Keywords
Dopamine, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), resveratrol, SIRT1, Sirtuin
Dopamine, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), resveratrol, SIRT1, Sirtuin
Dopamine (DA) transporter-mediated uptake of 1-methyl-4-phenylpyridine (MPP+), an oxidized metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), damage DA neurons by impairing mitochondrial respiratory chain and generating reactive oxygen species1. The DA neuron-selective toxicity is reproduced in animals by systemic administration of MPTP, which serve as models of Parkinson’s disease (PD)2. The neurotoxicity of MPTP is alleviated by pretreating mice with resveratrol (trans-3,5,4'-trihydroxystilbene) or other phytoalexins3–5. Besides directly suppressing oxidative stress of MPP+ as an antioxidant6, resveratrol modulates cytoprotective signaling molecules and enzymes that include nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase SIRT17. Given the pleiotropic cytoprotective potentials of SIRT1 through diverse substrates, resveratrol’s antagonism against MPTP may be mediated at least in part by SIRT17,8. SIRT1 alleviates animal and cellular models of amyotrophic lateral sclerosis (ALS), Huntington’s disease, Alzheimer’s disease, and an α-synuclein model of PD9. On the other hand, transgenic mice that overexpress SIRT1 in a neuronal subset via the neuron-specific enolase (NSE) gene promoter were not resistant to MPTP10.
We have established a distinct line of transgenic mice that overexpress SIRT1 in wider neuronal lineages and additionally in glial and vascular endothelial cells via the murine prion gene promoter (Prp)11,12. Unlike the NSE-SIRT1 mice, our Prp-SIRT1 mice are resistant to cerebral hypoperfusion by bilateral common carotid artery stenosis, due to vascular dilatation which is potentiated by SIRT1-mediated deacetylation of endothelial nitric oxide synthase (eNOS)12. Further, Prp-SIRT1 mice are resistant to proteotoxic stress by an ALS-linked mutant of superoxide dismutase 1 (SOD1), due partly to SIRT1-mediated deacetylation of the heat shock factor 1 (HSF1) and the resulting upregulation of HSP70i11. On the basis of the SIRT1-HSF1 axis, and the protective effects of HSF1/HSPs against neurodegenerative insults such as MPTP and α-synuclein9,13–16, we assessed whether Prp-SIRT1 mice is resistant to acute loss of DA neurons and gliosis by MPTP.
All animal procedures were done in accordance with the guidelines of the Animal Use and Care Committees of Kyoto University (MedKyo08097), Nagoya University (#13151), and Kanazawa University (AP-101606). A line of transgenic mice with a C57BL/6J background harboring the PrP-Sirt1cDNA transgene had been generated and deposited at RIKEN Bioresource Center (RBRC06467) as described elsewhere in detail (Watanabe et al., 2014). Mice were reared in a specific pathogen-free environment at 23 ± 2°C, and identified by PCR using a pair of primers, 5′-CAAGAGGTTGTTAATGAAGC-3′ and 5′-TTTCCTGTTGCCTTCAATCAGCTATCG-3′. All comparisons were made between 3–4-month-old, wild-type (WT) and transgenic (Tg) male littermates. Eight mice were subjected to intraperitoneal injection of MPTP (20 μg/g body weight) in saline or saline alone, each 4 times with 2 h-intervals17, followed by histological analysis 4 days later3.
Mice were deeply anesthetized with sodium pentobarbital (50 μg/g, i.p.), fixed with transcardial perfusion of 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Frozen-sectioned 10 μm-thick coronal brain sections were reacted with antibodies for tyrosine hydroxylase (TH, rabbit IgG, Chemicon) and glial fibrillary acidic protein (GFAP, mouse IgG, Sigma)17,19. The sections were reacted with Cy3-conjugated anti-rabbit IgG and FITC-conjugated anti-goat IgG (Jackson ImmunoResearch), and observed with a laser scanning confocal microscope (Eclipse TE2000U, Nikon) with the Nikon EZ-C1 software. We counted TH-positive neurons in the SNpc in three planes (−3.08, −3.16, and −3.40 mm from the bregma), and measured immunofluorescence intensity for TH in the striatum (caudoputamen; CPu) as described previously17,19.
We used the original Prp-SIRT1 Tg mouse line that chronically expresses murine Sirt1cDNA in the central nervous system (CNS) under control of the murine prion gene promoter11,18. The expression levels of SIRT1 in the midbrain and striatum assessed by immunoblot were approximately three times higher in heterozygous Tg mice than in the non-Tg (WT) littermates12. Four days after serial administrations of MPTP (20 μg/g body weight), we assessed acute histopathological changes of the nigrostriatal tract in the two genotypes (n = 8).
In the midbrain of Tg and WT mice without MPTP administration, the distribution and appearance of TH-positive cells (presumed DA neuronal somata and dendrites), surrounding GFAP-positive astrocytes, and the nuclei of these and other cells (consist mostly of non-DA neurons and microglia) were comparable (Figure 1A, top). MPTP administration induced acute, significant loss of TH-positive cells and reactive gliosis at comparable severity between the genotypes (Figure 1A, bottom). The numbers of TH-positive neuronal somata in the SNpc (identified in three serial sections) did not show statistically significant difference (Figure 1B). These data indicate that the supplementation of SIRT1 does not suppress the loss of DA neurons and reactive gliosis by acute MPTP toxicity.

(A) Representative immunofluorescence images of wild-type and Sirt1Tg mouse midbrain 4 days after intraperitoneal injection of saline with or without MPTP. Serial coronal sections triply labeled for tyrosine hydroxylase (TH), glial fibrillary acidic protein (GFAP), and nuclear DNA (DAPI) consistently exhibited no recognizable histopathological differences in the loss of TH-positive cells (presumed DA neuronal somata and dendrites) and in the proliferation of GFAP-positive astrocytes. Scale bars, 100 μm. (B) The number of TH-positive neurons identified in the three serial coronal sections of SNpc was comparable between the WT and Sirt1Tg littermates. The bars denote mean ± standard error of the mean (n = 4 × 4).
In the striatum/caudoputamen without MPTP administration, the staining patterns for TH (mostly axons and axon terminals of DA neurons), GFAP-positive astrocytes, and the nuclei of these and other cells were comparable between the Tg and WT littermates (Figure 2A, left). Loss of TH-positive neuropil and reactive gliosis after MPTP administration were also comparable between the genotypes (Figure 2A, middle; higher magnifications in the right). Fluorescence intensity for TH in the striatum after MPTP administration did not differ (Figure 2B), indicating that the supplementation of SIRT1 does not alleviate the loss of DA nerve terminals by acute MPTP toxicity.
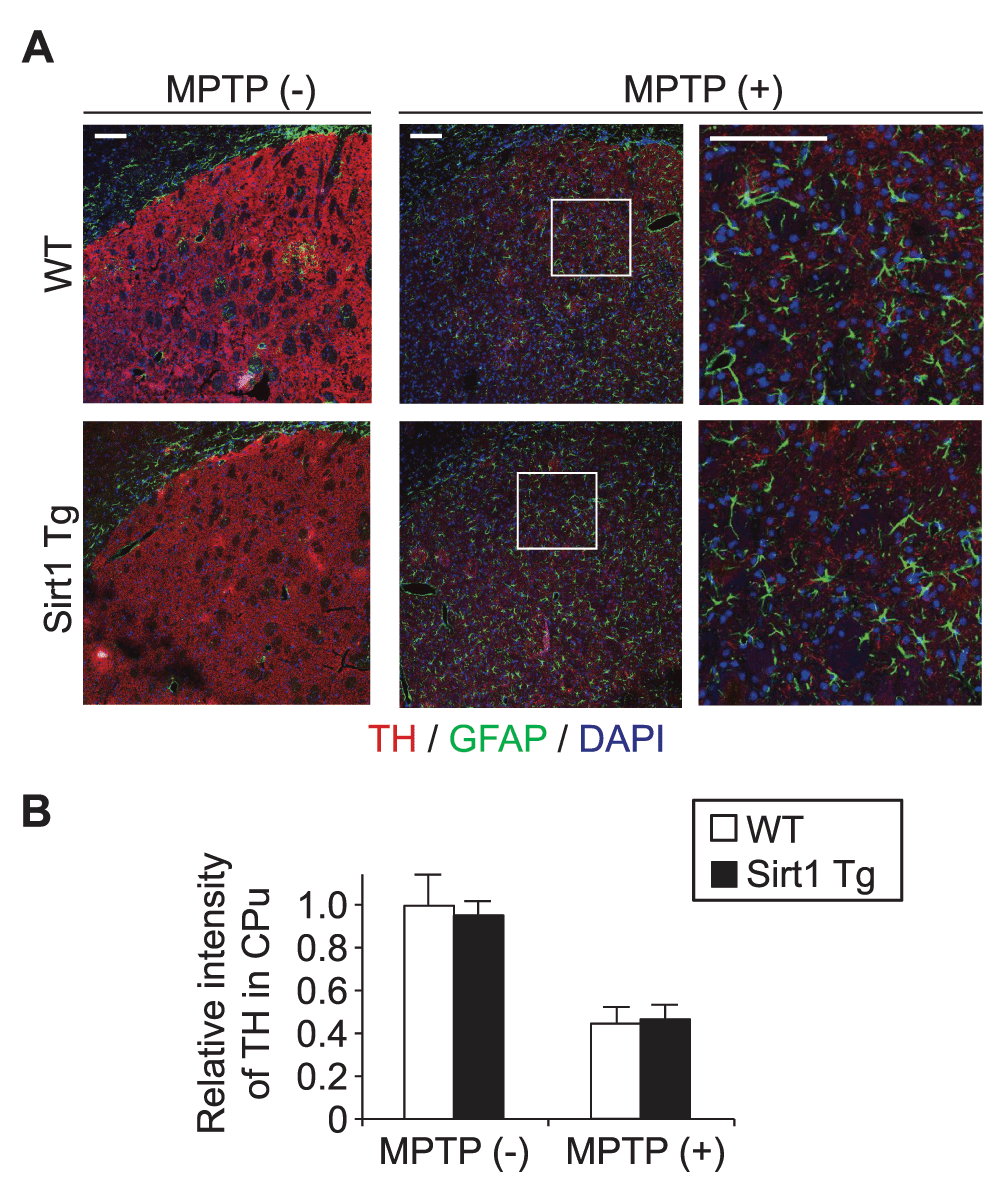
(A) Representative immunofluorescence images of wild-type and Sirt1Tg mouse striatum/caudoputamen 4 days after intraperitoneal injection of saline with or without MPTP. Coronal sections triply labeled for tyrosine hydroxylase (TH), glial fibrillary acidic protein (GFAP), and nuclear DNA (DAPI) exhibited no recognizable histopathological differences in the loss of TH-positive cells (presumed axons and axon terminals of DA neurons) and in the proliferation of GFAP-positive astrocytes. Scale bars, 100 μm. (B) The relative immunofluorescence intensity for TH in the striatum was comparable between WT and Tg littermates. The bars denote mean ± standard error of the mean (n = 4 × 4).
Overall, PrP-SIRT1 and WT male littermates exhibited similar responses to MPTP toxicity in terms of acute damages to nigrostriatal DA neurons, and proliferation/remodeling of neighboring astrocytes. These findings indicate that supplementation of SIRT1 in neurons and glia does not alleviate MPTP-induced DA neuronal damages and reactive gliosis.
Acute degeneration of DA neurons in the mouse MPTP model can be rescued by resveratrol pre-administration4,5, or by transgenic supplementation of peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) which controls mitochondrial biogenesis and oxidative phosphorylation19. The rescue effects had been attributed at least partly to SIRT17,8 on the basis that resveratrol directly or indirectly potentiates SIRT120, and that SIRT1 activates PGC-1α by deacetylation21. However, transgenic supplementation of SIRT1 either with the neuron-specific promoter10 or with the neuron/glia/vascular endothelial promoter (this study) did not confer tolerance to MPTP-induced pathology. The consistent results indicate that the resveratrol-mediated tolerance to MPTP is due to SIRT1-independent mechanisms (e.g., antioxidant activity as a polyphenol. See Introduction.), and that SIRT1-mediated activation of PGC-1α is insufficient to confer tolerance (i.e., the upregulation of PGC-1α is necessary). Thus, this study has made a case against the unproven notions that health benefits of resveratrol are attributed mostly to SIRT1, and that potentiation of SIRT1 in neurons and glia nonselectively suppresses neurodegeneration and gliosis. Nevertheless, it is worth testing whether Prp-SIRT1 mice are resistant to chronic neurotoxin models or genetic models of PD22,23, and whether PGC-1α/SIRT1-double Tg mice are more resistant than the original PGC-1α Tg mice19.
Our recent study with Prp-SIRT1 mice has demonstrated their resistance, albeit limited, to spinal cord degeneration caused by chronic overload of a mutant SOD111. The proteotoxic stress by misfolded SOD1 is alleviated at least in part by SIRT1-mediated deacetylation of a master transcription factor HSF1 and the resulting upregulation of HSP70i and perhaps other molecular chaperones11. Intriguingly, either transgenic supplementation of HSP70 or its heat shock-mediated upregulation (i.e., preconditioning) confers recognizable resistance to MPTP13–15. We therefore hypothesize that, in DA neurons of Prp-SIRT1 mice, the expression levels of the SIRT1 substrate HSF1 and the downstream effectors including HSP70i are insufficient to counter the toxicity of MPTP―as with the aforementioned situation of PGC-1α. Thus, an obvious subject for future studies is to test whether some preconditioning or milder insults (e.g., lower dose of MPTP or rotenone) could differentiate Prp-SIRT1 mice from wild-type mice.
All the original image data are accessible at Kanazawa University Repository, http://dspace.lib.kanazawa-u.ac.jp/dspace/handle/2297/41475?locale=en.
F1000Research: Dataset 1. Raw data of transgenic supplementation of SIRT1 in a mouse model of MPTP-induced parkinsonism. 10.5256/f1000research.6386.d4684124
YK and OH conducted the toxicological and histological analyses on mice bred by NA-I under the supervision of RT and MK. MK and OH designed the study and wrote the manuscript. All authors read and approved the manuscript.
This work was supported in part by CREST (Creation of a novel technology for prevention, diagnosis, and therapy for psychiatric and neurological disorders) from JST, Grant-in-Aid for Scientific Research on Innovative Areas, Comprehensive Brain Science Network, and Grants-in-Aid for Scientific Research from the MEXT of Japan.
We confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank D. Borchelt (University of Florida) for Mo-Prp plasmid DNA, S. Imai for murine Sirt1 cDNA, and A. Tanigaki, and R. Hikawa for technical help in the establishment of the PrP-Sirt1 transgenic line.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 27 May 15 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)