Keywords
Acne, biology, sebaceous gland, hair cycle, gene, immunology, wound healing, post inflammatory hyperpigmentation, vitamin C
Acne, biology, sebaceous gland, hair cycle, gene, immunology, wound healing, post inflammatory hyperpigmentation, vitamin C
Acne vulgaris (acne) is an inflammatory disease of the pilosebaceous gland1. It initially forms invisible micro (histopathological) comedones, then often appears in adolescents on the forehead as visible, clinically recognized blackheads or whiteheads (comedones) that develop into inflammatory red papules or pustules. Such lesions can become complicated with either atrophic or hypertrophic scars. Acne can develop into refractory cysts, nodules, and subcutaneous fistulas that are resistant to therapy. Acne most commonly appears on the face, neck, chest, and upper back, where sebaceous follicles predominate. In addition to acne vulgaris, related disorders include follicular occlusive diseases such as acne conglobata, perifolliculitis capitis abscedens et suffodiens (PCAS) (dissecting cellulitis or Hoffman disease), and hidradenitis suppurativa (acne inversa). These are all refractory and therapy-resistant diseases and commonly manifest as hypertrophic scars, cysts, and nodules.
The following factors are considered to be important to the classical etiology of acne vulgaris1: increased sebum excretion rates, endocrinological factors such as androgens, abnormal keratinization of the follicular infundibulum, bacterial proliferation, and subsequent inflammation. Recent genetic and immunological studies have now elucidated the pathogenesis of acne (Figure 1).
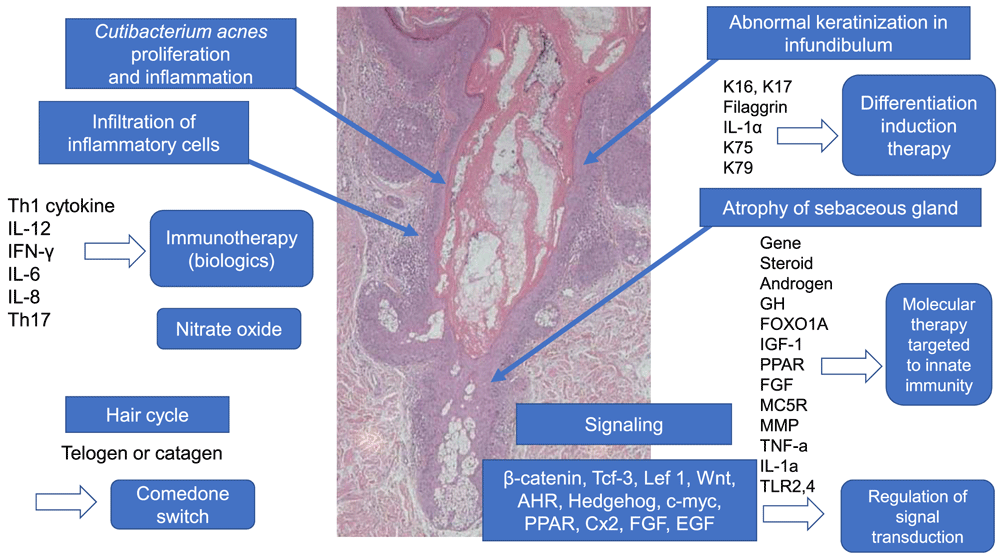
Atrophy of the sebaceous gland is induced by signal transduction due to various factors. In the hair cycle, anagen in normal skin changes to telogen or catagen in the “comedo switch”. Keratin (K) 75 and K79, which are present in the companion layer of normal hair follicles, are reduced in comedo. Immunological aspects show a T helper type 1 (Th1) and Th17 shift in acne. Diversified multiple genes are implicated in acne pathogenesis. According to the above-mentioned etiology, immunity induction therapy, molecular target therapy targeted on innate immunity, and the regulation of signal transduction could be alternative candidates for acne treatment in the future. AHR, aryl hydrocarbon receptor; Cx2, cyclooxygenase-2; EGF, epidermal growth factor; FGF, fibroblast growth factor; FOXO1A, forkhead box transcription factor class O1A; GH, Growth hormone; IFN, interferon; IL, interleukin; Lef, lymphoid enhancer-binding factor; MC5R, melanocortin 5 receptor; MMP, matrix metalloprotease; PPAR, peroxisome proliferation-activated receptor; Tcf, transcription factor; TLR, Toll-like receptor; TNF, tumor necrosis factor; Wnt, Wingless. This image was produced from the author’s clinic for this review.
Acne is a disease of sebaceous hair follicles that comprises multilobulated large sebaceous acini, tiny vellus hairs, and dilated follicular channels2. Sebaceous follicles are located in the face, chest, and upper back. The sebaceous gland is atrophic in acne lesions (Figure 1), meaning that sebum has already been discharged into follicular channels because of undifferentiated sebocytes becoming mature and differentiated. Cunliffe proposed cyclical comedo growth and explained the natural resolution of comedones3.
Stem cells in hair bulges can differentiate into sebocytes and outer root sheaths4. The progenitors of sebaceous glands in the junctional zone between the sebaceous duct and outer root sheath are leucine-rich repeat and immunoglobulin-like domain 1 (LRIG1)+ cells5 that can potentially differentiate into isthmus (epithelial) and sebaceous glands. These cells can also differentiate into sebocytes, sebaceous duct cells, and infundibular keratinocytes depending on the influence of diverse factors. We speculate that undifferentiated sebocytes in acne differentiate into sebaceous duct cells and infundibular keratinocytes instead of mature sebocytes, resulting in the abnormal keratinization of follicular channels. Saurat refers to this phenomenon as a “comedo switch”5. Saurat also stated that not all follicles are involved in acne of the face, neck, and trunk and that only 0.25% of sebaceous glands are involved in visible acne lesions5.
With respect to the molecular network signaling pathway, β-catenin, transcription factor (Tcf) 3, and lymphoid enhancer-binding factor (Lef)-1 are important4. High β-catenin levels stimulate hair follicle formation, whereas low levels stimulate the epidermis and sebaceous gland. Lef-1 cooperates with Indian hedgehog to control the proliferation and differentiation of sebaceous gland progenitors4. Peroxisome proliferation-activated receptor (PPAR), c-Myc, and Cx-2 differentiate towards sebocytes. The repression of β-catenin and activation of c-Myc and the hedgehog signaling cascade promote the differentiation and maturation of sebocytes6.
In addition, Wingless (Wnt) and hedgehog signals are important because the activation of Wnt signaling in LGR5+ bulge cells promotes hair growth. Loss of Wnt signaling in bulge cells expressing keratin (K) 15 promotes migration and differentiation into sebocytes. Wnt signaling regulates the proliferation and specification of junctional LRIG1+ cells, resulting in acne pathogenesis7. Androgens might promote sebocyte differentiation and inhibit Wnt signaling7. Aryl hydrocarbon receptors (AHRs) are linked to chloracne7. Exposure to AHR causes comedo formation, sebaceous gland atrophy, and the upregulation of AHR expression in humans and mice7. AHR inhibit sebaceous differentiation by promoting the differentiation of junctional zone stem cells into infundibular keratinocytes7. Fibroblast growth factor (FGF) 2 stimulates proliferation through the pilosebaceous unit7, and epidermal growth factor receptors (EGFRs) can enlarge the hyperproliferation of sebaceous glands and increase sebum production7.
The formation of acne is attributable to a decrease in linoleic acid in classical etiology1. Squalene peroxidated by lipoperoxidase and decreased vitamin E can induce inflammation. Lipoperoxidase can induce pro-inflammatory cytokines and keratinocyte proliferation and activate PPAR8. Monosaturated fatty acids can induce changes in keratinocyte proliferation and differentiation. AHR is metabolized to tetrachlorodibenzodioxin, which induces sebocytes to undergo epithelial-type differentiation. Insulin-like growth factor (IGF) 1 stimulates the formation of unsaturated lipids and neosynthesis of lipids9. The IGF axis is involved in acne pathogenesis10.
Recently, 11β-hydroxysteroid dehydrogenase type I (11β -HSD1) has been observed to promote lipid synthesis. Tumor necrosis factor (TNF)-α promotes lipogenesis in human sebocytes11. Transforming growth factor (TGF)-β maintains sebocytes in an undifferentiated state and decreases lipid accumulation12.
Recent genetic findings have clarified that genes encoding the enzymes 21-hydroxylase (CYP21A2), steroid 5α-reductase type I (SRD5A1), and androgen receptor (AR), the somatotropic axis (GH1, GHR, IGF1, IGFBP3, and IGF1R), and the forkhead box transcription factor class O1A (FOXO1A), peroxisome proliferator-activated receptor (PPARA, PPARB, PPARG, PPARD), FGF-2 (FGF2), melanocortin receptor (MC5R, MC1R), matrix metalloprotease (MMP1, MMP2, MMP3, MMP9, MMP13), TNF-α (TNF), IL-1α (IL1A), and Toll-like receptors (TLR2 and TLR4) are implicated in the pathogenesis of acne13,14.
Abnormal keratinization is an important factor in acne pathogenesis1. Expression of the hyperproliferative keratins (K6, K16, and K17) is increased in acne lesions15. Significant filaggrin expression in the infundibulum is closely associated with the abnormal keratinization involved in acne16. IL-1α is involved in abnormal keratinization17, and inflammation precedes keratinization1.
Hair cycles in acne have not been studied in detail. van Scott et al. stated that the hair cycles in acne are almost always either telogens or catagens18. However, keratin expression in the hair cycle in acne lesions has not been studied. A low microcomedo index in acne is associated with significantly higher K75 expression19. K75 is expressed in the companion layer between the inner (Henle) layer and outer root sheaths in the lower portion of normal hair follicles20. A companion layer is found in anagen but not in either telogen or catagen hair follicles. The prevalence of K75 depends on the hair cycle. Therefore, the hair cycle in acne with microcomedones is directed towards the follicular infundibulum and sebaceous duct instead of the lower portion of hair follicles that comprise companion layers. In addition, K79 is downregulated in comedonal acne lesions21 and is expressed in companion layers in normal anagen hair follicles19. Considering homeostasis in hair cycles in acne, progenitor cells in the junctional zone might differentiate not towards the outer root sheath of hair follicles below the sebaceous duct but towards the infundibulum and sebaceous duct cells in acne.
The immunological aspects of acne have become noteworthy. Malassezia and Demodex are related to the pathogenicity of folliculitis and rosacea, respectively. Cutibacterium acnes (C. acnes) and normal flora are involved in acne pathogenesis due to overgrowth in closed follicles. In acne, C. acnes in the follicular channel stimulates Langerhans cells in the infundibulum via TLR-2, resulting in the production of IL-12 and IL-8 by activating Th1 cells. C. acnes also stimulates follicular keratinocytes in the infundibulum via TLR-2, resulting in the production of IL-6 and IL-81,22 followed by the formation of inflammatory lesions such as red papules and pustules. Jeremy et al. proposed that initial inflammation caused by CD4, CD3, and macrophages induces comedones23: inflammation precedes keratinization23. A T helper type 1 (Th1) shift occurs in acne lesions, and Th1-positive cells are more prevalent in acne lesions than in normal skin24. From the aspect of host responses to C. acnes in acne pathogenesis, host immunological factors against C. acnes produced by PBMCs can be attributed more to bacteriological factors25.
In addition to Th1 cytokines, Th17 is also involved in acne pathogenesis. C. acnes is a potent inducer of Th17 and Th1, and significant numbers of cells express IL-17 in acne lesions26. IL-17 is reduced by vitamins A and D. IL-1β and TNF-α are involved in acne inflammation11. Corticotropin-releasing hormone (CRH) can increase IL-6 and IL-8 levels in vitro12. Therefore, regulating these cytokines might offer an alternative strategy for treating acne.
Acne has been treated with benzoyl peroxide (BPO) formulations, adapalene, antimicrobials, anti-androgen agents, and isotretinoin, which control abnormal follicular keratinization in the infundibulum, have bactericidal and bacteriostatic effects, inhibit inflammation, and decrease sebum excretion, according to the pathomechanism of classical etiological factors. From these immunological aspects in acne, Th1, Th17, and TNF-α are upregulated in acne.
Antimicrobials are useful in immunoregulation because they show not only antibacterial but also anti-inflammatory activities. However, antimicrobial use might increase bacterial resistance to antimicrobials and cause dysbiosis as well as side effects. Novel medicines without these adverse effects have been developed. Vaccination with antibodies against the Christie-Atkins-Munch-Petersen (CAMP) factor, which is associated with C. acnes cytotoxicity, decreases the growth of C. acnes and the production of murine MIP-227. The ability of a vaccine produced by Staphylococcus capitis E12 to prevent C. acnes overgrowth has been investigated28. A vaccine produced by S. capitis is expected for preventing C. acnes overgrowth and killing overgrown C. acnes. To suppress the inflammation completely, monoclonal antibodies blocking cytokines are needed because killed C. acnes induces inflammatory cytokines29.
Th1 and Th17 shift inhibitors and antibodies against IL-17 and TNF-α might offer alternative approaches to treating acne. Notably, a TNF-α antibody is presently used to treat acne conglobata30, hidradenitis suppurativa31, and PCAS32. Antibodies to IL-17, IL-23, and IL-1a will be used to treat acne conglobata, PCAS, and hidradenitis suppurativa, which will also be treated with apremilast33. Nitrate oxide can be an alternative treatment for acne in humans by reducing IL-1β, IL-8, TNF-α, and IL-6 induced by monocytes and IL-8 and IL-6 induced by keratinocytes via innate immunity34,35.
The most important complication in acne is scar formation. The rupture and breakdown of inflammatory red papules, pustules, and deep-seated subcutaneous abscesses in the deep dermis can lead to erosion and ulceration, resulting in scar formation. If ulceration is superficial, re-epithelialization proceeds without scarring. However, ulcers located deep below the reticular dermis form scars like deep dermal burns. Atrophic scars have been classified as icepick, boxcar, and rolling scars36, whereas elevated hypertrophic scars like acne conglobata sometimes form. Wound healing in acne should be taken into account when considering scar formation37. Scars form because of persistent inflammation and are associated with the depth of inflammation38. Scar formation is also associated with MMP, IL-6, TGF-β, macrophages, and B cells. Atrophic scars are dependent on B cells and macrophages39. Sebaceous duct cells can differentiate into epidermal keratinocytes and sebocytes in wounds. Thus, sebaceous ducts are bimodal, which is consistent with keratin expression between the infundibulum and sebocytes16.
Postinflammatory hyperpigmentation (PIH) is an important complication of acne vulgaris that occurs via damage to the basal cell layer. Non-surgical chemical glycolic acid (GA) peels and subsequent iontophoresis using vitamin C, vitamin A, and vitamin E are used to treat PIH, postinflammatory erythema (PIE), and atrophic scars37. GA loosens cellular adhesion, promotes loss from the cornified layer, as well as the regeneration of epidermal and dermal tissues, removes follicular cast in the infundibulum, and de-roofs pustules and red papules37. Vitamin C promotes re-epithelialization while inhibiting melanogenesis and reactive oxygen37. Vitamin C can induce self-renewal of the mesenchymal cell cycle program and fibroblast motility, promote fibroblast migration, confer anti-inflammatory effects, and induce macrophage inflammation40. Both PIH and PIE are treated using the vitamin C derivative, amphipathic vitamin C41,42. The mechanisms through which vitamin C improves atrophic scars are thought to be via self-renewal cell cycle progression, promoted fibroblast migration, matrix deposition and neo-vascularization, anti-inflammatory effects in macrophages, and attenuation mediators in wounds via IL-1β and TNF-α40. In addition, basic FGF (bFGF), an important factor in wound healing, can promote epithelialization and thus improve atrophic scars, resulting in flattening of the epidermis43. bFGF supplies epidermal defects with proliferating keratinocytes.
The pathogenesis of refractory nodulocystic lesions remains unclear. Significant amounts of filaggrin are expressed in cyst formation with retention hyperkeratosis44. Nodulocystic acne and acne conglobata are treated with isotretinoin45, but not in Japan. Kampo (traditional Japanese herbal medicine) such as Saireito can be very effective sometimes for treating nodulocystic acne46 and PCAS47. It works on cystic and alopecia lesions in PCAS, resulting in hair growth47. Hair cycles in PCAS are telogens or catagens as in acne vulgaris. Saireito exerts multiple effects on endogenous corticosteroids, inflammation, reactive oxygen species, coagulation, macrophages, neutrophils, and endothelial cells46. However, the mechanism of Saireito in acne pathogenesis awaits investigation in a basic research study.
Isotretinoin and anti-androgen therapy have not been applied in the treatment of acne in Japan. Therefore, severe acne is treated with oral antimicrobials combined with Kampo, topical BPO, and local steroid injections48. Although the mechanism of Kampo is not clear, Saireito can suppress Th1 shift and suppress B cell function, regulating Th1/Th2 balance in mice49. Jyumihaidokuto is available for acne vulgaris in Japan50. It contains Bokusoku, which inhibits rat 5α-reductase activity and testosterone-induced sebum synthesis in hamster sebocytes51. Keigairengyoto is also available for acne vulgaris in Japan50. It has anti-bacterial effects against C. acnes52 and inhibits reactive oxygen53. Comprehensive multiplexed therapy is recommended for therapy-resistant refractory acne.
This overview describes recent advances in acne pathogenesis. Understanding the multiple diverse, interdependent, and complicated etiological factors and the biology of sebaceous glands is extremely important. Progenitor cells in the junctional zone can differentiate into infundibulum, sebocytes, and outer root sheaths depending on the influence of genes and molecular signals. The altered hair cycle in acne remains of interest from the viewpoint of the biological homeostasis of pilosebaceous units. Immunological studies of acne have generated innovative immunity induction therapies such as antibodies to TNF-α and various cytokines. The most refractory complication of acne is scar formation, which involves the production of pro-inflammatory cytokines such as IL-1α, IL-1β, IL-6, TNF-α, and TGF-β54.
According to the etiopathogenesis of acne, potential treatments for acne in the future are topical anti-androgens, melanocortin receptor antagonists, IGF-1 inhibitors, PPAR modulators, acetylcholine inhibitors, topical retinoic and metabolism-blocking agents, monoclonal antibodies, antimicrobial peptides, antioxidants, phosphodiesterase inhibitors, IL-1β inhibitors, vitamin D analogues, dapsone, systemic antiandrogens, and immunotherapy55.
Immunity induction and wound healing therapies will become key strategies for preventing acne scars. Acne is a disease of the pilosebaceous unit. Based on acne pathogenesis, regulation of sebocyte differentiation is a novel therapeutic procedure56,57.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 29 Jul 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)