceptance of the disease could be challenging for patients80,81. Figure 1 (Gadelha et al.) summarizes the factors affecting QoL in acromegaly13.
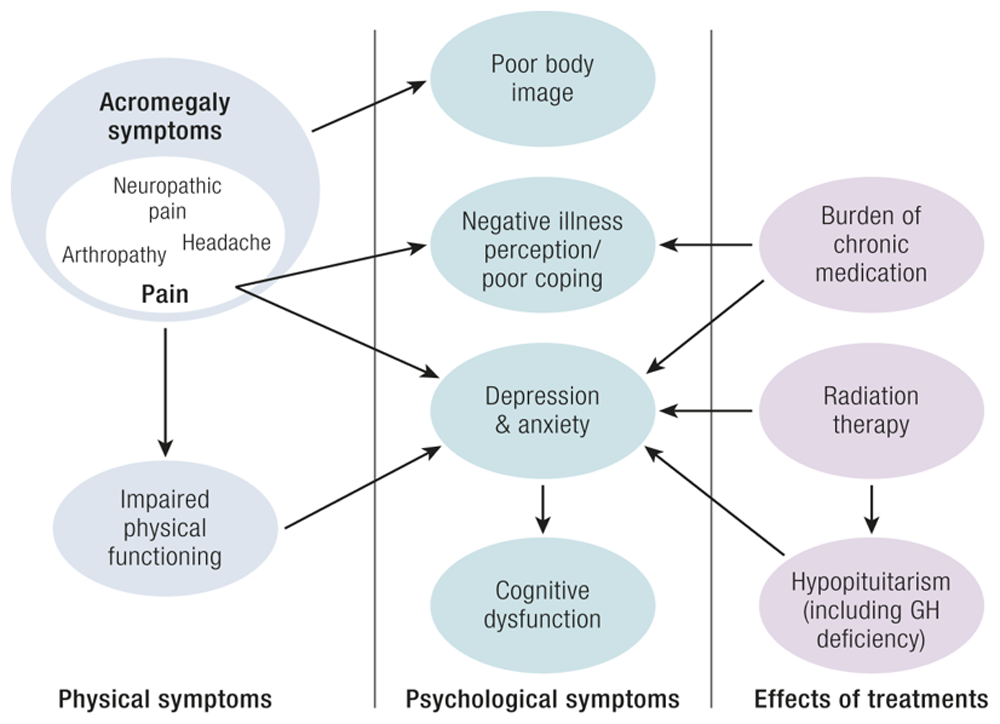
GH, growth hormone. The figure was reproduced from Gadelha MR et al., Systemic Complications of Acromegaly and the Impact of the Current Treatment Landscape: An Update, Endocrine Reviews, 2019, Volume 40, Issue 1, Pages 268–33213 by permission of the Endocrine Society.
QoL is defined as the patient’s perception of his or her physical and mental health, cognitive function, anxiety, depression, and subjective feelings of energy levels. QoL is considered an important endpoint in the evaluation of patients with acromegaly. It can be measured with questionnaires designed for use in the general population or aimed at specific disease dimension conditions, such as disease-generated or -specific questionnaires like the Acromegaly Quality of Life (AcroQoL) questionnaire75,82. Table 2 presents brief details of the following questionnaires: the Symptom Questionnaire83,84, Signs and Symptoms Score85,86, the Patient-Assessed Acromegaly Symptom Questionnaire85,86, Short-Form Health Survey (SF-36)87–89, the Multidimensional Fatigue Inventory (MFI-20)90,91, the Nottingham Health Profile92,93, Health-Related Quality of Life12,94, Psychological General Well-Being82,95, the Hospital Anxiety and Depression Scale96,97, the AcroQoL Questionnaire75,82,96,98, and the Acromegaly Treatment Satisfaction Questionnaire (Acro-TSQ)99,100.
| Questionnaires to evaluate quality of life in acromegaly | Description |
|---|---|
| Symptom Questionnaire | The Symptom Questionnaire is a 92-item questionnaire assessing four scales: anxiety, depression, somatic symptoms, and anger/hostility. Subjects indicate the frequency of symptoms in the past week on a scale from absent to very often. Higher scores indicate greater symptom severity. This questionnaire may be more sensitive in discriminating between distress levels than other tests83,84. |
| Signs and Symptoms Score (SSS) | The SSS questionnaire is designed specifically to measure acromegaly-related signs and symptoms such as headache, perspiration, joint pain, fatigue, and soft tissue swelling. Each of those five questions is evaluated by a score from 0 (absent) to 8 (severe) for a maximum of 40 indicative, very severe clinical manifestations85,86. |
| Patient-Assessed Acromegaly Symptom Questionnaire (PASQ) | The PASQ is a disease-specific questionnaire composed of seven questions. The first six questions evaluate physical symptoms (headache, perspiration, joint pain, fatigue, soft tissue swelling, and paresthesia of the extremities) and a seventh question evaluates overall well-being. Each aspect is scored from 0 to 8; a lower score reflects low disease impact and a higher score reflects a more severe disease burden85,86. |
| Short-Form Health Survey (SF-36) | The SF-36 is a validated self-administered questionnaire that evaluates physical and psychological symptoms and their impact on daily life. Eight domains are explored: physical functioning, role limitations due to physical functioning, general health perception, pain, vitality, emotional well-being, role limitations due to emotional health, and social functioning. SF-36 consists of 36 questions answered with either “yes/ no” responses or scaled responses ranging from three options (“yes, limited a lot”, “yes, limited a little”, or “no, not limited at all”) to six options (“all of the time”, “most of the time”, “a good bit of the time”, “some of the time”, “a little of the time”, or “none of the time”). Higher scores indicate a better quality of life87–89. |
| Multidimensional Fatigue Inventory (MFI-20) | The MFI-20 contains 20 statements to assess fatigue. There are five different dimensions of fatigue and each contains four items. The dimensions are general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue. Items are rated on a scale from 0 (absent) to 5 (extremely). A higher score indicates more fatigue experienced90,91. |
| Nottingham Health Profile (NHP) | The NHP is frequently used in patients with pituitary disease to assess general well-being and QoL. This survey consists of 38 “yes/no” questions that are subdivided into six scales assessing impairments: pain (eight items), energy level (three items), sleep (five items), emotional reactions (nine items), social isolation (five items), and disability/functioning (i.e., physical mobility) (eight items). A higher score is associated with more impairment92,93. |
| Health-Related Quality of Life (HRQoL) | This is a 15-item, standardized, self-administered HRQoL instrument that can be used both as a profile and as a single-index score measure. The 15 items evaluated are moving, seeing, hearing, breathing, sleeping, eating, speech, eliminating, usual activities, mental function, discomfort and symptoms, depression, distress, vitality, and sexual activity. For each item, the patient chooses one of the five levels that best describes his or her state of health at that moment12,94. |
| The Psychological General Well-Being (PGWB) | The PGWB Index is a 22-item questionnaire that evaluates six subscales: anxiety, depressed mood, positive well-being, self-control, general health, and vitality. Each item has six questions, which are scored from 0 to 5. A score of 110 represents a perfect QOL82,95 |
| Hospital Anxiety and Depression Scale (HADS) | This is a 14-item questionnaire evaluating anxiety and depression. Each item is scored on a 4-point scale. Scores range from 0 to 21 for the anxiety and depression subscale and from 0 to 42 for the total score. Higher scores indicate higher levels of anxiety and depression96,97. |
| AcroQoL Questionnaire | The AcroQoL Questionnaire has been used and validated in randomized clinical trials, leading to a better understating of the specific impairments in acromegaly. This questionnaire consists of 22 questions that evaluate physical and psychological dimensions. Answers are recorded on a scale from 1 (always or completely agree) to 5 (never or completely disagree). The maximum score of 110 reflects best possible QoL and is quoted as a percentage (e.g., 100%). The 22 items evaluate physical symptoms such as pain and fatigue and psychological function, such as impact on appearance, performance, and personal relationships75,82,96,98. |
| Acromegaly Treatment Satisfaction Questionnaire (Acro-TSQ) | This questionnaire is a novel tool to simultaneously evaluate patient satisfaction on disease and treatment with somatostatin receptor ligand. It measures treatment effectiveness, symptom burden, treatment side effects, convenience of treatment, and overall satisfaction over the past 4 weeks99,100. |
A systematic review confirmed that AcroQoL scores are significantly impaired in patients with acromegaly101. Geraedts et al. showed that the most significant factors associated with poor QoL were depression and higher BMI102. Other predictors have been inconsistently identified; longer disease duration, painful syndrome (musculoskeletal or headache), diabetes, older age, female gender, having received treatment with radiotherapy, and becoming GH-deficient after treatment75,102,103. Successful therapy might improve QoL but may not normalize QoL completely, even after biochemical cure75,104–107. Indeed, QoL has been shown to be severely impaired years after successful treatment in patients with acromegaly compared with the general population104,108,109.
Numerous studies have looked at the impact of different treatment modalities in first- or second-line therapies. Pituitary surgery compared with medical treatment seems superior in improving QoL in patients with acromegaly101 as in other types of pituitary tumor110. A French study found that patients who underwent surgery had a better QoL than if they had received medical treatment only (65 ± 18% versus 54 ± 14%, P = 0.009), and investigators concluded that neurosurgery was associated with greater improvement in QoL when compared with medical therapy alone111. Overall, greater GH suppression could be attained with surgery than with somatostatin receptor ligand (SRL) injections112. Indeed, patients with remission after surgery could avoid medication side effects and consequences of suboptimal biochemical control.
Types of medical therapy used rendered heterogeneous results on QoL, see Table 3113–125. Some studies with first-line SRL injections in treatment-naïve patients show improved QoL in patients who received long-acting lanreotide114,115, but the results were controversial106,116,124,126. On the other hand, a multicenter study showed that two thirds of patients controlled on SRL injections reported ongoing incommoding acromegaly symptoms127. In that study, gastrointestinal disturbances were very frequent (73%); this was possibly related to SRL side effects or disease activity itself127. A new questionnaire, Acro-TSQ, was developed to evaluate QoL specifically in patients who received injectable SRLs and provides further insight99.
| Therapy | Publication | Number of patients | Follow-up, Weeks | Disease status (for majority of patients) | Medical treatment in control group | Scales used | Effect on quality of life |
|---|---|---|---|---|---|---|---|
| Lanreotide Autogel | Sonino et al. (1999)113 | 10 | 8 | Uncontrolled | Octreotide LAR or bromocriptine | KSQ, CSKSLPP | ↑↑ |
| Lanreotide Autogel | Lombardi et al. (2009)114 | 51 | 52 | Uncontrolled | Treatment naïve | NHP | ↑↑ |
| Lanreotide Autogel | Caron et al. (2016)115 | 90 | 48 | Uncontrolled | Octreotide LAR | AcroQoL, PASQ | ↑↑ |
| Lanreotide Autogel | Schopohl et al. (2011)116 | 37 | 26–52 | Controlled | Octreotide LAR | AcroQoL | ↔ |
| Octreotide LAR intensification | Mangupli et al. (2014)117 | 28 | 24 | Uncontrolled | Treatment naïve or Octreotide LAR | AcroQoL | ↑↑ |
| Octreotide LAR | Chin et al. (2015)118 | 58 | 24 | Uncontrolled | Treatment naïve | AcroQoL | ↑ |
| Octreotide LAR | Ghigo et al. (2009)119 | 57 | 48 | Uncontrolled | Treatment naïve | AcroQoL, SSS | ↑↑ |
| Octreotide LAR every 6 weeks | Biermasz et al. (2003)120 | 14 | 36 | Controlled | Octreotide LAR every 4 weeks | NHP | ↔ |
| Octreotide LAR or Lanreotide Autogel intensification | Dal et al. (2018)121 | 61 | 52 | Controlled | Octreotide LAR or Lanreotide Autogel | AcroQoL, PASQ | ↔ |
| Pasireotide | Bronstein et al. (2016)122 | 119 | 52 | Uncontrolled | Octreotide LAR | AcroQoL | ↔ |
| Pegvisomant | Ghigo et al. (2009)119 | 56 | 48 | Uncontrolled | Treatment naïve | AcroQoL, SSS | ↑↑ |
| Pegvisomant | Trainer et al. (2009)123 | 52 | 40 | Uncontrolled | Octreotide LAR | AcroQoL, EQ-5D | ↑↑ (only AcroQoL) |
| Pegvisomant weekly or twice weekly | Higham et al. (2009)128 | 7 | 32 | Controlled | Pegvisomant daily | AcroQoL | ↔ |
| Pegvisomant - Octreotide LAR | Trainer et al. (2009)123 | 53 | 40 | Uncontrolled | Octreotide LAR | AcroQoL, EQ-5D | ↑↑ (only AcroQoL) |
| Pegvisomant - Octreotide LAR | Neggers et al. (2008)124 | 20 | 36 | Controlled | Octreotide LAR | AcroQoL, PASQ | ↑↑ |
| Pegvisomant - Octreotide LAR | Madsen et al. (2011)125 | 18 | 24 | Controlled | Octreotide LAR | EQ-5D, PASQ | ↔ |
↑, improvement in quality of life (QoL) subscale only; ↑↑, improvement in global QoL; ↔, no significant change in QoL; CSKSLPP, Cognitive Scale of Kellner’s Screening List for Psychosocial Problems; EQ-5D, European quality of life scale; KSQ, Kellner’s Symptom Questionnaire (psychological distress and well-being); LAR, long-acting release; NHP, Nottingham Health Profile; PASQ, Patient-Assessed Acromegaly Symptom Questionnaire; SF-36, Short-Form Health Survey; SSS, Signs and Symptoms Scale—acromegaly. This table was adapted from Gadelha MR et al., Systemic Complications of Acromegaly and the Impact of the Current Treatment Landscape: An Update, Endocrine Reviews, 2019, Volume 40, Issue 1, Pages 268–33213 by permission of the Endocrine Society.
Changing long-acting release (LAR) octreotide to pasireotide LAR did not improve QoL even if further disease control was obtained122. On the contrary, in one study, adding pegvisomant to SRLs led to improvement of QoL and clinical symptoms despite not showing any change in circulating IGF-1124,128, but studies with a similar design were negative125,129. This might suggest that improving biochemical control with a favorable safety profile and ease of administration would improve patient QoL. However, further research is required in this area.
Radiotherapy is usually indicated for patients with more aggressive cases of acromegaly, in particular those with residual disease after surgery and medical therapy75. Patients who underwent radiotherapy reported that it had a negative influence on energy, pain, social isolation, physical fatigue, and activity and motivation measured with the MFI-20130. During follow-up, the scores in 5 out of 26 QoL subscales significantly worsened75,90. This might be due to a direct brain effect induced by radiotherapy, by new hormonal deficits, by delay in biochemical control of excess GH, or by the disease severity itself or by a combination of these factors. Nonetheless, the results are important to take in account in the decision to irradiate a tumor, especially in young patients.
Development of GH deficiency after treatment of acromegaly has also been shown to affect QoL. Wexler et al. found that the mean scores on the Quality of Life Adult Growth Hormone Deficiency Assessment (QoL-AGHDA), SF-36, and Symptom Questionnaire Depression showed significantly impaired QoL in the GH-deficient group when compared with the GH-sufficient group. Peak GH levels after GH-releasing hormone-arginine stimulation were inversely associated with QoL-AGHDA scale scores (R = −0.53; P = 0.0005) and Symptom Questionnaire Depression subscale scores (R = −0.35; P = 0.031)131.
Osteoarthritis is a major cause of pain in acromegaly. Although improvement of hypertrophic articular and periarticular tissues has been noted with normalization of GH/IGF-1,132 degenerative joint disease is somewhat irreversible, and arthralgias might not resolve even after biochemical remission133–135. Fifty-eight patients with acromegaly were evaluated with the SF-36, Arthritis Impact Measurement Scales 2 (AIMS2), and AcroQoL questionnaires; 52 (90%) reported musculoskeletal pain, 29 (50%) reported neck pain, 49 (84%) had hip osteoarthritis, and 20 (34%) reported knee osteoarthritis. The SF-36, AIMS2, and AcroQoL scores were lower in patients with musculoskeletal pain. The conclusion was that patients with acromegaly had a significantly higher number of musculoskeletal problems that negatively affect their QoL at different levels136. Female patients and those with higher BMI seem to be the most affected137. In regard to vertebral fractures, no definite association has been observed with QoL; this is probably because many events are asymptomatic and many are missed unless patients had vertebral x-ray rather than bone densitometry138.
QoL is an important treatment objective besides biochemical remission and tumor control. Close attention to patient perception of wellness is essential and should prompt treatment modifications. Use of specific questionnaires helps to identify areas of improvement and monitor therapy effects. Other interventions such as cognitive behavioral therapy with a technique called “Think healthy and feel the difference” have proven sustained improvement over 9 months. 139 Moreover, physical activity programs140, and increased knowledge about the disease from physician-guided discussion groups or patient associations may also help to improve patient’s QoL141.
Recent population studies showed a longer life span for patients with acromegaly; this was most likely due to improvements in treatments, as the average delay in diagnosis seems to be largely unchanged over the decades. Raising awareness of complications of acromegaly even in biochemically controlled disease and introducing adequate screening programs in accordance with Acromegaly Consensus guidelines10,74 should improve both morbidity and mortality. Patient-reported outcome and QoL have become essential components of monitoring the disease, and improving QoL is an endpoint of the treatment in itself. Although many questionnaires and scales have been developed, more research is needed to determine how best to measure QoL in patients with pituitary disease in general and with acromegaly in particular.
The authors thank Shirley McCartney, PhD (Oregon Health & Sciences University Portland, Oregon, USA) for editorial assistance.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 29 Jul 20 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)