Keywords
microbiome, metagenomics, neurological infections, computational biology, next-generation sequencing, sequence alignment
This article is included in the Preclinical Reproducibility and Robustness gateway.
microbiome, metagenomics, neurological infections, computational biology, next-generation sequencing, sequence alignment
Metagenomic shotgun sequencing, in which DNA or RNA is extracted from a tissue sample and then sequenced, has the potential to detect a wide range of infections. Deep whole-genome shotgun (WGS) sequencing can detect bacteria, viruses, and eukaryotic pathogens with equal effectiveness, as long as the infectious agent is similar to a species that has been previously sequenced. Sequencing databases already contain thousands of known species, and as this number grows, the sensitivity of WGS will grow as well.
In 2014, a large outbreak of infection with enterovirus D68 was associated with both severe respiratory illness and acute paralysis, which the U.S. Centers for Disease Control and Prevention (CDC) named acute flaccid myelitis (AFM)1. Samples collected from 48 patients were sequenced and shown to form a novel strain, Clade B1, based on phylogenetic analysis of 180 complete enterovirus D68 sequences2. The same study conducted metagenomic sequencing of cerebrospinal fluid (CSF) and/or nasopharyngeal (NP) swabs from 22 of these patients and found enterovirus D68 in some NP samples that were positive based on PCR testing.
The identification of species from a WGS sample is a challenging problem that has spurred the development of multiple new computational methods3–5. Because of the large size of next-generation sequencing data sets, these methods need to be very fast, but in the context of clinical diagnosis, they also need to be accurate. We downloaded the 31 next-generation sequencing (NGS) samples from the Greninger et al.2 study (NCBI accession SRP055445) and re-analyzed them using a computational pipeline based on the recently developed Kraken metagenomic analysis software4, a very fast and sensitive system that can be customized to use a database containing any species whose sequences are available.
Among the 22 subjects for which NGS data were available, we found at least two that had far greater numbers of sequences (reads) from a bacterial pathogen than from enterovirus D68. Neither subject had been reported in 2 as having a bacterial infection.
In one subject, US/CA/09-871, reported by Greninger et al.2 as positive for enterovirus D68 through PCR and metagenomic NGS, we found in the NP swab sample an overwhelming presence of bacterial sequences from Haemophilus influenzae, a known cause of meningitis and neurological complications that was a common infection prior to the development of an effective vaccine.
Specifically, we identified 2,389,621 reads from H. influenzae in this subject, with the closest similarity to strain R2846. These reads comprise 93% of all microbial reads identified at the species level in the sample. Greninger et al.2 reported 2,742 reads (in their Supplementary Table 4) matching enterovirus D682 but did not report finding any bacterial reads from this sample. Our analysis found 1,330 reads matching enterovirus D68.
To confirm the identity of these reads, we aligned them separately to the complete genome of H. influenzae R2846, and we found that the reads completely covered the genome. Dividing the genome into 100 kilobase windows, depth of coverage varied from 266–828 reads/100Kbp, with far deeper coverage as expected at the 16S ribosomal RNA genes.
The enterovirus D68 isolated from patient US/CA/09-871 differed from the others in that it appeared in 2009, well before the 2014 outbreak, and that it grouped with Clade C, phylogenetically distinct from Clade B1 that was associated with AFM. This patient was reported2 as having encephalitis and severe respiratory illness but not AFM. The sequence evidence here suggests that the patient might have had complications from H. influenzae-associated encephalitis or encephalomyelitis, although no clinical or CSF data was available for our re-analysis.
In a second subject, US/CA/12-5837, we found a strikingly large number of reads from Staphylococcus aureus in the NP swabs. The two separate NGS files associated with this subject contained 6,858,453 and 1,343,806 reads, comprising 70% and 84% (respectively) of all non-human reads identified at the species level in each sample. The closest match was S. aureus subsp. aureus MRSA252, a methicillin-resistant strain. The coverage was deep enough, approximately 40X, that it would be possible to assemble this genome separately from the reads here (Figure 1). Greninger et al.2 reported 2,790 reads from enterovirus D68 in this subject (our analysis found 1,641) but did not report any from S. aureus.
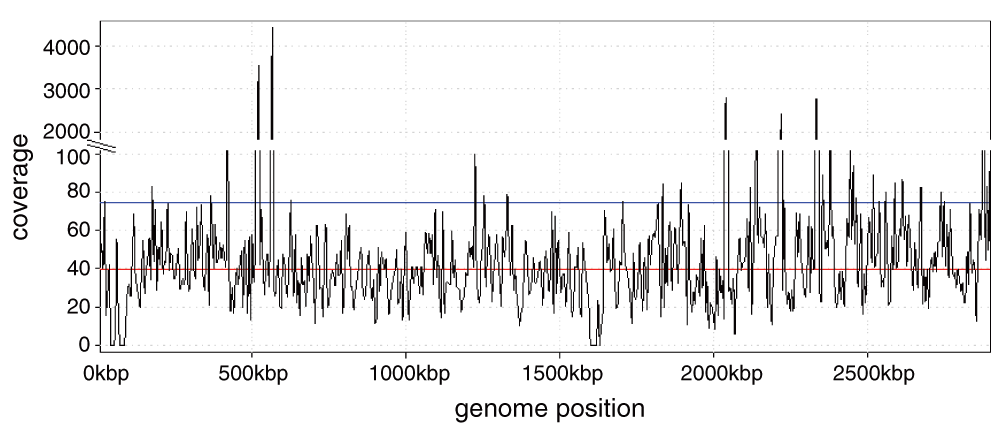
High peaks correspond to 16S rRNA genes. Red line: median coverage; blue line: mean coverage.
Patient US/CA/12-5837 was sampled in 2012, two years before the outbreak of AFM, although this patient was described in Greninger et al.2 as positive for enterovirus D68 based on clinical PCR testing and metagenomic sequencing. This patient is reported to be one of the first patients with enterovirus-D68-positive AFM2, but the sequence evidence indicates a severe S. aureus infection that might explain at least some of the patient’s symptoms. S. aureus has been implicated in neurological complications such as myelitis6 and meningitis7 by mechanisms that involve not only direct invasion into the central nervous system (CNS), but also immunopathogenic responses triggered by superantigens that can target the CNS8. At a minimum, S. aureus infection was overlooked by the previous analysis. Although the potential role of bacterial infection in the neurological disease that affected these two subjects is difficult to assess because of the lack of clinical and CSF information, its involvement as a pathogenic co-factor should be evaluated.
The metagenomics data (NCBI accession SRP055445) released by Greninger et al.2 comprise 43 files which cover 22 of the 48 subjects from their study (in their Supplementary Table 1); the study did not conduct NGS for all subjects. Our metagenomics pipeline identifies human reads at the same time that it searches for pathogens; therefore we scanned the data for human as well as microbial content. Greninger et al.2 reported that all human sequences had been removed from these files. We found, however, that all samples contained large numbers of human reads, ranging from a low of 18,215 to a high of 6,159,868. These comprised as few as 0.5% to as many as 95.6% of the reads in each sample, as shown in Table 1.
Shown are the number of reads in each sample that clearly match the human genome and do not match any microbial species. AFM: acute flaccid myelits; NP: nasopharyngeal swap; CSF: cerebrospinal fluid.
The inclusion of human sequence data in the files deposited at NCBI was likely a result of a computational method (SURPI5) that was insufficiently sensitive. Although the exact cause cannot be determined here, it is well known that sequence alignment algorithms often trade speed for sensitivity; e.g., by allowing fewer mismatches, an aligner can process reads at a much higher rate, at the cost of missing some alignments. It is less clear why the very large numbers of matches to two bacteria were missed; for both these bacteria, complete genomes from multiple strains are available in GenBank. We used both the Kraken system4 and the Bowtie2 aligner9 to ensure both sensitivity and speed in our analysis.
Release of sequence data is highly valuable, if not essential, for reproducibility and validation of sequencing-based studies. Failure to filter human reads from a sample is not uncommon; a recent study10 found that Human Microbiome Project samples, from which human DNA was supposed to have been removed, contain up to 95% human sequence. This suggests that future efforts to deposit microbiome data need to employ more sensitive computational screens in order to avoid the unintentional release of human sequence data.
Sequences were extracted from SRP055445 and each file was separately run through the Kraken program version 0.10.6-beta (https://github.com/DerrickWood/kraken)4, which identifies species by comparison with a database of all 31-bp sequences in all species. The database included the human genome (version GRCh38.p2), all complete bacterial and viral genomes, selected fungal pathogens, and known laboratory vector sequences from the NCBI UniVec database (http://www.ncbi.nlm.nih.gov/tools/vecscreen/univec). Percentages of bacterial and viral reads in each sample were re-computed after excluding human and vector sequences. Reads matching more than one species were classified at the genus level or above. Reads from H. influenzae and S. aureus were re-aligned using Bowtie2 version 2.2.59, a very fast and sensitive program for alignment of NGS reads to a reference genome, with the --local option. Bowtie2 was also used to re-align all reads from US/CA/12-5837 and US/CA/09-871 to the sequence of multiple enterovirus D68 strains (GenBank accessions JX101846.1, AY426531.1, KM851231.1, KM892500.1, KM892501.1, KM881710.2, KP745751.1, KP745755.1, KP745757.1, KP745760.1, KP745764.1, KP745766.1, and KP745767.1). We report the highest number of reads matching any one of these strains.
SLS conceived the study. FBP ran the computational analyses. SLS, CAP, and FBP jointly analyzed the computational results and wrote the manuscript.
This work was supported in part by the National Institutes of Health under grant R01-HG007196 and by the U. S. Army Research Office under grant number W911NF-14-1-0490.
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 13 Jul 15 |
read | read |
|
Version 1 02 Jul 15 |
||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
- “They don’t disagree with our finding, but say that they already
... Continue reading We thank Drs. Salzberg and Pardo for their response. Here are our replies addressing their new comments (in bulleted underline):- “They don’t disagree with our finding, but say that they already knew about it, pointing to their Supplementary Table 3. Suppl. Table 3 does indeed report large numbers of “bacterial reads” for these two samples, but it does not identify them further.”
Yes, we knew about the detection of Haemophilus influenzae in sample US/CA/09-871 and MRSA (methicillin-resistant Staphylococcus aureus) in sample US/CA/12-5837, both of which are nasopharyngeal / oropharyngeal (NP/OP) swabs. We would be happy to provide the actual output from the SURPI pipeline (Naccache, et al., Genome Research, 2014) that shows the presence of these two bacteria, which were easily detected. As we previously stated, we reported the bacterial reads from the SURPI pipeline (5,614,487 reads from US/CA/09-871 and 28,676,383 reads from US/CA/12-5837); a “re-analysis” using a different algorithm was not necessary.- “In contrast, Supplementary Table 5 in their paper includes specific read counts for 75 bacterial species that they found in most of their samples. These species include two species of Staphylococcus and one species of Haemophilus, but S. aureus and H. influenzae are not listed. Thus from the published paper, it is not possible to conclude that the authors were aware of either of these two species.”
We respectfully request that the authors read our paper and tables more carefully. In Supplementary Table 5, we report the bacterial species found in only the cerebrospinal fluid (CSF) samples, not in nasopharyngeal / oropharyngeal (NP/OP) swabs. Again, we specifically state in our paper: “. As this protocol reduces sensitivity of detection and speciation for non-viral microbes (i.e. bacteria, fungi, and parasites), only viral sequences are shown for the NP/OP samples. “- “Greninger, Naccache and Chiu also write that “the purpose of metagenomic NGS on NP/OP samples was to ‘aid in the recovery of enterovirus D68 genome sequences and detect potential co-infections from other viruses.” We agree that NGS can be valuable for this purpose, but we do not agree that findings of bacterial DNA - particularly when the bacteria dominate the sample, as in these two cases - should be ignored.”
We did not “ignore” the findings of bacterial RNA/DNA. First, we emphasize that the sample library preparation on the NP/OP samples was a RNA, not a DNA preparation. It was post-treated with post-DNase to enrich for RNA viral sequences. As such, interpretation of bacterial sequence data from this library is problematic. It is entirely possible that other bacteria were present but their DNA was selectively degraded, for instance. Also, such nuclease treatment will affect both the number and distribution of bacterial reads in the metagenomics sample, so attempting to derive quantitative information (“overwhelming presence of bacterial sequences”) and, even worse, attempting to attribute clinical significance from the metagenomics data is not valid.- Greninger et al. also make the point that they believe that “bacterial reads in NP/OP swabs from children most often reflect colonization/carriage and not infection.” We agree: we would expect any metagenomics sample from the nasopharyngeal tract to contain many bacterial species. However, as we explain in our paper, the two samples in question have an overwhelming number of reads from just one species: in US/CA/09-871, over 93% of the reads were H. influenzae - 2.4 million reads - and in the two files for the other patient, 70% and 84% of the reads were S. aureus - over 8 million reads. It is possible that these merely represent colonization and were not causing disease. Even if so, we still feel it would have been important to acknowledge the presence of these pathogens in these samples (assuming the authors were aware of them) and to discuss each of them, at least briefly.
We re-iterate that it is dangerous to over-interpret the data as it appears as if Salzberg and colleagues are doing here. Please see the previous paragraph on why the number and distribution of bacterial metagenomic reads in these cases are not reliable. As mentioned before, we do report the number of bacterial reads found in NP/OP samples in Supplementary Table 3. We did not discuss them given that (1) the focus for this study was on looking for clinical associations with AFM, (2) analysis of the NP/OP samples were problematic because the preparation was not suitable / reliable for bacterial metagenomics analysis and the difficulty in discriminating colonization from genuine infection, and (3) the presence of reads to many and multiple bacterial species across all of the NP/OP samples, not just the.two containing MRSA or Haemphilus influenza.Our focus was also on examination of “sterile sites” (e.g. cerebrospinal fluid and blood) for sequences to pathogens, since it is much easier to demonstrate that an infectious agent is associated or even causal if it is detected in a sterile site. We did not think that specifically mentioning Haemphilus influenzae from a positive control NP/OP sample would be relevant to the study, nor MRSA from the nose/throat of an AFM patient in part because it would be very difficult to distinguish colonization from infection. Also note that infectious disease diagnosis of MRSA infection is primarily made by positive cultures or detection of the bacteria from sterile or invasive sites such as blood, cerebrospinal fluid, and bronchoalveolar lavage fluid and not from nasal swabs which are mainly used to screen for MRSA colonization.
- “Greninger et al. express concern that our analysis suggests that MRSA or H. influenzae infections are involved in the pathogenesis of AFM. We do not suggest this, but we suggest that the role of bacterial infection as a co-factor should be evaluated in patients suspected of having AFM. Although not the point of our study or of the Greninger et al. study, this is an important issue in clinical practice and for future studies of patients suspected to have AFM, due to the potential role of bacteria in triggering inflammatory myelopathies, encephalomyelitis syndromes such as Acute Disseminated Encephalomyelitis (ADEM), or superantigen-induced disorders, which may present clinically as longitudinal extensive myelopathies that may resemble AFM.”
We agree that bacterial infection can be a co-factor. However, as previously mentioned, the library preparation from NP/OP was not suitable for bacterial metagenomics analysis. To evaluate this properly, the samples can and should be prepared as bacterial metagenomics libraries, and a complete analysis of the results, not just mentioning the Haemophilus influenzae and MRSA – as done here -- but also all of the other bacteria in the other samples, performed. Even after this analysis is done, much more study would need to be done to investigate the role of bacterial infection as a co-factor. The inadequate sample preparation for bacterial metagenomics analysis and non-sterile sample type (NP/OP) make our NP/OP data not suitable for investigating “the role of bacterial infection as a co-factor”.- The authors do point out one error in our paper, where we stated that patient US/CA/09-871 was reported (by them) as having “encephalitis and severe respiratory illness.” This was an error on our part, and we will submit a revised manuscript where we correct this to read “severe respiratory illness.”
We feel that this is a major error in the paper, as one of the two patients did not even have acute flaccid myelitis. Also, it is incorrect to say “severe respiratory illness”; this was a patient with an upper respiratory infection that may not have been clinically severe. We feel that it is inappropriate in general to report the results of metagenomics analyses in this fashion without understanding the clinical context and how the sample was collected and prepared. Note also that given this error, the title becomes even more misleading: “re-analysis of metagenomics sequences from acute flaccid myelitis patients reveals alternatives to enterovirus D68 infection.”- “Regarding our second main criticism - that the authors inadvertently left large numbers of human reads in their data, which were supposed to have been filtered to remove human DNA - the authors agree. As we mentioned in our paper, this problem has occurred elsewhere too: J. Allen and colleagues recently showed (Ames et al 2015, cited in our paper) that some of the Human Microbiome Project samples, all of which were supposed to have been cleaned of human reads, contain up to 95% human reads. We believe it is vitally important that the biomedical community be aware that the choice of computational methods is a very critical one, particularly when analyzing the large data sets that are becoming ever more common.”
We agree and have taken steps to ensure patient privacy (requesting that SRA data be removed from the database). However, we also believe that it is unfair to target our paper specifically, as many other earlier published studies including the Human Microbiome Project, as the authors point out, have the same issues. We will be erforming a bioinformatics analysis comparing the different computational methods for their ability to remove human reads.- We believe it is vitally important that the biomedical community be aware that the choice of computational methods is a very critical one, particularly when analyzing the large data sets that are becoming ever more common.
Although we agree with this statement, we believe that a “re-analysis” was not necessary for our paper as we detected both bacteria described by Salzberg and colleagues. We would have been happy to provide this data upon request. In addition, the re-analysis has numerous errors and issues of over-interpretation that did not take into account the clinical context and not understanding important details related to the study (e.g. upper respiratory infection does not imply “severe respiratory illness”; the NP/OP sample preparation was sub-optimal and problematic for bacterial metagenomics analysis; our patient with Haemphilus influenzae did not have acute flaccid myelitis). We believe that the re-analysis by Salzberg and colleagues simply confirms the results from our published analysis, although there are obvious differences in clinical interpretation.Alexander Greninger, MD/PhD
Samia Nacacche, PhD
Charles Chiu, MD/PhD
- “They don’t disagree with our finding, but say that they already knew about it, pointing to their Supplementary Table 3. Suppl. Table 3 does indeed report large numbers of “bacterial reads” for these two samples, but it does not identify them further.”
Yes, we knew about the detection of Haemophilus influenzae in sample US/CA/09-871 and MRSA (methicillin-resistant Staphylococcus aureus) in sample US/CA/12-5837, both of which are nasopharyngeal / oropharyngeal (NP/OP) swabs. We would be happy to provide the actual output from the SURPI pipeline (Naccache, et al., Genome Research, 2014) that shows the presence of these two bacteria, which were easily detected. As we previously stated, we reported the bacterial reads from the SURPI pipeline (5,614,487 reads from US/CA/09-871 and 28,676,383 reads from US/CA/12-5837); a “re-analysis” using a different algorithm was not necessary.- “In contrast, Supplementary Table 5 in their paper includes specific read counts for 75 bacterial species that they found in most of their samples. These species include two species of Staphylococcus and one species of Haemophilus, but S. aureus and H. influenzae are not listed. Thus from the published paper, it is not possible to conclude that the authors were aware of either of these two species.”
We respectfully request that the authors read our paper and tables more carefully. In Supplementary Table 5, we report the bacterial species found in only the cerebrospinal fluid (CSF) samples, not in nasopharyngeal / oropharyngeal (NP/OP) swabs. Again, we specifically state in our paper: “. As this protocol reduces sensitivity of detection and speciation for non-viral microbes (i.e. bacteria, fungi, and parasites), only viral sequences are shown for the NP/OP samples. “- “Greninger, Naccache and Chiu also write that “the purpose of metagenomic NGS on NP/OP samples was to ‘aid in the recovery of enterovirus D68 genome sequences and detect potential co-infections from other viruses.” We agree that NGS can be valuable for this purpose, but we do not agree that findings of bacterial DNA - particularly when the bacteria dominate the sample, as in these two cases - should be ignored.”
We did not “ignore” the findings of bacterial RNA/DNA. First, we emphasize that the sample library preparation on the NP/OP samples was a RNA, not a DNA preparation. It was post-treated with post-DNase to enrich for RNA viral sequences. As such, interpretation of bacterial sequence data from this library is problematic. It is entirely possible that other bacteria were present but their DNA was selectively degraded, for instance. Also, such nuclease treatment will affect both the number and distribution of bacterial reads in the metagenomics sample, so attempting to derive quantitative information (“overwhelming presence of bacterial sequences”) and, even worse, attempting to attribute clinical significance from the metagenomics data is not valid.- Greninger et al. also make the point that they believe that “bacterial reads in NP/OP swabs from children most often reflect colonization/carriage and not infection.” We agree: we would expect any metagenomics sample from the nasopharyngeal tract to contain many bacterial species. However, as we explain in our paper, the two samples in question have an overwhelming number of reads from just one species: in US/CA/09-871, over 93% of the reads were H. influenzae - 2.4 million reads - and in the two files for the other patient, 70% and 84% of the reads were S. aureus - over 8 million reads. It is possible that these merely represent colonization and were not causing disease. Even if so, we still feel it would have been important to acknowledge the presence of these pathogens in these samples (assuming the authors were aware of them) and to discuss each of them, at least briefly.
We re-iterate that it is dangerous to over-interpret the data as it appears as if Salzberg and colleagues are doing here. Please see the previous paragraph on why the number and distribution of bacterial metagenomic reads in these cases are not reliable. As mentioned before, we do report the number of bacterial reads found in NP/OP samples in Supplementary Table 3. We did not discuss them given that (1) the focus for this study was on looking for clinical associations with AFM, (2) analysis of the NP/OP samples were problematic because the preparation was not suitable / reliable for bacterial metagenomics analysis and the difficulty in discriminating colonization from genuine infection, and (3) the presence of reads to many and multiple bacterial species across all of the NP/OP samples, not just the.two containing MRSA or Haemphilus influenza.Our focus was also on examination of “sterile sites” (e.g. cerebrospinal fluid and blood) for sequences to pathogens, since it is much easier to demonstrate that an infectious agent is associated or even causal if it is detected in a sterile site. We did not think that specifically mentioning Haemphilus influenzae from a positive control NP/OP sample would be relevant to the study, nor MRSA from the nose/throat of an AFM patient in part because it would be very difficult to distinguish colonization from infection. Also note that infectious disease diagnosis of MRSA infection is primarily made by positive cultures or detection of the bacteria from sterile or invasive sites such as blood, cerebrospinal fluid, and bronchoalveolar lavage fluid and not from nasal swabs which are mainly used to screen for MRSA colonization.
- “Greninger et al. express concern that our analysis suggests that MRSA or H. influenzae infections are involved in the pathogenesis of AFM. We do not suggest this, but we suggest that the role of bacterial infection as a co-factor should be evaluated in patients suspected of having AFM. Although not the point of our study or of the Greninger et al. study, this is an important issue in clinical practice and for future studies of patients suspected to have AFM, due to the potential role of bacteria in triggering inflammatory myelopathies, encephalomyelitis syndromes such as Acute Disseminated Encephalomyelitis (ADEM), or superantigen-induced disorders, which may present clinically as longitudinal extensive myelopathies that may resemble AFM.”
We agree that bacterial infection can be a co-factor. However, as previously mentioned, the library preparation from NP/OP was not suitable for bacterial metagenomics analysis. To evaluate this properly, the samples can and should be prepared as bacterial metagenomics libraries, and a complete analysis of the results, not just mentioning the Haemophilus influenzae and MRSA – as done here -- but also all of the other bacteria in the other samples, performed. Even after this analysis is done, much more study would need to be done to investigate the role of bacterial infection as a co-factor. The inadequate sample preparation for bacterial metagenomics analysis and non-sterile sample type (NP/OP) make our NP/OP data not suitable for investigating “the role of bacterial infection as a co-factor”.- The authors do point out one error in our paper, where we stated that patient US/CA/09-871 was reported (by them) as having “encephalitis and severe respiratory illness.” This was an error on our part, and we will submit a revised manuscript where we correct this to read “severe respiratory illness.”
We feel that this is a major error in the paper, as one of the two patients did not even have acute flaccid myelitis. Also, it is incorrect to say “severe respiratory illness”; this was a patient with an upper respiratory infection that may not have been clinically severe. We feel that it is inappropriate in general to report the results of metagenomics analyses in this fashion without understanding the clinical context and how the sample was collected and prepared. Note also that given this error, the title becomes even more misleading: “re-analysis of metagenomics sequences from acute flaccid myelitis patients reveals alternatives to enterovirus D68 infection.”- “Regarding our second main criticism - that the authors inadvertently left large numbers of human reads in their data, which were supposed to have been filtered to remove human DNA - the authors agree. As we mentioned in our paper, this problem has occurred elsewhere too: J. Allen and colleagues recently showed (Ames et al 2015, cited in our paper) that some of the Human Microbiome Project samples, all of which were supposed to have been cleaned of human reads, contain up to 95% human reads. We believe it is vitally important that the biomedical community be aware that the choice of computational methods is a very critical one, particularly when analyzing the large data sets that are becoming ever more common.”
We agree and have taken steps to ensure patient privacy (requesting that SRA data be removed from the database). However, we also believe that it is unfair to target our paper specifically, as many other earlier published studies including the Human Microbiome Project, as the authors point out, have the same issues. We will be erforming a bioinformatics analysis comparing the different computational methods for their ability to remove human reads.- We believe it is vitally important that the biomedical community be aware that the choice of computational methods is a very critical one, particularly when analyzing the large data sets that are becoming ever more common.
Although we agree with this statement, we believe that a “re-analysis” was not necessary for our paper as we detected both bacteria described by Salzberg and colleagues. We would have been happy to provide this data upon request. In addition, the re-analysis has numerous errors and issues of over-interpretation that did not take into account the clinical context and not understanding important details related to the study (e.g. upper respiratory infection does not imply “severe respiratory illness”; the NP/OP sample preparation was sub-optimal and problematic for bacterial metagenomics analysis; our patient with Haemphilus influenzae did not have acute flaccid myelitis). We believe that the re-analysis by Salzberg and colleagues simply confirms the results from our published analysis, although there are obvious differences in clinical interpretation.Alexander Greninger, MD/PhD
Samia Nacacche, PhD
Charles Chiu, MD/PhD