Keywords
Lyme borreliosis, chronic Lyme disease, Borrelia burgdorferi, spirochetes, sexual transmission.
Lyme borreliosis, chronic Lyme disease, Borrelia burgdorferi, spirochetes, sexual transmission.
Lyme disease is the most common human tick-borne disease in the world today (Stricker & Johnson, 2014). It is transmitted by Ixodes ticks and is caused by the spirochete Borrelia burgdorferi (Bb) (Burgdorfer et al., 1982). Bb is phylogenetically related to the spirochetal agent of syphilis, Treponema pallidum (Gupta et al., 2013). T. pallidum is transmitted sexually between partners through contact of mucosal membranes, gaining access to the bloodstream through microabrasions and then disseminating systemically (Ho & Lukehart, 2011). The close phylogenic relationship of Bb to T. pallidum suggests that this mode of transmission might be possible for Bb.
In addition to theoretical considerations, evidence for non-vector transmission of Bb is based on animal models. Proof of contact transmission of Bb – without involvement of an arthropod vector – was established by two studies in mice. Burgess et al. (1986) caged uninfected deer mice with experimentally-infected deer mice and demonstrated transmission of Bb by seroconversion of contact-exposed mice from negative to positive and by the isolation of Bb from the blood of one contact-exposed mouse 42 days after initial contact. A study by Wright & Nielsen (1990) demonstrated that white-footed mice were susceptible to oral infection and transmitted infection to each other through direct contact. Furthermore, sexual transmission of Bb has been proposed in a canine model. Bb was transmitted to uninfected female dogs in estrus via semen by natural breeding with male dogs infected experimentally with Bb (Gustafson, 1993). Successful transmission of infection from male dogs to female dogs was shown by seroconversion of female dogs from negative to positive as well as the detection of Bb DNA in the tissue of fetuses from resulting pregnancies. If contact transmission of Bb occurs in mice and sexual transfer occurs in dogs, it is not unreasonable to postulate similar routes of infection in humans.
We sought to determine if viable Borrelia spirochetes could be recovered from human vaginal and seminal secretions, an important first step to investigate whether sexual transmission of these spirochetes among humans is possible.
Control subjects who were asymptomatic without a history of Lyme disease and patients with a history of Lyme disease were recruited for the study after written informed consent to collect and publish their data was obtained. Approval for sample collection was obtained from the Western Institutional Review Board, Olympia, WA (WIRB® #20141439). Further approval for sample testing was obtained from the Institutional Review Board of the University of New Haven, West Haven, CT. Serological testing of all participants was performed by IGeneX Reference Laboratories, Palo Alto, CA. Patients were considered positive for Lyme disease if they were serologically positive or if they had musculoskeletal, neurocognitive or cardiac symptoms clinically consistent with a Lyme disease diagnosis. None of the patients were taking antibiotics at the time of testing.
Borrelia spirochetes were cultured as previously described (Bankhead & Chaconas, 2007; Middelveen et al., 2013b; Middelveen et al., 2014a). The inoculum for blood culture was prepared as follows: 10 milliliters of whole blood was collected by sterile venipuncture from each patient. Samples sat at room temperature for 10 to 15 minutes allowing clotting to occur. Red blood cells (RBCs) were separated by low speed centrifugation. Barbour–Stoner–Kelly H (BSK-H) complete medium was used for cultures with the addition of 6% rabbit serum (Sigma Aldrich, #B8291) and the following antibiotics: phosphomycin (0.02 mg/ml), rifampicin (0.05 mg/ml), and amphotericin B (2.5 µg/ml) (Sigma Aldrich).
The culture medium described above was inoculated for blood culture with the spun serum containing white blood cells and some RBCs, and for genital culture with either ejaculated semen or vaginal secretions collected by intravaginal swabbing with a sterile cotton-tipped swab. Blood and genital cultures were incubated at 32°C in an Oxoid anaerobic jar (Thermo Scientific) containing an AnaeroGen sachet (Thermo Scientific) to provide an anaerobic environment. Cultures were incubated for four weeks and checked weekly by light and/or darkfield microscopy for visible motile spirochetes.
All cultures were processed for microscopic imaging and PCR by centrifuging the culture fluid at 15,000 g for 20 minutes to concentrate spirochetes. The supernatant was discarded and the pellet retained.
Dieterle silver staining was performed using two fixation methods. In the standard method, formalin-fixed, paraffin-embedded pellets were sectioned and stained with Dieterle silver stain as previously described (Aberer & Duray, 1991; Middelveen et al., 2013a). In the newer method, culture fluid was spread and dried on a SuperFrost™ Plus microscope slide (Fisher Scientific) and fixed by incubating the slide in acetone for 10 minutes at -20°C, as previously described (Sapi et al., 2013). Dieterle silver staining was performed on the acetone-fixed slide.
A. McClain Laboratories. Blood and genital culture pellets were processed for special staining at McClain Laboratories LLC, Smithtown, NY. Formalin-fixed, paraffin-embedded pellets were sectioned and stained with Dieterle silver stain or anti-Bb immunostains for spirochete detection, as previously described (Middelveen et al., 2013a; Middelveen et al., 2014a). In brief, immunostaining was performed using an unconjugated rabbit anti-Bb polyclonal antibody (Abcam ab20950), incubated with an alkaline phosphatase probe (Biocare Medical #UP536L), followed by a chromogen substrate (Biocare Medical #FR805CHC), and counterstained with hematoxylin. Positive and negative controls were prepared for comparison purposes with liver sections from Bb-inoculated mice and uninfected mice followed by Dieterle and immunostaining. Culture pellets from mixed Gram-positive bacteria and mixed Gram-negative bacteria were also prepared for comparison purposes as negative controls to exclude cross-reactivity with commonly encountered microorganisms. Staining was titrated to determine optimal antibody dilutions to achieve positive staining of spirochetes while minimizing background staining (Middelveen et al., 2013a; Middelveen et al., 2014a).
B. University of New Haven. Samples were processed for Bb immunostaining as previously described (Sapi et al., 2013). Culture fluid was spread and dried on a SuperFrost™ Plus microscope slide (Fisher Scientific) and fixed by incubating the slide in acetone for 10 minutes at -20°C. Dried, fixed culture fluid was submerged under 100 μl of polyclonal FITC-labeled rabbit anti-Bb antibody (Thermo Scientific #PA-1-73005) diluted 1:50 in 1× PBS buffer with 1% BSA (Sigma Aldrich #A9418). For negative controls, the antibody was omitted and replaced with normal rabbit serum. The slides were then incubated for 1 hour at 37°C in a humidified chamber, washed with 1× PBS for 5 minutes at room temperature, rinsed twice in double distilled water and dried in a laminar air-flow hood for 10 minutes. The slides were mounted with Vectashield mounting medium with DAPI counterstain (Vector Labs) and viewed with fluorescent microscopy at 400× magnification with a Leica DM2500 microscope (Sapi et al., 2013).
The Bb molecular beacon DNA probe was generously provided by Dr. Alan MacDonald. Probe FlaB (sequence of 23 mer) was derived from the Bb open reading frame (ORF) BB0147 (approximately 1100 mer) of the flagellin B gene. A nucleotide Basic Local Alignment Search Tool (BLAST) search of the 23 mer sequence disclosed no matches in the human genome or in any other life form other than the Bb sequence of BB0147.
Bb detection with the molecular beacon was performed as previously described (Middelveen et al., 2014a) using the following protocol: paraffin sections were dewaxed by baking at 60°C, then immersed in serial 100% xylene baths followed by serial immersion through baths of 100% ethanol, 90% ethanol, 80% ethanol, and finally in distilled H2O, and then air-dried. Fixed sections were immersed in 20 μl of the working DNA beacon solution. The sectioned specimen was covered with a layer of plastic cut from a Ziploc® freezer bag and was heated at 90°C for 10 minutes to denature DNA and RNA. The heat was first reduced to 80°C for 10 minutes, then the slides were removed from heat and allowed to gradually cool to 24°C. The slides were washed in PBS, covered with 30% glycerol and a glass coverslip, then examined under an EPI Fluor microscope. Staining of test specimens was performed alongside staining of positive and negative controls. The positive control was prepared by embedding a known Bb strain in agarose, formalin-fixing the specimen then blocking in paraffin and staining sections as described above.
The specificity of the FlaB probe was validated in studies performed at the University of New Haven (Sapi E., unpublished observation 2014; see Supplemental Figure 1). The FlaB probe hybridized to Bb sensu stricto, yet failed to hybridize with B. afzelii, B. garinii, B. hermsii, Treponema denticola and Escherichia coli. Thus the probe appears to be specific for detection of Bb sensu stricto.
Blood and genital culture pellets were first dissolved in 200 μl of Qiagen buffer, then forwarded to the University of New Haven, Department of Biology and Environmental Science, West Haven, CT, USA and Australian Biologics, Sydney, NSW, Australia for PCR detection of Borrelia.
A. Australian Biologics. Detection of Borrelia by PCR was performed as previously described (Mayne et al., 2012) using the Eco™ Real-Time PCR system with primers targeted to the genes encoding 16S rRNA (Borrelia), flA (T. denticola) and fliG1 (T. pallidum) and analyzed with the software version 3.0.16.0. DNA was extracted from the dissolved culture pellets using the QIAamp DNA Mini Kit (Qiagen) and 20 μl were used for each reaction. The thermal profile involved incubation for 2 minutes at 50°C, polymerase activation for 10 minutes at 95°C then PCR cycling for 40 cycles of 10 seconds at 95°C dropping to 60°C sustained for 45 seconds. All samples were run in duplicate with positive and negative controls. Positive controls were genomic DNA samples from B. burgdorferi, B. garinii, and B. afzelii (Amplirun DNA/RNA amplification controls, Vircell S.L, Granada, Spain). Negative controls were samples of non-template DNA in molecular-grade water. The magnitude of the PCR signal generated (∆R) for each sample was interpreted as positive or negative compared to positive and negative controls.
In samples with sufficient DNA for sequencing, endpoint PCR amplification and Sanger sequencing of the Borrelia gene target from cultures was followed by BLAST comparison with known Borrelia sequences, as previously described (Mayne et al., 2012).
B. University of New Haven. DNA samples were extracted from blood, vaginal or seminal cultures by lysing cells overnight in 180 µl tissue lysis buffer (Qiagen) and 20 µl Proteinase K (Qiagen) at 56°C in a shaking water bath followed by phenol:chloroform extraction the next day. The DNA was resuspended in 50–100 µl 1×TE buffer.
A published TaqMan assay targeting a 139-bp fragment of the gene encoding the Borrelia 16S rRNA was used for the detection of Borrelia in DNA extracted from patient samples (O’Rourke et al., 2013). All reactions were carried out at a final volume of 20 µl and consisted of 900 nM of each primer, 200 nM of probe, and 10 µl of 2× TaqMan Universal PCR Master Mix (Applied Biosystems) and 1 nanogram of DNA. Amplifications were carried out on a CFX96 Real-Time System (Bio-Rad), and cycling conditions consisted of 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds. Fluorescent signals were recorded with CFX96 Real-Time software and Cq threshold was set automatically. The reactions were performed in triplicate with positive and negative controls.
Nested PCR primers for the genes encoding the Borrelia 16S rRNA, fla and pyrG loci were used as previously described (Clark et al., 2013; Margos et al., 2010; Sapi et al., 2013). Reactions were carried out in a final volume of 50 µl using 10 µl template DNA. Final concentrations were 2× Buffer B (Promega), 2 mM MgCl2, 0.4 mM dNTP mix, 2 µM of each primer, and 2.5 U Taq polymerase (Invitrogen). “Outer” primers were used in the first reaction. “Inner” primers were used for the nested reaction, in which 1 µl of PCR product from the first reaction was used as template for the second. Cycling parameters were as follows: 94°C for 5 minutes followed by 40 cycles of denaturation at 94°C for 1 minute, annealing for 1 minute (temperature based on the primer set used), and extension at 72°C for 1 minute, with a final extension step at 72°C for 5 minutes. PCR products were visualized on 1–2% agarose gels. Sanger sequencing was used for gene analysis, as previously described (Margos et al., 2010).
All patient data are shown in Table 1. The control group included four asymptomatic patients (two males and two females). All four were seronegative for Bb.
Patients 6 & 7 (*), 8 & 9 (**), 10 & 11 (†), and 12 & 13 (††) are sexual partners. Patients 8 and 11 were seronegative but clinically diagnosed with Lyme disease.
The patient group included six male subjects and seven female subjects, including four pairs of partners (Patients 6 and 7, 8 and 9, 10 and 11, and 12 and 13, respectively). Eleven of the 13 patients selected for the study were serologically positive for Lyme disease. Patient 1 was serologically equivocal and patient 8 was seronegative, although Bb plasmid DNA was detected in whole blood and serum from this patient.
Blood cultures from 11 patients were incubated for four weeks and checked weekly for spirochete growth using light and darkfield microscopy. Motile spirochetes and/or motile spherules were observed in the culture fluid from all 11 patients after four weeks (Table 2). Genital cultures from the four controls were incubated for four weeks. None of the control cultures contained visible spirochetes, and the cultures were sent for PCR testing. Genital cultures from the 11 patients were incubated for four weeks and checked weekly. Motile spirochetes were observed in the culture fluid from all 11 patients after four weeks (Figure 1A). See Dataset, data file 1.
See Dataset, data file 1. ND, not done.
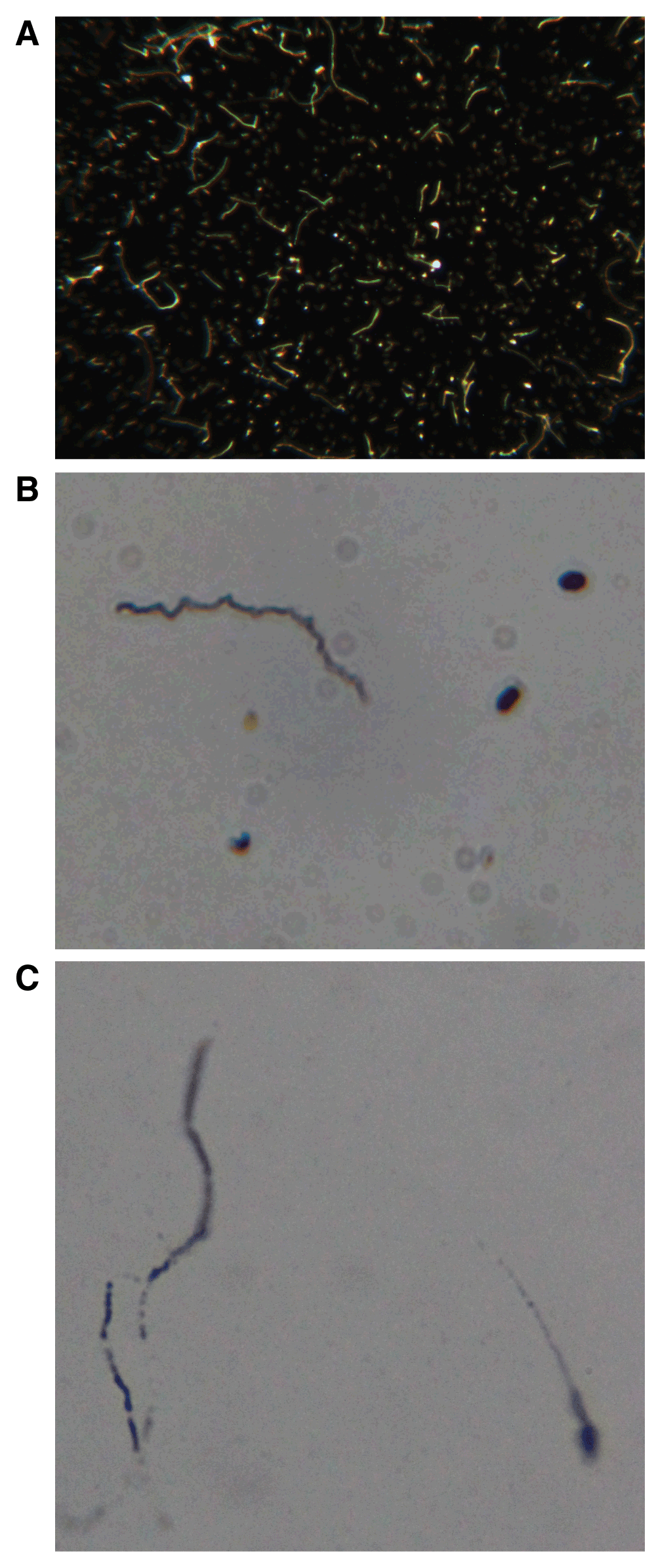
A: Darkfield image of genital culture from Patient 1. Note numerous spirochetes. 400× magnification. See Dataset, data file 1. B: Dieterle silver stain of genital culture from Patient 12. Note darkly staining spirochete. Formalin fixed slide, 400× magnification. See Dataset, data file 2. C: Semen sample from Patient 10 showing B. burgdorferi spirochetes (left) and sperm cell (right). Dieterle silver stain of acetone fixed slide, 1000× magnification. See Dataset, data file 2.
Most genital cultures grew very well and contained abundant spirochetes, but some blood cultures contained few spirochetes. Therefore, to better document the presence of spirochetes in culture, the culture fluid was concentrated into pellets by centrifugation (Table 3). Spirochetes and/or spherules were detected by sectioning and special staining of paraffin blocked pellets in all the patient blood and genital cultures concentrated by centrifugation, except for blood and genital culture pellets from Patient 1 that were lost during paraffin blocking (Table 3). Control genital culture samples were sent directly for PCR testing and were not subjected to light and darkfield microscopy.
See Dataset, data file 2.
A. Dieterle silver staining. Using standard Dieterle staining, spherules and/or spirochetal forms were visible in all patient genital cultures (Figure 1B). Spirochetes were detected in all patient genital culture pellets except for Patient 1, whose pellet was lost during processing (Table 3). Using the newer fixation method, spirochetes and sperm cells were visible in semen samples and showed distinct morphology (Figure 1C). Sperm cells are known to stain with silver stains (Pathak et al., 1979; Schmid et al., 1983). Sperm cells were seen in all semen samples except for Patients 2 and 6, who had vasectomies (data not shown). Since control genital cultures had no visible spirochetes, the control samples were sent directly for PCR testing and were not subjected to Dieterle silver staining. See Dataset, data file 2.
B. Anti-Bb immunostaining.
I. Culture fluid – University of New Haven
Genital culture fluid from Patient 1 was fixed on a SuperFrost™ Plus microscope slide and was stained with FITC-labelled polyclonal anti-Bb antibody. Staining was strongly positive, revealing well-defined spirochetes morphologically consistent with Bb (Figure 2A). The polyclonal antibody was not reactive to T. denticola (data not shown).
II. Culture pellets – McClain Laboratories
Anti-Bb immunostaining was positive for all genital cultures except for Patient 1, whose pellet was lost during processing (Table 4). Immunostaining revealed both spiral and globular Bb forms (Figure 2B). Since control genital cultures had no visible spirochetes, the control samples were sent directly for PCR testing and were not subjected to immunostaining. See Dataset, data file 3.
See Dataset, data files 3 and 4. ND, not done.
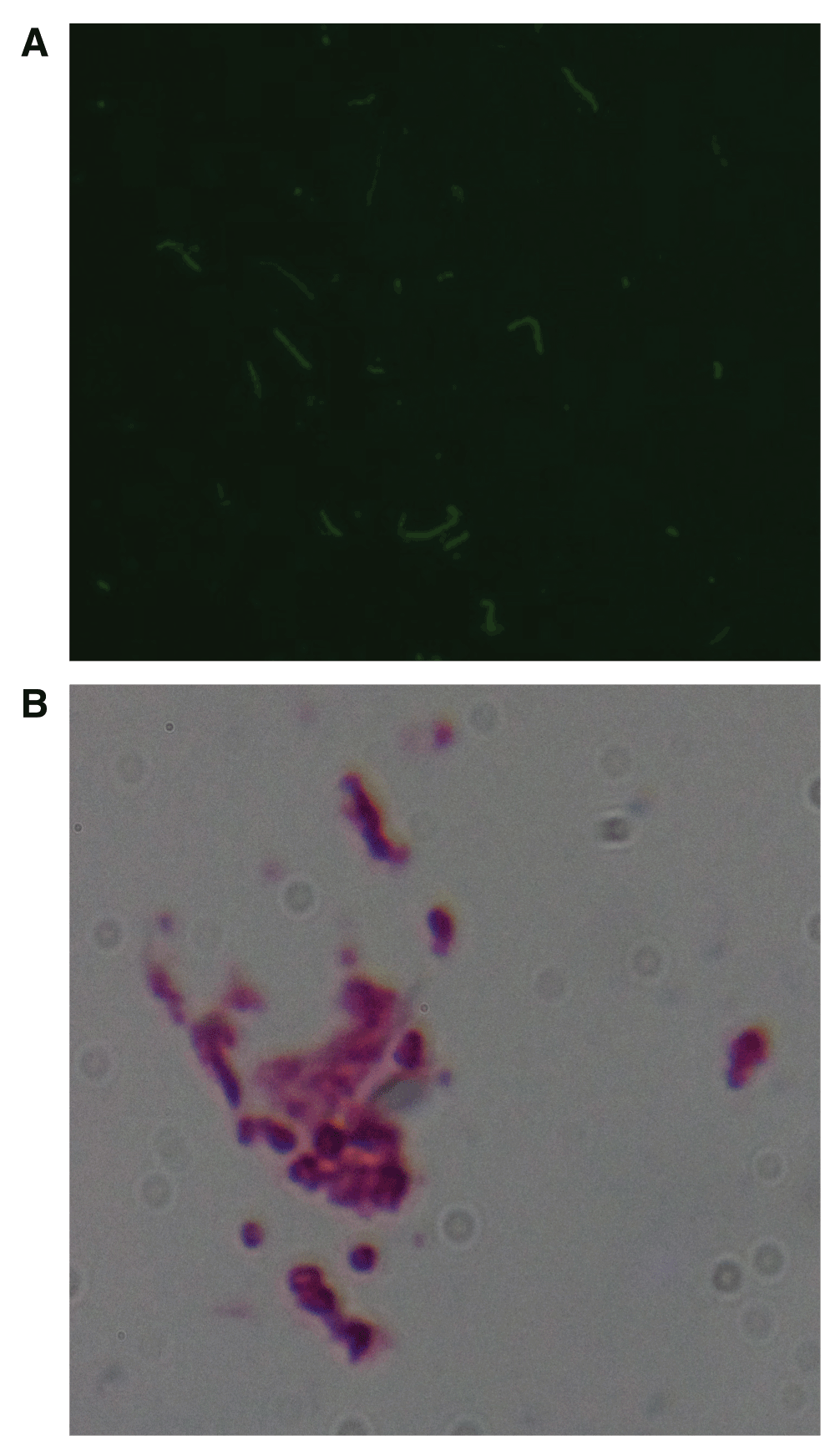
A: B. burgdorferi immunostaining of vaginal culture from Patient 1. Note intensely staining spiral and round forms in culture. 400× magnification. B: B. burgdorferi immunostaining of seminal culture from Patient 6. Note intensely staining spiral and round forms in culture. 400× magnification. See Dataset, data file 3.
Hybridization with the Fla B probe was positive for genital culture pellets from Patients 2–9 (Table 4). The culture pellet from Patient 1 was lost during processing. The molecular probe showed intense staining in vaginal secretions and less intense staining in semen samples (Figure 3A and 3B). See Dataset, data file 4.
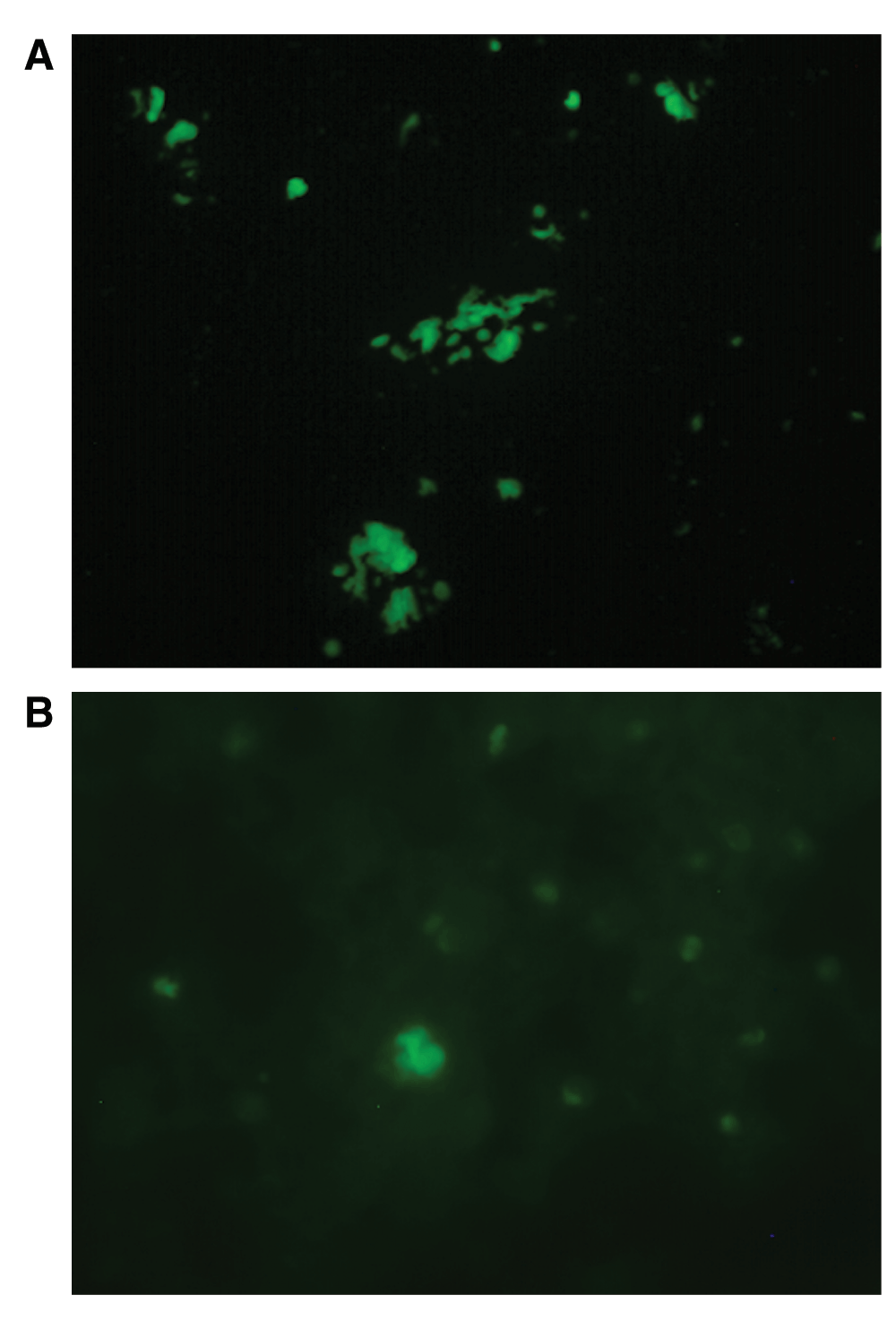
A: Molecular hybridization of B. burgdorferi-specific FlaB probe with seminal culture from Patient 6. Note intensely staining spiral and round forms in culture. 400× magnification. B: Molecular hybridization of B. burgdorferi-specific FlaB probe with vaginal culture from Patient 7. Note intensely staining spiral and round forms in culture. 400× magnification. See Dataset, data file 4.
A. Australian Biologics. Borrelia 16S rRNA sequence was not detected by real-time PCR in any of the control genital culture pellets. In contrast, Borrelia 16S rRNA sequence was detected in genital culture pellets from 11 of 13 patients (Table 5A). Patient 2 had equivocal test results and Patient 3 had negative test results in seminal cultures. See Dataset, data file 5. Real-time PCR failed to detect treponemal gene sequences in any of the control or patient genital culture pellets. See Dataset, data file 5a. The 16S rRNA isolates from six patients were sequenced and subjected to BLAST analysis (see below).
B. University of New Haven. PCR testing using the TaqMan assay for Borrelia 16S rRNA sequence was positive in blood culture pellets from seven of nine patients tested (Table 5B). Patients 1 and 5 had negative results in blood culture pellets using the TaqMan assay, but both were positive by nested PCR for the pyrG gene. In addition, nested PCR targeting the fla gene was performed on blood culture pellets from Patients 2, 3 and 4, and nested PCR targeting the 16S rRNA gene was performed on the blood culture pellet from Patient 6. The samples were positive, and sequencing revealed 99–100% homology with Bb sensu stricto strain B-31 (Table 5B). See Dataset, data file 7.
Table 5A: Real-time PCR – Australian Biologics.
Table 5B. PCR – University of New Haven.
PCR testing using the TaqMan assay for Borrelia 16S rRNA sequence was negative in all four control genital culture pellets, and nested PCR targeting the pyrG and fla genes was negative in all four control samples, confirming the results of the TaqMan assay (Table 5B). In contrast, eight of nine patients were positive for TaqMan 16S rRNA sequence in the genital culture pellets. Patient 6 was negative using the TaqMan assay for 16S rRNA sequence but positive using nested PCR targeting a different portion of the 16S rRNA gene (Table 5B). Nested PCR targeting the fla gene (Patient 3) and the 16S rRNA gene (Patients 3 and 7) was also performed on genital culture pellets and was positive in those patients, confirming the results of the TaqMan assay. Patient 12 had positive PCR targeting the pyrG gene with confirmatory sequencing (see below).
PCR isolates of the vaginal culture from Patient 1 (Australian Biologics) and the seminal culture from Patient 3 (University of New Haven) were subjected to Sanger sequencing and BLAST analysis and showed 97–99% homology with Bb sensu stricto strain B-31 (Table 5A and Table 5B). See Datasets, data files 6 and 7. PCR isolates of blood cultures from Patients 2, 3, 4 and 6 were subjected to Sanger sequencing and BLAST analysis at University of New Haven and showed 99–100% homology with Bb sensu stricto strain B-31 (Table 5B). See Dataset, data file 7.
PCR isolates of genital cultures from three couples having unprotected sex (Patients 6-7, 10-11 and 12-13) were subjected to Sanger sequencing and BLAST analysis. Patients 6, 7, 10, 11 and 13 had sequencing done at Australian Biologics, while Patient 12 had sequencing done at University of New Haven. Sequencing revealed that the first and third couples had Borrelia strains that matched Bb sensu stricto strain B-31 (Table 6). In contrast, the second couple had PCR sequences that matched B. hermsii strain YOR. Thus the Borrelia strain shared by this couple differed significantly from the strains identified in the other couples. See Dataset, data file 6.
Sequencing for Patients 6, 7, 10, 11 and 13 was done at Australian Biologics. Sequencing for Patient 12 was done at University of New Haven. See Dataset, data file 6.
In this study using standard and published culture, immunohistochemical, molecular hybridization and PCR techniques, we have shown that Borrelia strains are present in semen and vaginal secretions from patients with Lyme disease. Simultaneous testing for treponemal spirochetes was negative in genital secretions of all Lyme disease patients, confirming the specificity of Borrelia detection in these patients. Furthermore we have shown that couples having unprotected sex have virtually identical strains of Borrelia in their genital secretions, suggesting that Borrelia spirochetes might be transmitted from person to person without a tick vector.
As expected, PCR sequencing of cultured Borrelia from semen and vaginal secretions yielded primarily Bb sensu stricto strains, reflecting the North American origin of our study subjects. In addition, PCR sequencing of genital secretions from one couple yielded identical strains of Bb sensu stricto strains in two different laboratories. However, we were surprised to find one couple with identical strains of B. hermsii in their genital secretions. The presence of a distinct Borrelia strain in semen and vaginal secretions from a sexually active couple that differs from strains found in other couples supports the premise of Borrelia transmission via shared genital secretions. The finding is analogous to sharing distinct human immunodeficiency virus (HIV) strains, which is well recognized in sexual partners with HIV/AIDS (Shaw & Hunter, 2012).
Animal models have provided compelling evidence for contact transmission of Bb without a tick vector in mice, ducks, cats and dogs (Burgess et al., 1986; Burgess & Patrican, 1987; Burgess, 1989; Burgess, 1992; Wright & Neilsen, 1990). Bb has been shown to survive in stored semen from dogs, rams and bulls (Kumi-Diaka & Harris, 1995). Furthermore, seminal transmission of Bb has been noted in dogs, as described above (Gustafson, 1993). In contrast, contact transmission of Bb could not be demonstrated in Lewis rats and Syrian golden hamsters (Moody & Barthold, 1991; Woodrum & Oliver, 1999). Technical limitations in the study of these highly inbred rodents may have contributed to the negative results.
While it is not possible to perform controlled sexual transmission studies in humans, several investigators have speculated that this mode of transmission is possible (Bach, 2001; Harvey & Salvato, 2003; Stricker et al., 2004). The suggestion that Bb could be transmitted sexually was initially proposed by Bach in 2001. He observed that sexually active patients had a marked propensity for antibiotic failure and speculated that re-infection occurred by intimate person-to-person contact. Bb DNA was detected by PCR technology in human breast milk, umbilical cord blood, semen and vaginal secretions taken from patients presenting at his practice (Bach, 2001).
The study of a group of chronically ill Bb-seropositive and PCR-positive patients in Houston, Texas – a non-endemic area – provided epidemiological evidence that Lyme disease could spread in the absence of a suitable vector (Harvey & Salvato, 2003). In the absence of infected ticks, intimate person-to-person transfer was implicated as the probable means of transmission (Harvey & Salvato, 2003). A study by Stricker et al. provided clinical and immunological evidence for Bb transmission from partner to partner. In heterosexual seropositive couples with Lyme disease in which only one partner had a documented tick bite, the partner with the documented tick bite tended to have more severe clinical manifestations of the disease and a lower CD57 natural killer (NK) cell level (Stricker et al., 2004). This difference in clinical severity and CD57 NK cell level was not noted in seropositive couples diagnosed with Lyme disease in which both partners had a documented history of tick bite (Stricker et al., 2004). Sexual transfer of Borrelia infection through mucosal contact therefore seems possible in humans. The fact that we have been able to culture motile, actively reproducing, viable spirochetes from human genital secretions reinforces this hypothesis.
Recent reports from the Centers for Disease Control and Prevention (CDC) indicate that more than 300,000 cases of Lyme disease are diagnosed yearly in the USA (CDC, 2013). Sexual transmission of Borrelia may partly explain the large number of annual cases that is almost two times higher than breast cancer and six times higher that HIV/AIDS (Stricker & Johnson, 2014). Recognition of possible sexual transmission of Borrelia in both humans and animals is fundamentally important because of the epidemiological implications. If sexual transmission of Borrelia occurs in both animals and humans, this mode of transmission is a possible means of introducing Borrelia infection into areas not considered endemic and of introducing the spirochete to new reservoirs. Borrelia would also join the list of other spirochetes that are either proven or postulated to be sexually transmitted, including the spirochetal agents of syphilis and leptospirosis (Harrison & Fitzgerald, 1988; Maatouk & Moutran, 2014).
Lyme disease diagnosis is based largely upon serological testing using CDC-sanctioned two-tier surveillance criteria supported by FDA-approved commercial test kits. While most patients in this study did have positive serological test results for Lyme borreliosis, some were considered serologically negative, and the majority of our study subjects did not meet the positive standard as defined by the CDC surveillance criteria (CDC, 2014a). We were able to detect Borrelia spirochetes in the blood and/or genital secretions of all patients who were clinically diagnosed with Lyme disease, demonstrating that the CDC surveillance protocol is inadequate diagnostically. Inadequate diagnostic methodology undoubtedly results in under-reporting of Lyme disease, and at least one group has speculated that this substandard methodology is considered acceptable because Borrelia is not sexually transmitted (Lange & Sayyedi, 2002). In addition, if Borrelia spirochetes were transmitted sexually, then patients with false-negative results may unknowingly spread the infection to sexual partners.
The 2011 CDC case definition for Lyme disease states that a positive Bb culture confirms the diagnosis of the disease (CDC, 2014b). Although culture of Borrelia genital isolates may be a useful diagnostic laboratory methodology in the future, detecting and characterizing cultured Borrelia isolates is not straightforward, and both false-positive and false-negative results could occur. In our experience, human clinical isolates from genital secretions frequently propagate prolifically in culture, but on occasion they do not. In such instances, the culture must be concentrated and specific staining should be conducted to ascertain the presence of spirochetes. Once detected, spirochetes must be characterized genetically for specific identification. PCR is currently the most reliable means for correctly identifying cultured isolates, but even this methodology has drawbacks and limitations (Lange & Sayyedi, 2002; Nolte, 2012).
There are currently no standardized FDA-approved PCR protocols or kits available for Bb detection, so commercial PCR testing constitutes an array of “home brew” assays using different methodologies such as real-time PCR and nested PCR, with various primers targeting different genes, yielding wide differences in sensitivity and specificity (Nolte, 2012; Schmidt, 1997; Yang et al., 2012). False negatives can result because primers may be strain-specific and may not detect all Borrelia genotypes, and fluids such as blood, semen and vaginal secretions may contain substances inhibitory to the PCR process (Lange & Sayyedi, 2002; Nolte, 2012; Yang et al., 2012). The potential for false-positive PCR testing may also arise if there is DNA contamination in the laboratory, and appropriate positive and negative controls must be included in the assay (Nolte, 2012; Lange & Sayyedi, 2002). We experienced differences in primer specificity in our clinical isolates and also found that inhibition occurred, particularly in semen cultures.
Another complicating factor in Borrelia isolation is the morphological variation of the spirochete, which includes spherical, granular or cystic forms. Morphological variants of Bb, some of which are not culturable, are well documented in the medical literature (Barthold et al., 2010; Hodzic et al., 2014; Kurtti et al., 1987; Preac Mursic et al., 1996). These variants may play a role in infection, enabling Bb and other pathogenic spirochetes to evade the immune system (Döpfer et al., 2012; Menten-Dedoyart, 2012; Preac Mursic et al., 1996). Limited Bb growth and non-spiral morphology are thought to be induced by unfavorable environmental conditions (Brorson et al., 2009), and these features appear to be consistent with our observations. We found that Borrelia growth was more vigorous with more long slender morphological variants in cultures of genital secretions compared to cultures of blood, and we speculate that the human circulatory system is a more hostile environment for Borrelia than the human reproductive system.
Several questions have been raised about the likelihood of Borrelia sexual transmission (Craig, 2014). First, according to the CDC surveillance system Lyme disease occurs most commonly in children and older adults. However, the CDC surveillance system only captures about 10% of Lyme disease patients, and the other 90% may have a different demographic distribution consistent with sexual transmission, as shown in a recent study from Australia (Mayne, 2015). Second, while sexually transmitted diseases like herpes simplex virus (HSV) and gonorrhea show an urban predominance, Lyme disease has a more rural distribution (Craig, 2014). However, Lyme disease is acquired in more ways than HSV and gonorrhea, and the rate of sexual transmission is unknown at present. Thus the epidemiology of Lyme disease may differ from other sexually transmitted diseases based on these undefined variables. Third, the transmission of HIV can be traced from one sex partner to another using HIV strain typing. Based on our study, a similar transmission pattern using Borrelia strain typing may be seen once larger studies are performed among couples having unprotected sex. In summary, sexual transmission of Borrelia is plausible in light of our limited knowledge about the risk of acquiring Lyme disease.
In conclusion, we have shown that Borrelia spirochetes are present in semen and vaginal secretions of patients with Lyme disease. Furthermore, virtually identical strains of Borrelia are present in couples having unprotected sex, suggesting that transmission via intimate contact without a tick vector may occur. The epidemiology and clinical risk of Borrelia sexual transmission remain to be determined.
F1000Research: Dataset 1. Raw data of Borrelia spirochetes in human vaginal and seminal secretions, 10.5256/f1000research.5778.d40491 (Middelveen et al., 2014b).
Written informed consent to publish clinical details and study results was obtained from each participant.
MJM recruited patients, performed the spirochete cultures and wrote the original manuscript. CB, KRF, AT and ES performed the IFA and PCR studies. JB, YW and AF performed the PCR studies. HAS and PJM provided patient samples and edited the manuscript. RBS recruited patients, coordinated all studies, revised the manuscript and edited it for publication. All authors approved the manuscript for publication.
The authors have no competing interests to declare. Preliminary results of the study were presented at the Western Regional Meeting of the American Federation for Medical Research, Carmel, CA, on January 25, 2014, and published in abstract form (J Invest Med 2014;62:280–1).
Supported in part by a grant to MJM from the Lindorf Family Foundation, Newark, OH. This work is dedicated to the memory of Dr. Willy Burgdorfer.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors thank Drs. Stewart Adams, Gordon Atkins, Robert Bransfield, George Chaconas, Douglas Demetrick, Dorte Dopfer, Christopher Hardy, Nick Harris, Doug Kahn, Alan MacDonald, Steve McClain, Kary Mullis, Jyotsna Shah, Leo Shea and Janet Sperling for helpful discussion. We are grateful to Dr. Robert B. Allan, Joel Israel and Anita Vieyra for technical support, and we thank Lorraine Johnson for manuscript review.
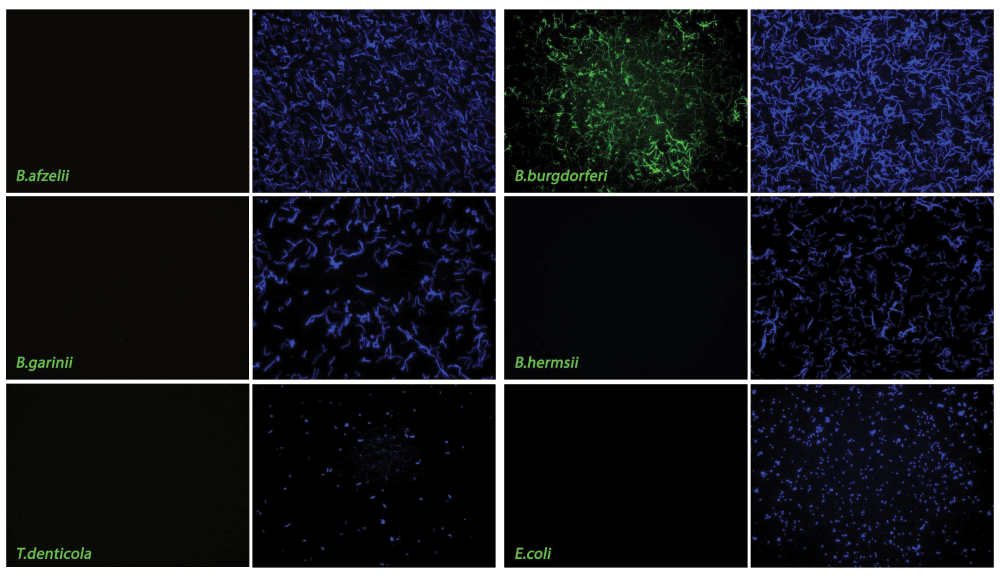
FlaB hybridization is shown in green, while DAPI counterstain of bacterial targets is shown in blue. Note specific hybridization of FlaB probe with B. burgdorferi sensu stricto and lack of hybridization with other Borrelia strains, T. denticola or E.coli. 400× magnification. See Dataset, data file 4.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 3 (revision) 27 Apr 15 |
read | read | read | |
|
Version 2 (revision) 20 Jan 15 |
read | read | ||
|
Version 1 18 Dec 14 |
read | |||
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (5)
[References]
[[1|title=Untersuchungen Über die Aetiologie der Wundinfectionskrankheiten|authors=Koch/R|vol= Leipzig: F.C.W. Vogel|year=1878]]
[[2|title=Zur Ätiologie und Pathogenese des Abdominaltyphus|authors=Jürgens/GJ|vol=Ztschr. f. klin. Med., Berl.|year=1904]]
[[3|title=Chapter 3 Infectious Diseases Related To Travel: Typhoid & Paratyphoid Fever In CDC Health Information for International Travel|authors=Newton/AE;Mintz/E |source=CDC|year=August 01, 2013|url=http://wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-diseases-related-to-travel/typhoid-and-paratyphoid-fever]]
[[4|title=DNA sequencing diagnosis of off-season spirochetemia with low bacterial density in Borrelia burgdorferi and Borrelia miyamotoi infections|authors=Lee/SH;Vigliotti/JS;Vigliotti/VS;Jones/W;Moorcroft/TA;Lantsman/K|source=Int J Mol Sci|vol=15|issue=7|year=2014|fpage=11364|lpage=11386|type=journal|doi=10.3390/ijms150711364|pmid=24968274]]
References
1. Koch R: Untersuchungen Über die Aetiologie der Wundinfectionskrankheiten. 1878; Leipzig: F.C.W. Vogel.
2. Jürgens GJ: Zur Ätiologie und Pathogenese des Abdominaltyphus. 1904; Ztschr. f. klin. Med., Berl..
3. Newton AE, Mintz E: Chapter 3 Infectious Diseases Related To Travel: Typhoid & Paratyphoid Fever In CDC Health Information for International Travel. CDC. August 01, 2013. Reference Source
4. Lee SH, Vigliotti JS, Vigliotti VS, Jones W, et al.: DNA sequencing diagnosis of off-season spirochetemia with low bacterial density in Borrelia burgdorferi and Borrelia miyamotoi infections. Int J Mol Sci. 2014; 15 (7): 11364-11386 PubMed Abstract | Publisher Full Text
Most recent data from the CDC still continue to show that, unlike the reported incidence of sexually transmitted diseases, reported cases of Lyme disease are concentrated in the same 13 States as in previous years. Obviously, the epidemiology is the same whether the reported number of cases is 30K or 300K per year.
Most recent data from the CDC still continue to show that, unlike the reported incidence of sexually transmitted diseases, reported cases of Lyme disease are concentrated in the same 13 States as in previous years. Obviously, the epidemiology is the same whether the reported number of cases is 30K or 300K per year.
We appreciate Phillip Baker's interest in our study showing live, culturable Borrelia spirochetes in semen and vaginal secretions from patients with Lyme disease. We used microscopy, ... Continue reading Co-written with Marianne J. Middelveen
We appreciate Phillip Baker's interest in our study showing live, culturable Borrelia spirochetes in semen and vaginal secretions from patients with Lyme disease. We used microscopy, immunochemistry, molecular hybridization and polymerase chain reaction (PCR) techniques to demonstrate the presence of these live spirochetes in cultures of genital secretions, and as stated in the study our results suggest that Lyme disease could be sexually transmitted. All of the concerns in Baker’s commentary were previously aired on the Internet, and these concerns were addressed in our report. His objection has little merit for the reasons described below.
Baker represents a group of researchers who cling to the concept that Lyme disease is a trivial illness that is "hard to catch and easy to cure", and that chronic Lyme disease due to persistent infection with the Lyme spirochete, Borrelia burgdorferi, is rare or non-existent[ref-1],[ref-2]. As discussed elsewhere, this "Lyme denialist" philosophy and disregard for opposing viewpoints has been a major factor in the epidemic spread of Lyme disease, which accounts for more than 300,000 new cases per year in the USA according to the latest Centers for Disease Control and Prevention (CDC) estimates[ref-2]. As stated in our article, this alarming epidemic is twice as common as breast cancer and six times more common than HIV/AIDS, and its prevalence is hard to explain by tickbite transmission alone. Thus from an epidemiological perspective, sexual transmission of the live Borrelia spirochetes found in genital secretions is a plausible mechanism for the spread of Lyme disease.
Baker's commentary on our article uses selective information that supports his point of view while ignoring contradictory evidence. He starts with two animal models that appear to refute contact or sexual transmission of Lyme disease, and he concludes with selective epidemiological evidence from the CDC that obliquely supports his view. Sandwiched in between is a flawed discussion of maternal-fetal transmission of Lyme disease that has no bearing on sexual transmission of the spirochetal infection. Although we have addressed the animal model and epidemiological issues in our article, we will address these issues in greater detail below. We will leave it to readers to find the flaws in Baker's denial of maternal-fetal transmission of Lyme disease, since this form of transmission has been documented in canine, bovine, murine and human reports from the peer-reviewed medical literature[ref-3]-[ref-8].
Baker cites two animal studies as proof that Lyme disease cannot be sexually transmitted[ref-9],[ref-10]. The study by Moody and Barthold was performed using Lewis rats, while the study by Woodrum and Oliver was performed using Syrian hamsters. In limiting his analysis to these two studies, Baker ignores the compelling observations in mice, birds, cats and dogs that support contact or sexual transmission of Lyme disease without a tick vector, as outlined in our report[ref-4],[ref-11]-[ref-13]. The Lewis rat and Syrian hamster studies had significant flaws. First, these rodents are highly inbred with all Lewis rats descended from a single breeding colony, and all Syrian hamsters descended from a single female breeder[ref-14],[ref-15]. The effect of inbreeding on contact transmission of Borrelia is unknown. Second, these rodents are poor models for human Lyme disease. The Lewis rat develops a transient arthritis that fades without treatment following intraperitoneal injection of Borrelia, while the Syrian hamster may be colonized by Borrelia for long periods without showing any signs of disease[ref-16],[ref-17]. Since the disease pattern in these animals differs significantly from the human pattern, it is not surprising that transmission of Borrelia would differ as well. In fact, the immunocompetent Syrian hamster has been abandoned as a model for human Lyme disease because of these differences. Third and perhaps most important, both studies used limited culture and immunological methods to determine sexual transmission of Borrelia, and more sensitive detection of spirochetes by molecular techniques might have yielded positive results, as shown in other rodent experiments using PCR[ref-4]. Thus the selective animal models cited by Baker fail to refute the possibility of sexual transmission of Lyme disease in humans.
Baker attempts to use CDC surveillance statistics to further disprove sexual transmission of Lyme disease. He points to the fact that 95% of reported CDC surveillance cases occur in 12 states in the USA, and that these reported cases are seasonal and have a male and child predominance. As pointed out in our article, however, CDC surveillance cases account for less than 10% of total Lyme cases (about 30,000 cases out of more than 300,000 annual cases in the USA). Thus the epidemiology of the vast majority of Lyme cases is not covered by CDC surveillance reporting and may differ significantly from the epidemiology reported by the CDC. To support this view, as noted in our study, a recent report from Australia found that the prevalence of Lyme disease cases aligned with the sexually active population on that continent[ref-18]. Baker also questions whether our Lyme disease testing was accurate since it differs from the serological parameters used for CDC surveillance criteria. However the CDC surveillance criteria rely on testing that misses more than half of Lyme disease cases[ref-19], and these criteria are not recommended for diagnosis of Lyme disease[ref-20]. The reason why Baker thinks that insensitive and incomplete epidemiology statistics trump microscopy, immunochemistry, molecular hybridization and PCR testing that demonstrates live, culturable Borrelia spirochetes in genital secretions from our Lyme disease patients is unclear.
Baker bemoans the fact that because of the inconvenient evidence presented in our study, Lyme disease patients may suddenly be concerned about sexual transmission of their disease. Because Baker does not represent or treat patients, he may be unaware that this question is being asked by patients in clinicians’ offices every day as spouses and sexual partners become symptomatic. Should Lyme disease patients be concerned about sexual transmission? Absolutely. We currently treat Lyme disease with 60-year-old antibiotics that are marginally effective against a chronic infectious disease[ref-1],[ref-21]. Baker and his research associates should focus on the risk of sexual transmission of Borrelia and strive to develop new treatments for this alarming epidemic infection along the lines of the global "Manhattan Project" that has successfully dealt with the HIV/AIDS epidemic[ref-2],[ref-19]. Our suffering Lyme disease patients deserve nothing less.
[References]
[[1|title=Lyme disease: The next decade|authors=Stricker/RB;Johnson/L|source= Infect Drug Resist|vol=4|year=2011|fpage=1|lpage=9|type=journal|pmid=21694904|pmcid= PMC3108755]]
[[2|title=Lyme disease: Call for a “Manhattan Project” to combat the epidemic|authors=Stricker/RB;Johnson/L|source=PLoS Pathog|vol=10|issue=1|year=2014|fpage=e1003796|type=Journal|doi=10.1371/journal.ppat.1003796|pmid=24391491|pmcid=PMC3879353]]
[[3|title=Intrauterine transmission of Borrelia burgdorferi in dogs|authors=Gustafson/JM;Burgess/EC;Wachal/MD;Steinberg/H|source=Am J Vet Res|vol=54|issue=6|year=1993|fpage=882|lpage=890|type=journal|pmid= 8323057]]
[[4|title=Borrelia burgdorferi infection in dairy cows, rodents, and birds from four Wisconsin dairy farms|authors=Burgess/EC;Wachal/MD;Cleven/TD|source=Vet Microbiol|vol=35|issue=1-2|year=1993|fpage=61|lpage=77|type=journal|pmid=8362496]]
[[5|title=Maternal-fetal transmission of the Lyme disease spirochete, Borrelia burgdorferi|authors=Schlesinger/PA;Duray/PH;Burke/BA;Steere/AC;Stillman/MT|source= Ann Intern Med|vol=103|issue=1|year=1985|fpage=67|lpage=68|type=journal|pmid=4003991]]
[[6|title=Lyme Borrelia positive serology associated with spontaneous abortion in an endemic Italian area|authors=Carlomagno/G;Luksa/V;Candussi/G;Rizzi/GM;Trevisan/G|source=Acta Eur Fertil|vol=19|issue=5|year=1988|fpage=279|lpage=281|type=Journal|pmid=3252658]]
[[7|title=Borrelia burgdorferi in a newborn despite oral penicillin for Lyme borreliosis during pregnancy|authors=Weber/K;Bratzke/HJ;Neubert/U;Wilske/B;Duray/PH|source=Pediatr Infect Dis J|vol=7|issue=4|year=1988|fpage=286|lpage=289|type=journal|pmid=3130607]]
[[8|title=Gestational Lyme borreliosis: implications for the fetus|authors=MacDonald/AB|source=Rheum Dis Clin North Am|vol=15|issue=4|year=1989|fpage=657|lpage=677|type=journal|pmid=2685924]]
[[9|title=Relative infectivity of Borrelia burgdorferi in Lewis rats by various routes of inoculation|authors=Moody/KD;Barthold/SW|source= Am J Trop Med Hyg|vol=44|issue=2|year=1991|fpage=135|lpage=139|type=journal|pmid=2012256]]
[[10|title=Investigation of venereal, transplacental, and contact transmission of the Lyme disease spirochete, Borrelia burgdorferi, in Syrian hamsters|authors=Woodrum/JE;Oliver/JH|source=J Parasitol|vol=85|issue=3|year=1999|fpage=426|lpage=430|type=journal|pmid=10386432]]
[[11|title=Experimental inoculation of Peromyscus spp. with Borrelia burgdorferi: Evidence of contact transmission|authors=Burgess/EC;Amundson/TE;Davis/JP;Kaslow/RA;Edelman/R|source=Am J Trop Med Hyg|vol=35|issue=2|year=1986|fpage=355|lpage=359|type=journal|pmid=3513648]]
[[12|title=Experimental infection of the white-footed mouse with Borrelia burgdorferi|authors=Wright/SD;Nielsen/SW|source=Am J Vet Res|vol=51|issue=12|year=1990|fpage=1980|lpage=1987|type=journal|pmid=2085225]]
[[13|title=The in utero and seminal transmission of Borrelia burgdorferi in Canidae (PhD thesis, University of Wisconsin, Madison; Accessed: December 30, 2014)|authors=Gustafson/JM|year=1993|url=http://search.library.wisc.edu/catalog/ocm29551629]]
[[14|title=Lewis rat (Accessed: December 30, 2014)|source=Wikipedia|url=http://en.wikipedia.org/wiki/Laboratory_rat]]
[[15|title=Golden hamster (Accessed: December 30, 2014)|source=Wikipedia|url=http://en.wikipedia.org/wiki/Golden_hamster]]
[[16|title=Experimental chronic Lyme borreliosis in Lewis rats|authors=Moody/KD;Barthold/SW;Terwilliger/GA;Beck/DS;Hansen/GM;Jacoby/RO|source=Am J Trop Med Hyg|vol=42|issue=2|year=1990|fpage=165|lpage=174|type=journal|pmid=2138431]]
[[17|title=Experimental infection of the hamster with Borrelia burgdorferi|authors=Johnson/RC;Codner/C;Russell/M;Duray/PH|source=Ann N Y Acad Sci|vol=539|year=1988|fpage=258|lpage=263|type=journal|doi=10.1111/j.1749-6632.1988.tb31859.x]]
[[18|title=Clinical determinants of Lyme borreliosis, babesiosis, bartonellosis, anaplasmosis and ehrlichiosis in an Australian cohort|authors=Mayne/PJ|source=Int J Gen Med|vol=8|year=2015|fpage=15|lpage=26|type=journal|doi=10.2147/IJGM.S75825|url= http://www.dovepress.com/clinical-determinants-of-lyme-borreliosis-babesiosis-bartonellosis-ana-peer-reviewed-article-IJGM#]]
[[19|title=Lyme disease diagnosis and treatment: lessons from the AIDS epidemic|authors=Stricker/RB;Johnson/L|source=Minerva Med|vol=101|issue=6|year=2010|fpage=419|lpage=425|type=journal|pmid=21196901]]
[[20|title= Lyme disease: 2011 case definition (Accessed: December 30, 2014)|source=CDC|url=http://wwwn.cdc.gov/NNDSS/script/casedef.aspx?CondYrID=752andDatePub=1/1/2011%2012:00:00%20AM.]]
[[21|title=Relevance of chronic Lyme disease to family medicine as a complex multidimensional chronic disease construct: a systematic review|authors=Borgermans/L;Goderis/G;Vandevoorde/J;Devroey/D|source=Int J Family Med|vol=2014|year=2014|type=journal|doi=10.1155/2014/138016|url=http://www.hindawi.com/journals/ijfm/2014/138016/]]
We appreciate Phillip Baker's interest in our study showing live, culturable Borrelia spirochetes in semen and vaginal secretions from patients with Lyme disease. We used microscopy, immunochemistry, molecular hybridization and polymerase chain reaction (PCR) techniques to demonstrate the presence of these live spirochetes in cultures of genital secretions, and as stated in the study our results suggest that Lyme disease could be sexually transmitted. All of the concerns in Baker’s commentary were previously aired on the Internet, and these concerns were addressed in our report. His objection has little merit for the reasons described below.
Baker represents a group of researchers who cling to the concept that Lyme disease is a trivial illness that is "hard to catch and easy to cure", and that chronic Lyme disease due to persistent infection with the Lyme spirochete, Borrelia burgdorferi, is rare or non-existent1,2. As discussed elsewhere, this "Lyme denialist" philosophy and disregard for opposing viewpoints has been a major factor in the epidemic spread of Lyme disease, which accounts for more than 300,000 new cases per year in the USA according to the latest Centers for Disease Control and Prevention (CDC) estimates2. As stated in our article, this alarming epidemic is twice as common as breast cancer and six times more common than HIV/AIDS, and its prevalence is hard to explain by tickbite transmission alone. Thus from an epidemiological perspective, sexual transmission of the live Borrelia spirochetes found in genital secretions is a plausible mechanism for the spread of Lyme disease.
Baker's commentary on our article uses selective information that supports his point of view while ignoring contradictory evidence. He starts with two animal models that appear to refute contact or sexual transmission of Lyme disease, and he concludes with selective epidemiological evidence from the CDC that obliquely supports his view. Sandwiched in between is a flawed discussion of maternal-fetal transmission of Lyme disease that has no bearing on sexual transmission of the spirochetal infection. Although we have addressed the animal model and epidemiological issues in our article, we will address these issues in greater detail below. We will leave it to readers to find the flaws in Baker's denial of maternal-fetal transmission of Lyme disease, since this form of transmission has been documented in canine, bovine, murine and human reports from the peer-reviewed medical literature3-8.
Baker cites two animal studies as proof that Lyme disease cannot be sexually transmitted9,10. The study by Moody and Barthold was performed using Lewis rats, while the study by Woodrum and Oliver was performed using Syrian hamsters. In limiting his analysis to these two studies, Baker ignores the compelling observations in mice, birds, cats and dogs that support contact or sexual transmission of Lyme disease without a tick vector, as outlined in our report4,11-13. The Lewis rat and Syrian hamster studies had significant flaws. First, these rodents are highly inbred with all Lewis rats descended from a single breeding colony, and all Syrian hamsters descended from a single female breeder14,15. The effect of inbreeding on contact transmission of Borrelia is unknown. Second, these rodents are poor models for human Lyme disease. The Lewis rat develops a transient arthritis that fades without treatment following intraperitoneal injection of Borrelia, while the Syrian hamster may be colonized by Borrelia for long periods without showing any signs of disease16,17. Since the disease pattern in these animals differs significantly from the human pattern, it is not surprising that transmission of Borrelia would differ as well. In fact, the immunocompetent Syrian hamster has been abandoned as a model for human Lyme disease because of these differences. Third and perhaps most important, both studies used limited culture and immunological methods to determine sexual transmission of Borrelia, and more sensitive detection of spirochetes by molecular techniques might have yielded positive results, as shown in other rodent experiments using PCR4. Thus the selective animal models cited by Baker fail to refute the possibility of sexual transmission of Lyme disease in humans.
Baker attempts to use CDC surveillance statistics to further disprove sexual transmission of Lyme disease. He points to the fact that 95% of reported CDC surveillance cases occur in 12 states in the USA, and that these reported cases are seasonal and have a male and child predominance. As pointed out in our article, however, CDC surveillance cases account for less than 10% of total Lyme cases (about 30,000 cases out of more than 300,000 annual cases in the USA). Thus the epidemiology of the vast majority of Lyme cases is not covered by CDC surveillance reporting and may differ significantly from the epidemiology reported by the CDC. To support this view, as noted in our study, a recent report from Australia found that the prevalence of Lyme disease cases aligned with the sexually active population on that continent18. Baker also questions whether our Lyme disease testing was accurate since it differs from the serological parameters used for CDC surveillance criteria. However the CDC surveillance criteria rely on testing that misses more than half of Lyme disease cases19, and these criteria are not recommended for diagnosis of Lyme disease20. The reason why Baker thinks that insensitive and incomplete epidemiology statistics trump microscopy, immunochemistry, molecular hybridization and PCR testing that demonstrates live, culturable Borrelia spirochetes in genital secretions from our Lyme disease patients is unclear.
Baker bemoans the fact that because of the inconvenient evidence presented in our study, Lyme disease patients may suddenly be concerned about sexual transmission of their disease. Because Baker does not represent or treat patients, he may be unaware that this question is being asked by patients in clinicians’ offices every day as spouses and sexual partners become symptomatic. Should Lyme disease patients be concerned about sexual transmission? Absolutely. We currently treat Lyme disease with 60-year-old antibiotics that are marginally effective against a chronic infectious disease1,21. Baker and his research associates should focus on the risk of sexual transmission of Borrelia and strive to develop new treatments for this alarming epidemic infection along the lines of the global "Manhattan Project" that has successfully dealt with the HIV/AIDS epidemic2,19. Our suffering Lyme disease patients deserve nothing less.
References
1. Stricker RB, Johnson L: Lyme disease: The next decade. Infect Drug Resist. 2011; 4: 1-9 PubMed Abstract | Free Full Text
2. Stricker RB, Johnson L: Lyme disease: Call for a “Manhattan Project” to combat the epidemic. PLoS Pathog. 2014; 10 (1): e1003796 PubMed Abstract | Free Full Text | Publisher Full Text
3. Gustafson JM, Burgess EC, Wachal MD, Steinberg H: Intrauterine transmission of Borrelia burgdorferi in dogs. Am J Vet Res. 1993; 54 (6): 882-890 PubMed Abstract
4. Burgess EC, Wachal MD, Cleven TD: Borrelia burgdorferi infection in dairy cows, rodents, and birds from four Wisconsin dairy farms. Vet Microbiol. 1993; 35 (1-2): 61-77 PubMed Abstract
5. Schlesinger PA, Duray PH, Burke BA, Steere AC, et al.: Maternal-fetal transmission of the Lyme disease spirochete, Borrelia burgdorferi. Ann Intern Med. 1985; 103 (1): 67-68 PubMed Abstract
6. Carlomagno G, Luksa V, Candussi G, Rizzi GM, et al.: Lyme Borrelia positive serology associated with spontaneous abortion in an endemic Italian area. Acta Eur Fertil. 1988; 19 (5): 279-281 PubMed Abstract
7. Weber K, Bratzke HJ, Neubert U, Wilske B, et al.: Borrelia burgdorferi in a newborn despite oral penicillin for Lyme borreliosis during pregnancy. Pediatr Infect Dis J. 1988; 7 (4): 286-289 PubMed Abstract
8. MacDonald AB: Gestational Lyme borreliosis: implications for the fetus. Rheum Dis Clin North Am. 1989; 15 (4): 657-677 PubMed Abstract
9. Moody KD, Barthold SW: Relative infectivity of Borrelia burgdorferi in Lewis rats by various routes of inoculation. Am J Trop Med Hyg. 1991; 44 (2): 135-139 PubMed Abstract
10. Woodrum JE, Oliver JH: Investigation of venereal, transplacental, and contact transmission of the Lyme disease spirochete, Borrelia burgdorferi, in Syrian hamsters. J Parasitol. 1999; 85 (3): 426-430 PubMed Abstract
11. Burgess EC, Amundson TE, Davis JP, Kaslow RA, et al.: Experimental inoculation of Peromyscus spp. with Borrelia burgdorferi: Evidence of contact transmission. Am J Trop Med Hyg. 1986; 35 (2): 355-359 PubMed Abstract
12. Wright SD, Nielsen SW: Experimental infection of the white-footed mouse with Borrelia burgdorferi. Am J Vet Res. 1990; 51 (12): 1980-1987 PubMed Abstract
13. Gustafson JM: The in utero and seminal transmission of Borrelia burgdorferi in Canidae (PhD thesis, University of Wisconsin, Madison; Accessed: December 30, 2014). 1993. Reference Source
14. Lewis rat (Accessed: December 30, 2014). Wikipedia. Reference Source
15. Golden hamster (Accessed: December 30, 2014). Wikipedia. Reference Source
16. Moody KD, Barthold SW, Terwilliger GA, Beck DS, et al.: Experimental chronic Lyme borreliosis in Lewis rats. Am J Trop Med Hyg. 1990; 42 (2): 165-174 PubMed Abstract
17. Johnson RC, Codner C, Russell M, Duray PH: Experimental infection of the hamster with Borrelia burgdorferi. Ann N Y Acad Sci. 1988; 539: 258-263 Publisher Full Text
18. Mayne PJ: Clinical determinants of Lyme borreliosis, babesiosis, bartonellosis, anaplasmosis and ehrlichiosis in an Australian cohort. Int J Gen Med. 2015; 8: 15-26 Publisher Full Text | Reference Source
19. Stricker RB, Johnson L: Lyme disease diagnosis and treatment: lessons from the AIDS epidemic. Minerva Med. 2010; 101 (6): 419-425 PubMed Abstract
20. Lyme disease: 2011 case definition (Accessed: December 30, 2014). CDC. Reference Source
21. Borgermans L, Goderis G, Vandevoorde J, Devroey D: Relevance of chronic Lyme disease to family medicine as a complex multidimensional chronic disease construct: a systematic review. Int J Family Med. 2014; 2014. Publisher Full Text | Reference Source
To determine if borreliosis can be transmitted by direct contact, Moody and Barthold 2 housed three-day-old - or three-week-old- Lewis rats, deliberately infected with B. burgdorferi, with normal, uninfected rats for 30 days. As expected, all deliberately infected rats continued to be actively infected, 30 days later; however, none of the uninfected rats acquired infection after 30 days of intimate direct contact with their
infected house mates.
In other experiments, Moody and Barthold 2 were unable to demonstrate venereal transmission of borreliosis from seven infected females-or six infected males - to uninfected rats of the opposite sex.
In the work of Woodrum and Oliver 3, six female Syrian hamsters infected with B.burgdorferi were mated with six uninfected males; conversely, three infected males were mated with six uninfected females. None of the uninfected hamsters became infected after mating with an infected partner of the opposite sex, indicating that borreliosis is not sexually transmitted. These investigators also failed to demonstrate contact transmission of B. burgdorferi between infected female -or male-hamsters and uninfected hamsters of the opposite sex. Also, it was not possible to transmit borreliosis to uninfected hamsters with urine or feces from infected hamsters.
Sadly, preliminary oral reports of the observations of Middelveen et al.1 have already generated an inordinate amount of fear and anxiety within the lay community due to sensationalized reports of their unconfirmed findings by an uncritical - and often naïve - press. This has already caused much harm. To date, I have received numerous inquiries from distraught individuals, wondering if they now should even consider marrying their spouse-to-be for fear of contracting Lyme disease that some mistakenly believe to be incurable. Some fear the possibility of giving birth to an infected or congenitally deformed child, because their spouse or spouse-to-be had been diagnosed and treated for Lyme disease in the past.
To examine the issue of in utero transmission of infection, Moody and Barthold 2 inoculated pregnant female Lewis rats with viable B. burgdorferi, at four days of gestation. All of the inoculated pregnant females became seropositive as expected, and B. burgdorferi could be cultured from their spleens at 20 days of gestation; however, their placentas and fetuses were culture negative, indicting the lack of in utero transmission.
Moody and Barthold 2 used two different experimental protocols to determine if transplacental transmission of B. burgdorferi occurs. One protocol involved six non-pregnant infected females that were subsequently mated and became pregnant. Three of the females were allowed to carry to full term, whereas the remaining three were sacrificed just prior to parturition. All offspring and offspring-to-be were found to be culture negative for B. burgdorferi, as well as seronegative for antibody specific for B. burgdorferi, indicating that transplacental transmission of infection does not occur.
In the second protocol, six females were infected via tick bite after becoming pregnant, and were allowed to carry their fetuses to birth; all were negative for infection. The results of these studies likewise failed to provide evidence for the transplacental transmission of naturally acquired borreliosis.
Other investigators examined the possibility of congenital birth defects in humans with Lyme disease by doing a rather large comparative study involving 5,000 infants, half from an area in which Lyme disease was endemic and half as controls from an area without Lyme disease 5. They found no significant differences in the overall incidence of congenital malformations between the two groups.
In another study, involving 1,500 subjects including controls, no increased risk of giving birth to a child with a congenital heart defect was noted in women who had either been bitten by a tick or had been treated for Lyme disease during or before pregnancy 6.
Finally, an extensive analysis of the world literature revealed “that an adverse outcome due to maternal infection with B. burgdorferi at any point during pregnancy in humans is at most extremely rare” 7.
In view of the above considerations, the work by Middelveen et al. 1 is not credible and must be view with much skepticism. There is no evidence to indicate that vaginal and seminal secretions provide a suitable environment for the growth of Borrelia 8 and that the mere presence of Borrelia in vaginal and seminal secretions – if such an observation is indeed valid and can be confirmed independently – results in disease. In the absence of such proof, no conclusions can be drawn as to the sexual transmission of Lyme disease by of B. burgdorferi.
Addendum added in proof:
It should be noted that the basic epidemiology of vector-borne Lyme disease is strikingly different from that for sexually transmitted diseases (STDs). For example, annual data provided by the CDC indicate that about 95% of all reported cases of Lyme disease occur in 12 States, and the incidence of Lyme disease is seasonal and peaks with increased tick activity. Also, in the case of STDs, there are more husband-wife paired cases rather than the preponderance of cases of males and children with Lyme disease. Most important, Middelveen et al. state that “… the majority of our study subjects did not meet the positive standard as defined by the CDC surveillance criteria.” One must therefore wonder if their study subjects ever had Lyme disease in the first place.
References
To determine if borreliosis can be transmitted by direct contact, Moody and Barthold 2 housed three-day-old - or three-week-old- Lewis rats, deliberately infected with B. burgdorferi, with normal, uninfected rats for 30 days. As expected, all deliberately infected rats continued to be actively infected, 30 days later; however, none of the uninfected rats acquired infection after 30 days of intimate direct contact with their
infected house mates.
In other experiments, Moody and Barthold 2 were unable to demonstrate venereal transmission of borreliosis from seven infected females-or six infected males - to uninfected rats of the opposite sex.
In the work of Woodrum and Oliver 3, six female Syrian hamsters infected with B.burgdorferi were mated with six uninfected males; conversely, three infected males were mated with six uninfected females. None of the uninfected hamsters became infected after mating with an infected partner of the opposite sex, indicating that borreliosis is not sexually transmitted. These investigators also failed to demonstrate contact transmission of B. burgdorferi between infected female -or male-hamsters and uninfected hamsters of the opposite sex. Also, it was not possible to transmit borreliosis to uninfected hamsters with urine or feces from infected hamsters.
Sadly, preliminary oral reports of the observations of Middelveen et al.1 have already generated an inordinate amount of fear and anxiety within the lay community due to sensationalized reports of their unconfirmed findings by an uncritical - and often naïve - press. This has already caused much harm. To date, I have received numerous inquiries from distraught individuals, wondering if they now should even consider marrying their spouse-to-be for fear of contracting Lyme disease that some mistakenly believe to be incurable. Some fear the possibility of giving birth to an infected or congenitally deformed child, because their spouse or spouse-to-be had been diagnosed and treated for Lyme disease in the past.
To examine the issue of in utero transmission of infection, Moody and Barthold 2 inoculated pregnant female Lewis rats with viable B. burgdorferi, at four days of gestation. All of the inoculated pregnant females became seropositive as expected, and B. burgdorferi could be cultured from their spleens at 20 days of gestation; however, their placentas and fetuses were culture negative, indicting the lack of in utero transmission.
Moody and Barthold 2 used two different experimental protocols to determine if transplacental transmission of B. burgdorferi occurs. One protocol involved six non-pregnant infected females that were subsequently mated and became pregnant. Three of the females were allowed to carry to full term, whereas the remaining three were sacrificed just prior to parturition. All offspring and offspring-to-be were found to be culture negative for B. burgdorferi, as well as seronegative for antibody specific for B. burgdorferi, indicating that transplacental transmission of infection does not occur.
In the second protocol, six females were infected via tick bite after becoming pregnant, and were allowed to carry their fetuses to birth; all were negative for infection. The results of these studies likewise failed to provide evidence for the transplacental transmission of naturally acquired borreliosis.
Other investigators examined the possibility of congenital birth defects in humans with Lyme disease by doing a rather large comparative study involving 5,000 infants, half from an area in which Lyme disease was endemic and half as controls from an area without Lyme disease 5. They found no significant differences in the overall incidence of congenital malformations between the two groups.
In another study, involving 1,500 subjects including controls, no increased risk of giving birth to a child with a congenital heart defect was noted in women who had either been bitten by a tick or had been treated for Lyme disease during or before pregnancy 6.
Finally, an extensive analysis of the world literature revealed “that an adverse outcome due to maternal infection with B. burgdorferi at any point during pregnancy in humans is at most extremely rare” 7.
In view of the above considerations, the work by Middelveen et al. 1 is not credible and must be view with much skepticism. There is no evidence to indicate that vaginal and seminal secretions provide a suitable environment for the growth of Borrelia 8 and that the mere presence of Borrelia in vaginal and seminal secretions – if such an observation is indeed valid and can be confirmed independently – results in disease. In the absence of such proof, no conclusions can be drawn as to the sexual transmission of Lyme disease by of B. burgdorferi.
Addendum added in proof:
It should be noted that the basic epidemiology of vector-borne Lyme disease is strikingly different from that for sexually transmitted diseases (STDs). For example, annual data provided by the CDC indicate that about 95% of all reported cases of Lyme disease occur in 12 States, and the incidence of Lyme disease is seasonal and peaks with increased tick activity. Also, in the case of STDs, there are more husband-wife paired cases rather than the preponderance of cases of males and children with Lyme disease. Most important, Middelveen et al. state that “… the majority of our study subjects did not meet the positive standard as defined by the CDC surveillance criteria.” One must therefore wonder if their study subjects ever had Lyme disease in the first place.
References