Keywords
MnO nanoparticles, epithelial cells, gastrointestinal, inflammatory bowel disease, mitochondrial function
This article is included in the Nanoscience & Nanotechnology gateway.
This article is included in the Iowa State University collection.
MnO nanoparticles, epithelial cells, gastrointestinal, inflammatory bowel disease, mitochondrial function
Manganese oxide nanoparticles (MnO NP), intestinal epithelial cells (IECs), gastrointestinal (GI), inflammatory bowel disease (IBD), magnetic resonance imaging (MRI), tight junction (TJ), oxygen consumption rates (OCR), interleukin 6 (IL-6), reactive oxygen species (ROS)
The gut mucosa continuously encounters a barrage of biological challenges including those that are derived from the host, microbial and foreign/food antigens, environmental toxicants, and/or biological toxins. The intestinal epithelial barrier is also the first line of physical defense in the intestine. Intestinal epithelial cells (IECs) sense foreign and endogenous danger signals and plays an important role in the maintenance of mucosal immune homeostasis by regulating inflammation and restricitng the uptake of phlogistic components from the lumen1–4. Consequently, IECs are at the forefront of the physiological barrier separating the host from the inflammatory components present in the intestinal lumen. As the intestinal barrier is constantly faced with steady insult from microbial and other environmental influences, there are many challenges that affect the maintenance of mucosal homeostasis. For example, the resident microbiota, that inhabit the gut, plays a critical role in several IEC processes, including the rate of intestinal epithelial cell turnover, promotion of epithelial restitution after injury, and reorganization of tight junction (TJ) proteins which all contribute to a healthy epithelial barrier3. However, there are also deleterious outcomes that can be induced by microbial components and excreted proteins. Microbial enterotoxin have been shown to interact and interfere with TJ integrity which negatively affects the epithelial integrity by increasing the exposure of the host to microbial-derived inflammatory components4.
As chronic inflammation is one of the main pathological features of inflammatory bowel disease (IBD), the intestinal epithelial barrier integrity plays a major role in maintaining a balance between host–microbial interactions that can affect susceptibility to and the pathogenesis of IBD5. Disruption of the intestinal barrier, along with increased levels of proinflammatory cytokines, has been shown to lead to or be present at the onset of intestinal inflammation and disorders including IBD6–8. The presence of proinflammatory cytokines in a healthy intestine are beneficial (i.e., cytoprotective) based on their role in regulating a variety of biological processes including IEC activation, replication, differentiation, as well as the regulation of innate and adaptive immunity9. However, the imbalance between pro-inflammatory and anti-inflammatory cytokines impedes the resolution of inflammation and adversely affects the maintenance of the intestinal homeostasis leading to the perpetuation of disease and tissue destruction10.
Nanotechnology is a rapidly growing field that has been shown to provide many advantages in various areas of medicine. With the goal to improve several technological areas, such as drug delivery and magnetic resonance imaging, the usage of nanoparticles, specifically metal nanoparticles including manganese oxide nanoparticles (MnO NP), have played in important role in advancing these fields11–16. Despite the potential benefits, there are also concerns about the induction of adverse health effects17. With the growing concerns regarding the unintentional environmental effects of nanoparticles on the gastrointestinal (GI) tract, there needs to be further evaluation of the effects that MnO NP may have on biological systems18. For example, metal-based NPs could contribute to adverse health conditions resulting from alterations of gene expression, dysregulation of physiological processes due to improper intracellular trafficking, and accumulation of NPs at toxic concentrations19. More complete understanding of the basis of metal-based NPs toxicity is essential to elucidate the occupational, environmental, and health risk-assessments. Regardless of whether it is intentional or unintentional ingestion, the GI tract has been shown to be the primary route of entry for metal nanoparticles including MnO NP. Individuals can be exposed to MnO NPs as a result of their occupation (e.g. welding) or medical treatment (e.g. magnetic resonance imaging (MRI))20–22. Given the proximity to the GI lumen, assessing the effects of nanoparticles on IECs in the presence of bacterial components presents a more holistic model of exposure when assessing the effects of MnO NP on gut IECs as the gut microbiota influence cellular physiology, metabolism, and immune function23. However, published studies that take into consideration the interaction between host IECs, nanoparticles, and microbiota are extremely limited. Therefore, the objective of this study is to investigate the cytotoxicity of environmentally related MnO NP on IECs. To mimic the in vivo environment, these studies were designed to examine the interactions of IECs with MnO NP in conjunction with a bacterial lysate derived from E. coli LF82. Results indicate there are synergistic interactions between MnO NPs and bacterial components that adversely affect IEC function.
Manganese oxide nanoparticles (MnO NP (US Research Nanomaterials, Inc., Houston, TX, USA)) used in all experimental procedures were kindly provided by Dr. A. Kanthasamy (Department of Biomedical Sciences, Iowa State University, Ames, IA). Based on transmission electron microscopy the size of MnO NP were 16.8 ± 2.6 nm in diameter.
The MODE-K intestinal epithelial cell line generated from the small intestine of a C3H mouse was used in all experimental procedures described below (kind gift from Dr. C. Elson, Division of Gastroenterology and Hepatology, School of Medicine, University of Alabama at Birmingham, AL). In this study, MODE-K cells were grown in high glucose (4.5 g/L) DMEM medium containing 10% FBS, 2 mM L-glutamine, 50 units of penicillin, and 50 µg/mL streptomycin. In general, cells were inoculated into a tissue culture plate (6–96 wells) depending on the experimental requirements and were cultured overnight in a humidified atmosphere of 5% CO2 at 37°C. The length of each experiment and MODE-K cell density for each experiment has been provided in the methods section for each experimental procedure. For the majority of experiments, there were four treatment groups: control, 1 µg/mL E. coli LF82 lysate, 10 µg/mL MnO NP, and 10 µg/mL MnO NP plus 1 µg/mL E. coli LF82 lysate. At 24 hours before initiating experimental treatments, the culture medium was switched from high-glucose medium to low-glucose DMEM medium. Dose titration experiments were performed to identify the minimal dose of E. coli LF82 lysate and the MnO NPs that did not affect cell viability after 24 hours, and these doses were chosen to assess the interactions between the MnO NPs and E. coli LF82 lysate.
An adherent invasive E. coli (AIEC) strain LF82 that has been associated with ileal Crohn’s disease was cultivated in BHI broth at 37°C in an aerobic environment. Bacteria were harvested after overnight replication, washed with PBS, frozen, lyophilized, and stored at -20°C until used. The lyophilized bacterial cells were weighed and resuspended in sterile PBS (2 mg/mL) prior to being subjected to two freeze-thaw cycles, followed by sonication at 50 amps, 75 amps, and 100 amps for 30 seconds at each amperage. This sonication cycle was repeated three times. The lysates were sterilized by ultraviolet irradiation and sterility confirmed bacteriologically. With minor modifications, this method was used as previously described24.
Cell viability was measured using the CellTiter 96 Aqueous Non-Radioactive Cell Proliferation (MTS assay kit from Promega Corporation, Madison, WI). MODE-K cells were inoculated into 24-well plates with 120,000 cells/mL per well one day prior to treatment. The next day, MODE-K cell monolayers were washed twice with low glucose DMEM before addition of the respective stimulants diluted in low glucose DMEM medium and cultured for an additional 48 hours. Following treatment, 20 µL of MTS solution reagent mix was added to each well and incubated at 37°C for 30 minutes. At the end of incubation, readings were taken at a wavelength of 490 nm and another reference reading for each well was taken at 670 nm to eliminate background. This method was previously described and adopted to fit our experimental procedure25. Data shown is represented as the mean ± SEM collected from two independent experiments.
Prior to assessing cell viability, supernatants were collected from MODE-K cell cultures at 24 and 48 hours after addition of the MnO NP and/or E. coli LF82 lysate and analyzed for the presence of cytokines. A multiplex bead-based assay was used to measure the presence of IL-6, TNFα, and IFNγ. The assay was performed using Bio-Plex Pro COOH magnetic beads (Luminex Corp., Austin TX) conjugated with the following monoclonal antibodies (Fisher Scientific Co. LLC) to capture IL-6 (Cat # 14-7061-85; clone MP5-20F3), TNFα (Cat # 14-7313-81, clone AN-18), or IFNγ (Cat. # 14-7325-85; clone 1F3F3D4). Biotinylated monoclonal antibodies (Fisher Scientific Co. LLC) were used for detection of the bound (i.e., captured) IL-6 (Cat. # 13-7062-85; clone MP5-32C11), TNFα (Cat # 13-7326-85; clone MP6-Xt3), and IFNγ (Cat. # 13-7312-85; clone R4-6A2). Mean fluorescence intensity was measured using Strep-Avidin conjugated phycoerythrin (PE) (Fisher Scientific Company, LLC) and a Bio-Plex System 200 (BioRad Life Science, Hercules, CA). Standard curves were generated using commercially available cytokine standards (Peprotech, Inc., Cranbury, NJ) and Bio-Plex Manager Software Version 6.0 (Bio-Rad Laboratories, Inc., Carlsbad, CA, USA). Results are representative of two independent experiments containing six replicates per treatment.
For one-dimensional migration assays, 300,000 MODE-K cells per well were suspended in DMEM (low glucose, 1.0 g/L) inoculated into 12 well plates and incubated at 37 °C in 5 % CO2 overnight. The next day a (+) shaped scratch (i.e., monolayer wound) was created in each well using a P-200 pipette tip, creating a cell free gap that, in time, would be covered by replicating/migrating MODE-K cells. Treatments were then added and cells were then incubated for 24 hours. Images were taken at 0 and 24 hours post-wounding. The four treatment groups were: medium alone (i.e., control), 1 µg/mL E. coli LF82 lysate, 10 µg/mL MnO NP, and 10 µg/mL MnO NP plus 1 µg/mL E. coli LF82 lysate. After 24 hours, experiments were terminated. Images were taken using a 5x lens on an inverted microscope Leica DMi1 (Leica Microsystems, IL, US) using the software Leica Application Suite 4.5.0 (Leica Microsystems, IL, US) from 10 representative wounded areas per treatment. Images and gap spacing were then measured and analyzed with an automation and image analysis software Metamorph 5.0.7 (Molecular Devices LLC, CA, US). (ImageJ is a freely available alternative that can be used for the same purposes as Metamorph.) Gap closure was quantified by determining the mean distance of closure (i.e., healing) of the gap. This was done by measuring the area of the cell-free space separating the two edges of the cellular monolayer at 24 hours after addition of the treatments, and then subtracting this value from starting value that was measured at the 0-hour time point. This method was previously described and adopted to fit our experimental procedure26. Results are a representation of two independent experiments containing six replicates per treatment.
Mitochondrial superoxide generation was measured using MitoSOXTM Red Mitochondrial Superoxide Indicator (Thermo Scientific). The four treatment groups were: medium alone, 1 µg/mL E. coli LF82 lysate, 10 µg/mL MnO NP, and the 10 µg/mL MnO NP plus 1 µg/mL E. coli LF82 lysate. MODE-K cells were plated in 96-well plates with 8,000 cells/well one day prior to treatment and allowed to attach overnight. The next day, cells were washed twice with low glucose DMEM medium before addition of the indicated treatments suspended in low glucose DMEM medium and the cells were incubated for an additional 24 hours. Following treatment, MitoSox reagent (Thermo Scientific) was added to each plate well at a 1:1000 concentration. Relative fluorescence intensity was then acquired using a microplate reader per manufacturers instructions. Data is representative of two independent experiments containing six wells per treatment per experiment and is presented as the mean ± SEM.
Mitochondrial respiration was measured using a Seahorse XF24e Extracellular Flux analyzer (Seahorse Bioscience, North Billerica, MA). The Seahorse XF24e Extracellular Flux analyzer is a high-throughput instrument that yields real-time measurements of cellular respiration rates. In our studies, we were interested in assessing the effects of four treatments on mitochondrial respiration and their effects on oxygen consumption rates (OCR). MODE-K cells were seeded at a density of 300,000/mL into a T-25 culture flask and incubated overnight in 5% CO2 at 37.8ºC. The next day, cells were treated with respective treatments for 24 hours. Stimulated cells were recovered from the plates by scraping and added to the Seahorse plates using CellTac at 60,000 cells per well and incubated for 1 hour to allow the cells to adhere. At this time, the mito-stressor reagents oligomycin (1 µM), FCCP (2 µM) and rotenone/antimycin A (0.5 µM) were loaded into the corresponding injection ports of the Seahorse FluxPak cartridge and placed in the Seahorse analyzer to be equilibrated. After equilibration, the plate containing treated cells was then placed in the Seahorse analyzer covered with the Flux Pak cartridge. The analyzer was then programmed to measure the basal OCR readouts at five specified time intervals before progressing to inject the mito-stressors every three cycles of measuring OCR. Measuring mitochondrial respiration with the Seahorse analyzer allowed the measurement of the effect of each treatment at the three critical stages of cellular respiration. Data shown is the mean ± SEM and are representative of five independent wells.
All statistical data analyses were performed using Prism 4.0 (GraphPad Software, San Diego, CA). Data were analyzed using one-way ANOVA with the Tukey post-hoc test for comparing all treatment groups with that of the control. Multiple comparisons test was also performed to analyze differences between treatment groups. Differences with p < 0.05 were considered significant. All data is representative of two independent experiments containing six wells per treatment.
The induction of reactive oxygen species (ROS) may negatively impact the crucial barrier function of the intestinal epithelium. Reactive oxygen species are increased in the inflamed mucosa of IBD patients and may contribute to loss of intestinal barrier function27. Furthermore, during inflammation, oxidative stress can lead to the induction of cell injury and apoptosis of intestinal epithelial cells28.
To assess the induction of mitochondrial ROS in the presence of MnO NPs, the MitoSOX assay was used. After 24 hours of incubation with the indicated treatments, measurements were taken and data was normalized as percent control (Figure 1). Although there was no significant difference, there was approximately a 15% or 25% increase in mitochondrial superoxide generation in treatment groups receiving MnO NP or MnO NP + bacterial lysate, respectively (Figure 1). While there was a slight additive effect, this data suggests that mitochondrial superoxide generation by the MODE-K cells was primarily driven by the presence of MnO NP as opposed to the E. coli LF82 lysate.
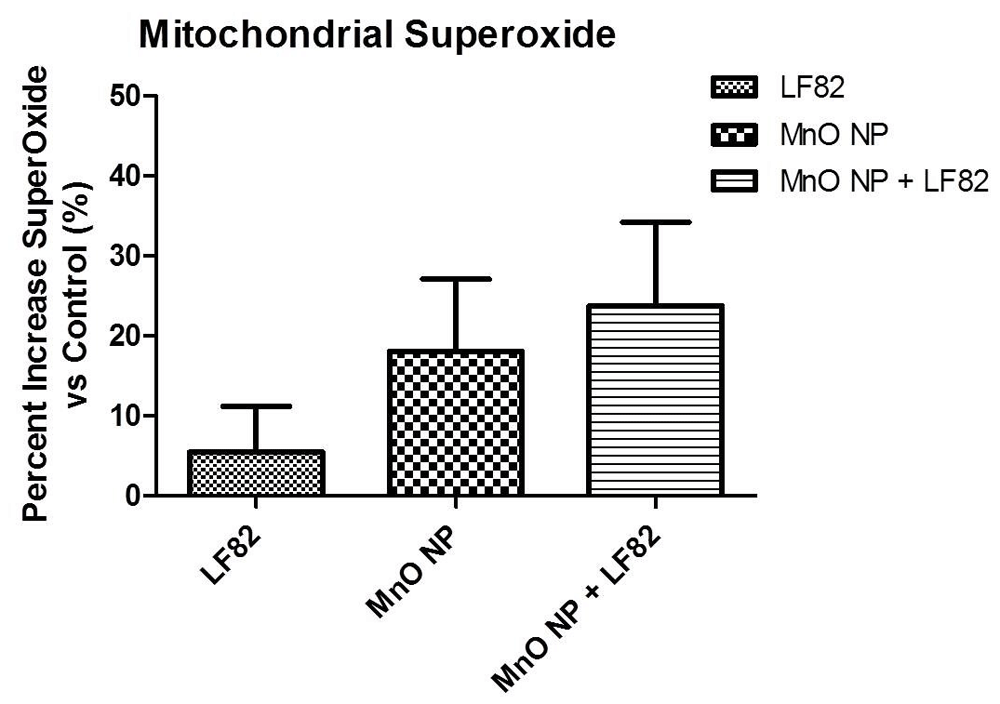
Data was normalized to percent control. MODE-K cells were incubated in the presence of 1 µg/mL E. coli LF82 lysate (LF82), 10 µg/mL MnO nanoparticles (NP), or a combination of both. Data is representative of two independent experiments containing six wells per treatment per experiment and is presented as the mean ± SEM.
Damage and impairment of the intestinal epithelial barrier may result in an increased permeability and absorption of toxic and phlogistic factors from the intestinal lumen leading to inflammation and disequilibrium of epithelial homeostasis29. This inflammatory response can lead to the induction of cellular injury and apoptosis of intestinal epithelial cells28,29. To assess the secretion of inflammatory cytokines from the MODE-K cells, culture supernatants were harvested 24 hours after the addition of the treatments. IL-6 was the only cytokine detected (i.e., no TNFα or IFNγ) and this was associated with exposure to the E. coli LF82 lysate as opposed to the MnO NP alone. However, a significant in IL-6 secretion was detected when the MODE-K cells were treated with both the E. coli LF82 lysate + MnO NP (p < 0.05) (Figure 2A). Similar results were also obtained in culture supernatants collected after 48 hours of incubation (Figure 2B); however, there was a threefold increase in the secretion of IL-6 at 48 hours when the MODE-K cells were exposed to both stimulants.
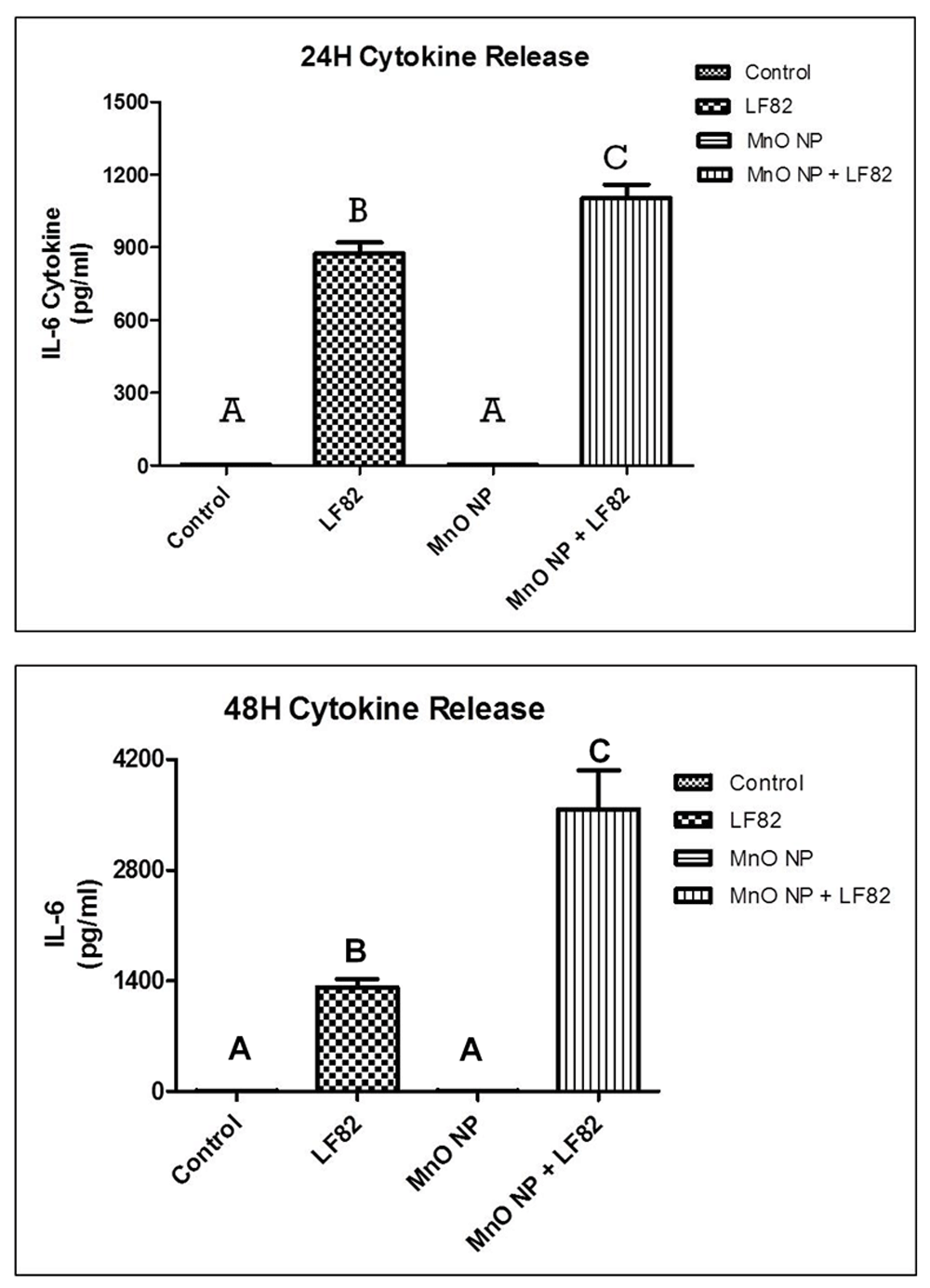
(A) Culture supernatants were harvested at 24 hours post-treatment. (B) Culture supernatants were harvested at 48 hours post-treatment. Results are representative of two independent experiments containing six replicates per treatment. Different letter denotes difference in significant (p < 0.05) change. The error bars represent the SEM.
Wound healing is a crucial function of the intestinal epithelium as it can be injured by toxic luminal substances, inflammation, interactions with microbes, oxidative stress, and pharmaceuticals27,30. Upon injury, the intestinal epithelium undergoes a wound healing process which is dependent on the balance of three cellular events; restitution, proliferation, and differentiation of immature epithelial cells adjacent to the wounded area31. To assess the effects of MnO NP on the wound healing process (i.e., replication and migration), a scratch wound assay using MODE-K cell monolayers was performed. Using a one-way ANOVA, a significant effect was observed between treatment groups and controls (p < 0.05) (Figures 3A and 3B).
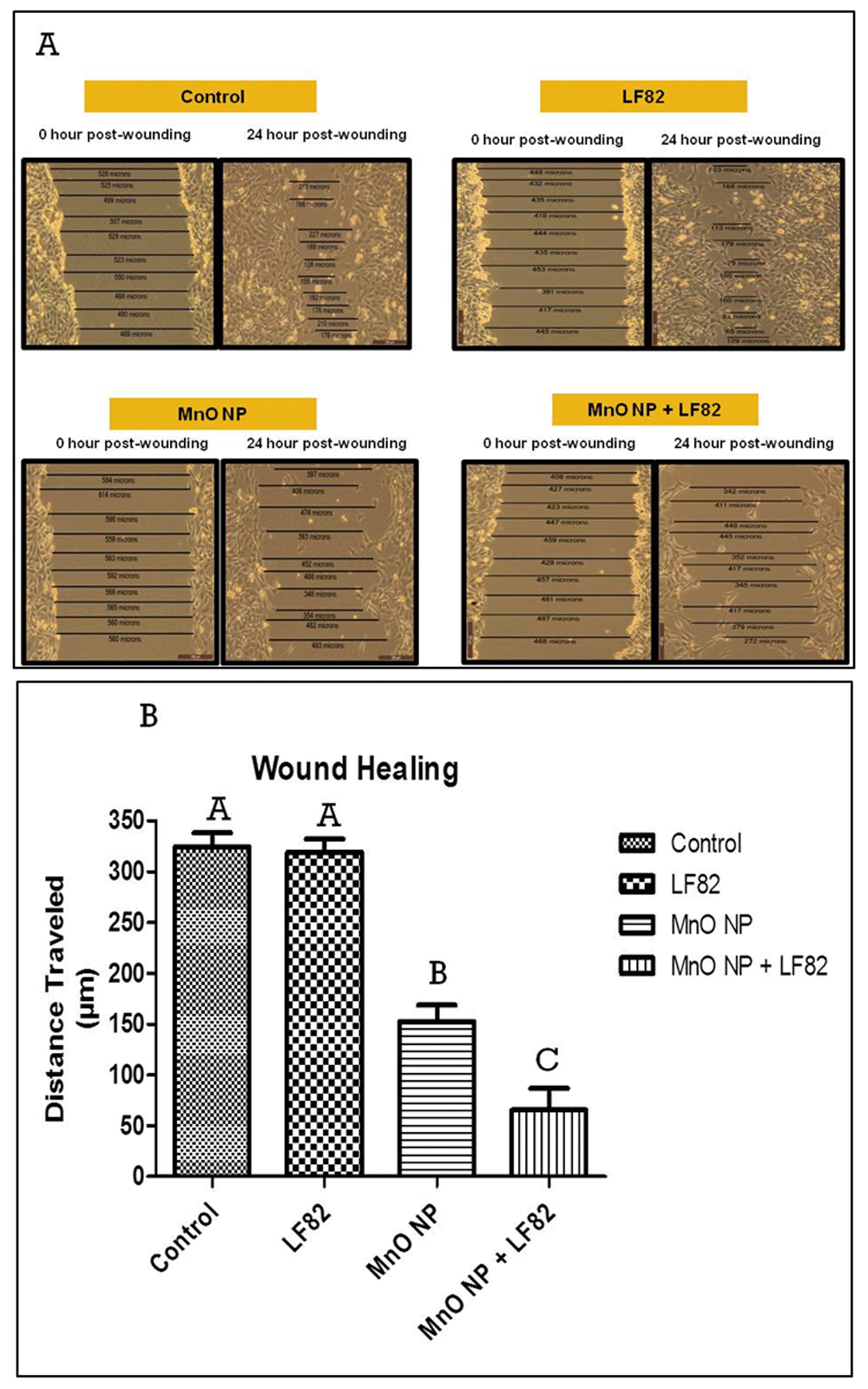
Measurements of the gaps created by the scratch were taken at 0 and 24 hours post-treatment. (A) Photographs of representative monolayers taken 24 hours post-treatment. (B) Histogram depicting the distance traveled (µm) by MODE-K cells to close the gap between edges of the wound. Results are a representation of two independent experiments containing six replicates per treatment. Different letter denotes difference in significant (p < 0.05) change. Error bars represent the SEM.
Post-hoc analysis indicated a significantly (p < 0.05) slower wound healing response when the control cells (medium alone) was compared to MODE-K cells treated with either 10 µg/mL MnO NP alone or 10 µg/mL MnO NP + 1 µg/mL E. coli LF82 lysate. In contrast, the addition of the E. coli LF82 lysate alone to the cultures had no effect on the replication and migration of the MODE-K cells. However, analysis revealed a significant (p < 0.05) decrease in wound healing when cells were treated with 10 µg/mL MnO NP + 1 µg/mL E. coli LF82 lysate compared to the 10 µg/mL MnO NP alone treatment group suggesting a synergistic effect on wound healing.
Mitochondrial function plays a critical role in maintaining intestinal stemness and homeostasis32. As mitochondrial dysfunction is associated with a loss of proliferative capacity by intestinal epithelial cells, the effects on crucial factors of mitochondrial respiration were measured using a mitostress test. Utilizing the Seahorse XFe24 Analyzer, changes in basal respiration, maximal respiration, proton leak, ATP production, and spare respiration capacity were measured (Figure 4).
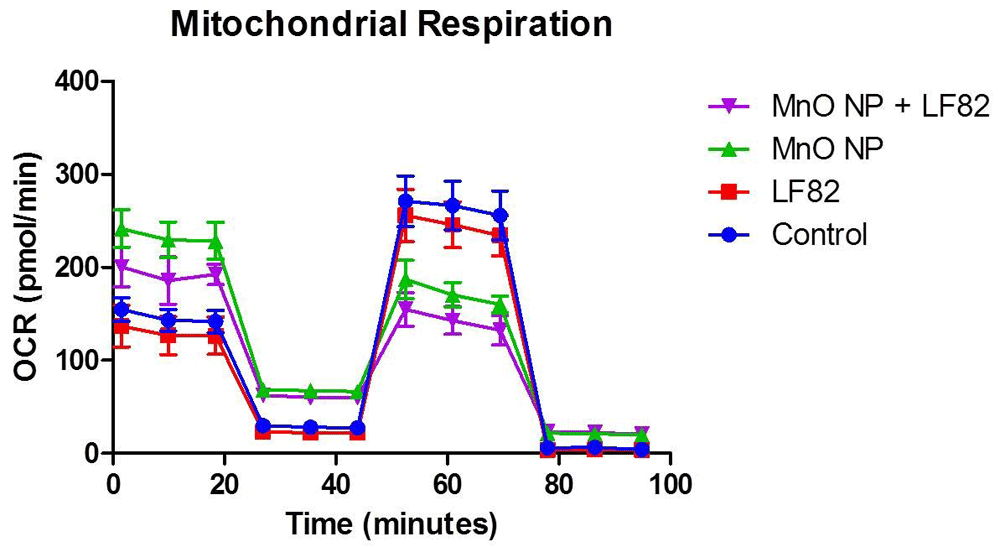
After incubation in the presence of 1 µg/mL E. coli lysate, 10 µg/mL MnO NP or both, metabolic responses of MODE-K cells was measured as described in the Methods. Mitochondrial stress test (MST) was used to assess the oxygen consumption rate (OCR) including the basal respiration (Figure 5), maximal respiratory capacity (Figure 6), proton leak (Figure 7), ATP production (Figure 8) and spare respiratory capacity (Figure 9). Data shown is a single experimental replicate that is representative of two independent experiments.
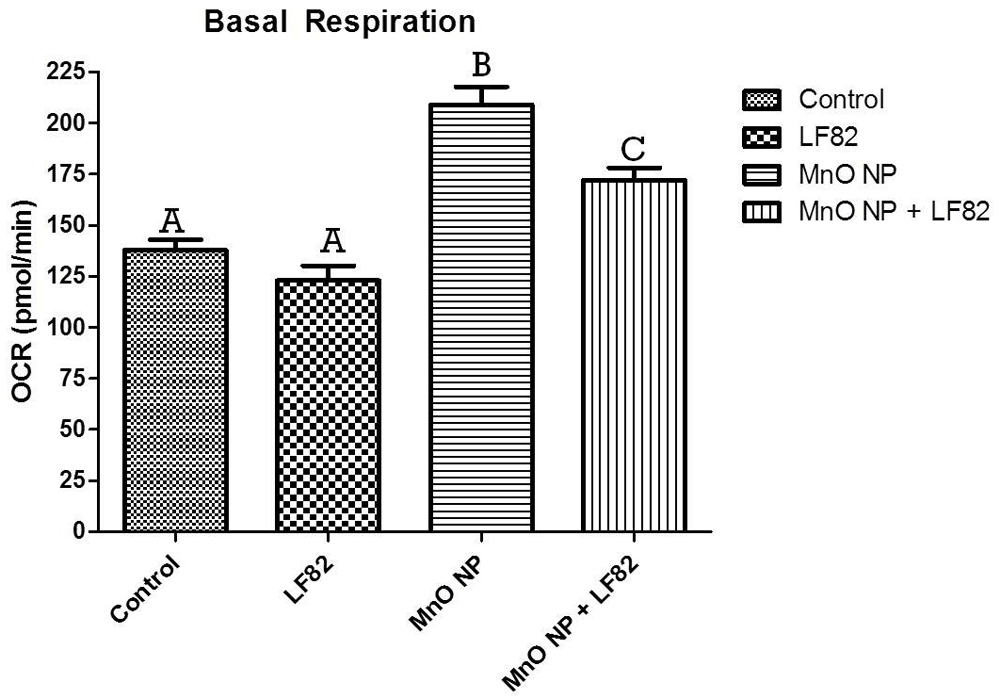
After incubation in the presence of 1 µg/mL E. coli LF82 lysate, 10 µg/mL MnO NP or both, metabolic responses of MODE-K cells was measured as described in the Methods. Data shown is the mean ± SEM of five independent wells. Significant (p < 0.05) difference is denoted by difference in letter.
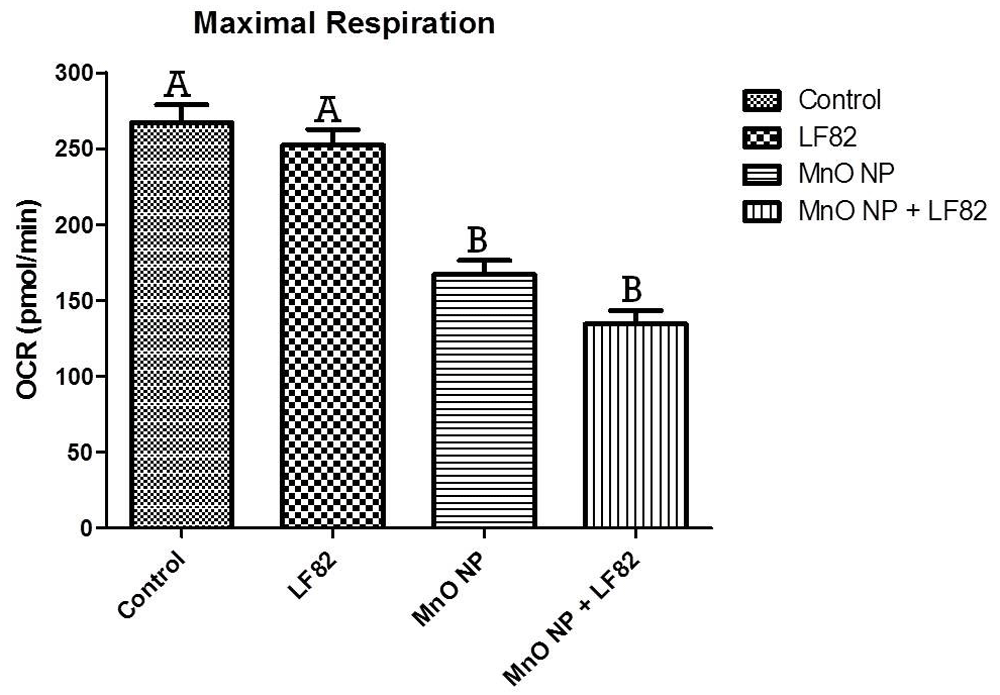
After incubation in the presence of 1 µg/mL E. coli LF82 lysate, 10 µg/mL MnO NP or both, metabolic responses of MODE-K cells was measured as described in the Methods. Data shown is the mean ± SEM and are representative of five independent wells. Significant (p < 0.05) difference is denoted by difference in letter.
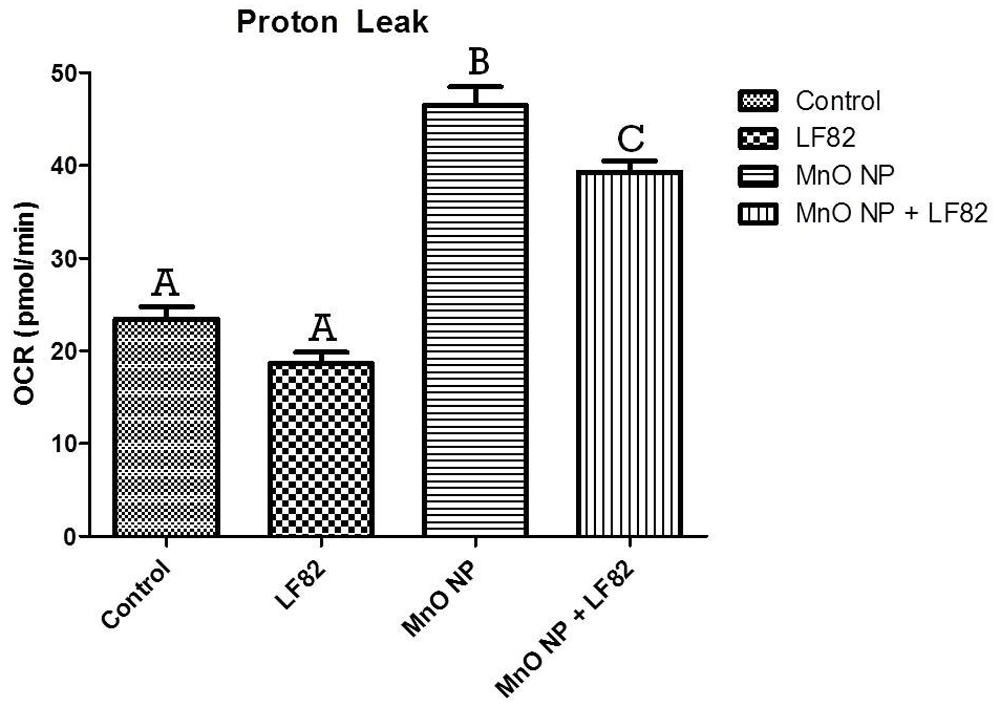
After incubation in the presence of 1 µg/mL E. coli LF82 lysate, 10 µg/mL MnO NP or both, metabolic responses of MODE-K cells was measured as described in the Methods. Data shown is the mean ± SEM and are representative of five independent wells. Significant (p < 0.05) difference is denoted by difference in letter.
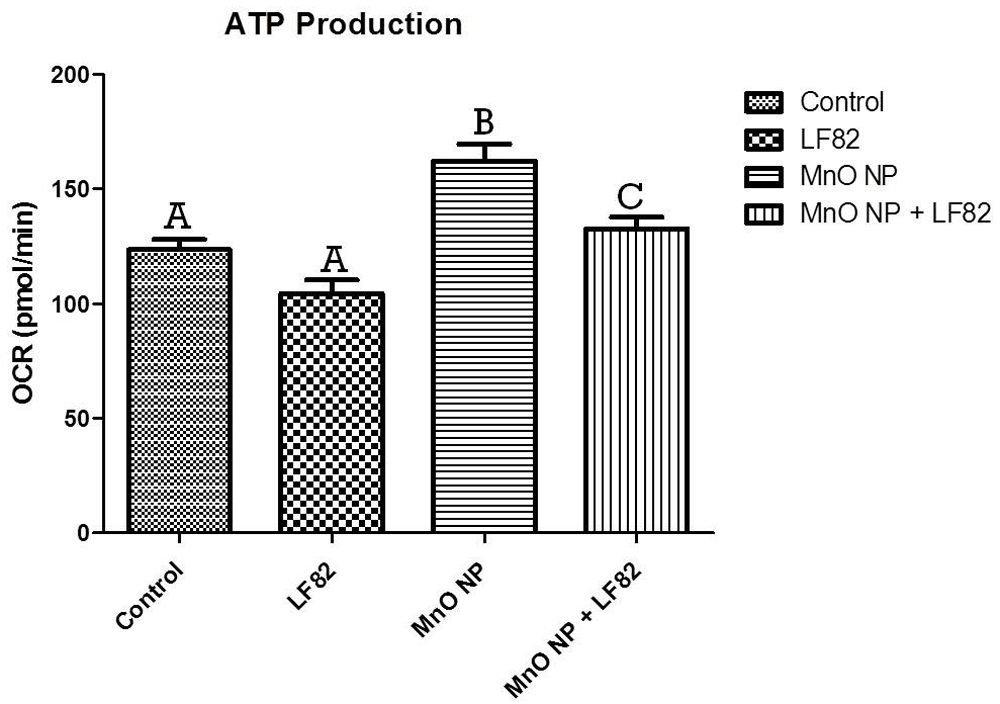
After incubation in the presence of 1 µg/mL E. coli LF82 lysate, 10 µg/mL MnO NP or both, metabolic responses of MODE-K cells was measured as described in the Methods. Data shown is the mean ± SEM and are representative of five independent wells. Significant (p < 0.05) difference is denoted by difference in letter.
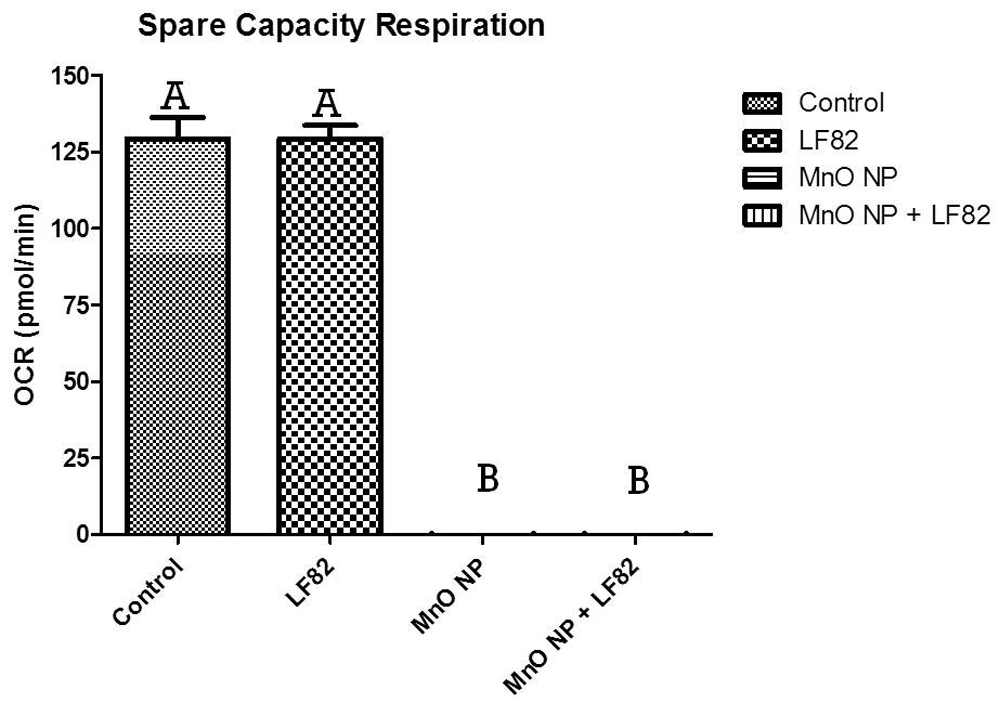
After incubation in the presence of 1 µg/mL E. coli LF82 lysate, 10 µg/mL MnO NP or both, metabolic responses of MODE-K cells was measured as described in the Methods. Spare capacity of MODE-K cells were measured at 24 hours post-treatment initiation. Data shown is a representation of five independent wells. Significant (p < 0.05) difference is denoted by difference in letter.
Basal respiration is a measurement that shows the energetic demand of the cell under baseline conditions. Analysis showed a significant (p < 0.05) elevation of basal respiration in both treatments groups containing MnO NP when compared to medium and E. coli LF82 lysate alone, with the highest increase induced by the MnO NP alone (Figure 5).
Maximal mitochondrial respiration, which is a measurement of the maximum rate of respiration that cells can achieve when operating at maximum capacity was alsao measured. Significantly (p < 0.05) lower levels of maximal respiration were seen in cells treated with the MnO NP alone and an even more severe affect was observed in the MnO NP + E. coli LF82 lysate treated group (Figure 6). While not significant, MODE-K cells treated with both MnO NP + E. coli LF82 lysate, experienced a greater decrease in maximal respiration indicating that the presence of bacterial lysate further impacted mitochondrial respiration.
Another parameter measured in the mitostress test (MST) is mitochondrial proton leak. Mitochondrial proton leak can have a major impact on mitochondrial oxidative phosphorylation coupling efficiency and production of reactive oxygen species. The presence of MnO NP caused a significant increase (p < 0.05) in mitochondrial proton leak either alone or in combination with the E. coli LF82 lysate (Figure 7).
ATP production was also measured during the MST and it was observed that MnO NP induced higher oxygen consumption rates, as evidenced by the significant (p < 0.05) increase in ATP production when compared to control and MODE-K cells treated with the E. coli LF82 lysate alone. In addition, the presence of the bacterial lysate appeared to attenuate the metabolic activity induced MnO NP alone (Figure 8). Any treatment group containing MnO NP experienced an increase in ATP production suggesting this to be a MnO NP-mediated effect.
The final parameter measured in the MST was spare respiratory capacity, which measures the capability of the cell to respond to an energetic demand, which can be an indicator of the cell’s fitness or flexibility. When compared to control MODE-K cells, any group treated with MnO NP had a significant (p < 0.05) decrease in the spare respiratory capacity suggesting that a decrease in respiratory spare capacity was mediated by the presence of the MnO NPs independent of the presence of E. coli LF82 lysate (Figure 9).
Mitochondrial dysfunction is associated with a loss of proliferative capacity in intestinal epithelial cells32. As the MTS assay takes into account three parameters (cytotoxic, cytostatic, and antiproliferative effects), any decrease in the presence of viable cells may be a result from either one or a combination of these three parameters and would provide crucial insight about cell viability and health. After 48 hours of incubation, a significant decrease was observed in the MnO + E. coli treated MODE-K cells (p < 0.05) (Figure 10) which is consistent with the wound healing data shown in Figure 3. There was no significant impact measured by the MTS when the cells were treated with either the MnO NP or E. coli LF82 lysate alone.
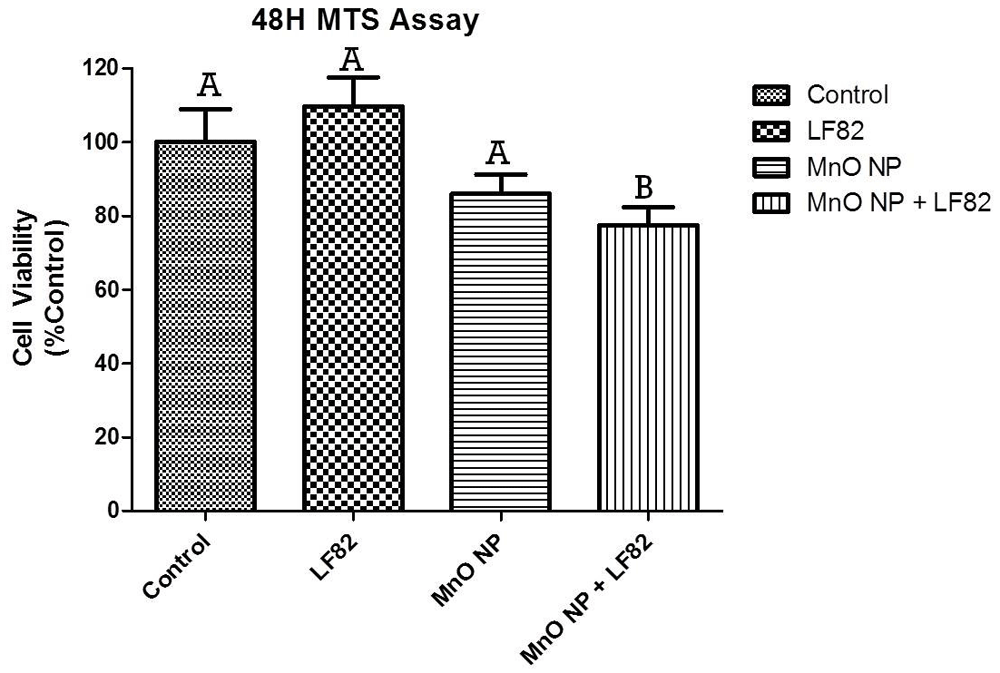
The viability of MODE-K cells was measured 48 hours after the addition of 1 µg/mL E. coli LF82 lysate, 10 µg/mL MnO NP or both. Data shown is represented as the mean ± SEM collected from two independent experiments. In each experiment, six replicate wells were measured per treatment with significant (p < 0.05) difference denoted by difference in letter.
The intestinal epithelial barrier, which consists of several elements, including intestinal epithelial cells and tight junctions, prevents microorganisms, undesirable solutes, toxins, and luminal antigens from entering the body33–37. It is suggested that disruption of the intestinal barrier function and repeated intestinal epithelial damage are key features of IBD, as well as other intestinal disorders29,38. Furthermore, it is known that environmental irratants (e.g., pollutants) negatively impact GI IECs. Environmental exposure to MnO NPs can occur as a result of occupational exposure in manganese ore processing, the metallurgy industry, metalworking, welding, and various potential biomedical applications20–22,39. MnO NP have been shown to cause impairment of dopaminergic neurons and induce inflammation in the brain, increase in oxidative stress and apoptosis in alveolar epithelial cells, and increase oxidative stress and DNA damage in mouse embryonic stem cells, human breast cancer epithelial cells, and human fibrosarcoma epithelial cells39–42. However, few studies have evaluated at the effects of MnO NP toxicity on intestinal epithelial cells, even though the primary route of exposure would be oral or ingestion. Therefore, the goal of this study was to investigate the cytotoxicity of environmentally related MnO NP on IECs. As most studies evaluate the biological effects of MnO NP alone, this approach may underestimate their in vivo toxicity in the GI tract given the potential interactions with the resident microbiota. In this study, new insights regarding the toxicity of MnO NP for IECs was evaluated in combination with bacterial components (e.g., E. coli LF82 lysate). The presence of the bacterial products seem to have both negative and cytoprotective effects on the MODE-K cells. By themselves, MnO NP negatively affected MODE-K epithelial cells, but in the presence of the bacterial lysate, synergistic effects were observed in several biological functions including secretion of IL-6, wound healing, and mitochondrial processes.
In this study, IL-6 secretion was synergistically elevated in the culture supernatant of MODE-K cells stimulated with MnO NP + E. coli LF82 lysate. IL-6 is a pleiotropic, pro-inflammatory cytokine which plays an important role in promoting inflammatory response in the gut and in the systemic circulation10,38. Elevated IL-6 serum levels have been detected in acute and chronic inflammation GI disease. Moreover, elevated IL-6 production has been associated with Crohn’s disease (CD) and ulcerative colitis (UC)10. As elevated levels of localized IL-6 in the intestine may be a characteristic feature of active IBD, IECs are one of the major cell types responsible for the production of IL-643. Modulation of IL-6 levels in the GI tract can undermine the integrity of the intestinal epithelial barrier by increasing intestinal barrier permeability44,45.
Because manganese ions were detected in culture supernatants (1853 – 2585 ppb), it is possible that excessive Mn++ ions in the culture medium had an impact mitochondrial functions but published data indicates that cytotoxicity occurs at mM concentrations46. Mn++ ions have an essential role in survival and death mechanisms of cells from all organisms because Mn++ ions regulate Mn-containing enzymes such as manganese superoxide dismutase and regulate expression and activity of caspases. Accumulation of Mn++ ions to excessive levels (≥ mM concentrations) has been shown to cause cellular dysfunction and cell death46. Data suggest the presence of MnO NP led to an accumulation and excess level of Mn++ in IECs that disrupted the balance of the redox interaction and caused mitochondrial dysfunction. This would in turn lead to deleterious effects in wound healing response that was observed in this study. Control of intestinal epithelial stemness is crucial for tissue homeostasis and the mitochondrial function plays a critical role in maintaining intestinal stemness32. Dysfunction of the mitochondria was accompanied by impairment in ATP production which resulted in reduced proliferation of the intestinal epithelium and loss of stemness32. The results of this study demonstrated that combination of MnO NP and E. coli LF82 lysate caused impairment of mitochondrial respiration of MODE-K cells. These results suggest that the presence of MnO NP in the gut could affect mitochondrial energy capacity and consequently mucosal homeostasis resulting in impairment of barrier function and development of leaky gut syndrome.
Mitochondria play an important role during the interaction of gut microbiota with host cells and mitochondrial activity may be an important factor that modulates microbiota diversity and quality47. A study performed using colonic biopsy samples showed that induced mitochondrial dysfunction affected epithelial barrier function, which allowed transepithelial flux of bacteria across the intestinal epithelium48. An extraordinary amount of energy is required by IEC during the inflammatory and immune responses49. For example, the first contact of the gut microbiota with the intestinal epithelium stimulates Toll-like receptors in IECs which results in the recruitment of B cells and plasma blasts that subsequently secrete IgA to limit the over-colonization of gut microbiota50. Based on the described in vitro studies, it is predicted that environmental exposure to MnO NP will dysregulate the mitochondrial function leading to a loss in epithelial barrier integrity and the infiltration of gut microbes initiating an inflammatory response50.
The GI tract has been shown to be the primary route of ingestion and excretion for metal nanoparticles. Results of this study suggest that the addition of bacterial components to IEC cultures in combination with MnO NP might be predictive of the cellular response seen in vivo as this more appropriately represents the context of exposure to environmental irratants and toxicants in the GI tract. Currently, the toxicity of nanoparticles is evaluated with various approaches that include both in vitro and in vivo methods of assessment, but rarely do they take into consideration the presence of the microbial community that exist in the body. Assessing the effects of nanoparticles on intestinal epithelial cells in the presence of bacterial components presents a more holistic, and representative model of exposure when assessing the effects of nanoparticles on gut epithelial cells as the gut microbiota can influence cellular physiology, metabolism, and immune function23. The results presented herein indicate that it is important to integrate the contributions of multiple stimuli on IEC function and ultimately intestinal barrier integrity in order to obtain a more representative impact of metal-based nanoparticles on the health and homeostasis of the GI tract.
Open Science Framework: Cytotoxic effects of manganese oxide nanoparticles in combination with microbial components on intestinal epithelial cells, https://doi.org/10.17605/OSF.IO/2RKEA51
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors thank Mary Jane Long and Meghan Wymore-Brand for technical assistance and Dr. Anumantha Kanthasamy for the kind gift of manganese oxide nanoparticles.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Titma T, Shimmo R, Siigur J, Kahru A: Toxicity of antimony, copper, cobalt, manganese, titanium and zinc oxide nanoparticles for the alveolar and intestinal epithelial barrier cells in vitro.Cytotechnology. 2016; 68 (6): 2363-2377 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Analytical biochemistry, nanoparticle characterisation and use in medicine, toxicology, medicine.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Wang A, Keita ÅV, Phan V, McKay CM, et al.: Targeting mitochondria-derived reactive oxygen species to reduce epithelial barrier dysfunction and colitis.Am J Pathol. 2014; 184 (9): 2516-27 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Epithelial barrier function; Inflammatory Bowel Disease; Intestinal physiology.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 12 Aug 20 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)