Introduction
Alarm signals are signals that are emitted by individuals in the presence of predators. These signals may not only divert predator attention or discourage it to attack but also alert conspecifics1. Alarm signals can be transmitted via different communication pathways: alarm calls are widespread among social terrestrial animals (rodents2, birds3 and primates4). Other animal species use alarm pheromones for the same purpose (insects5,6 or mice7). In aquatic environments, chemical alarm signals are widespread especially due to the large number of water-soluble compounds8 (insects9, crustaceans10, asteroids11,12, gastropods13,14, amphibians15,16 and fishes17). Signaling is usually costly for the individual sender18 and often appears to primarily benefit the receivers. However, to evolve and be maintained by natural selection, the fitness benefits for the signaling individual must override its costs.
The evolution of alarm cues in fishes is a particularly puzzling example of alarm signaling whose evolution has intrigued evolutionary ecologists since the 1960’s19,20 until today21. Alarm cues, also termed ‘Schreckstoff’’22,23 are located in specialized epidermal ‘club cells’24–26. They are released following injuries by predators, and widespread among different fish taxa including cichlids25,27. Signal-receiving conspecifics benefit because the presence of alarm cues reliably indicates high predation risk, thus allowing them to respond to predator presence in order to increase survival28,29. However, the benefits to the signaler are unclear because it often may not survive a predatory attack. Furthermore, alarm cue production is energetically demanding30.
Several hypotheses have been proposed to explain the phenomenon of alarm cues in fishes31,32. First, alarm cues might benefit the survival of the producing individual. In this context, it has been proposed that alarm pheromones may function to attract secondary predators33. During the following interference between competing predators prey might be able to escape. Mathis et al. showed that pike Esox lucius and predatory diving beetles were indeed attracted by minnow alarm cues33. In the presence of such secondary predators (pike), escape probabilities of minnows were actually increased34. Accordingly, the signaling individual can increase its own fitness by producing alarm cues, provided that it survives an initial predator attack.
Other authors proposed that the alarm function is a mere by-product and instead suggested the primary function of alarm cues to be anti-pathogenic agents35 or promote the healing of injuries36. A recent study supported this hypothesis; club cell production was unrelated to predation risk but stimulated by skin penetrating pathogens and parasites37. Moreover they found that UV radiation also affected club cell production, providing evidence for a general immune function of alarm cues.
A further hypothesis relying on Hamilton’s inclusive fitness theory predicts that individuals can increase their fitness indirectly by supporting the survival of genetically related individuals38, coined ‘kin selection’ by Maynard-Smith39. Generally, costly communication is facilitated when interacting individuals are related40. While kin selection has been suggested to play an important role in the evolution of mammalian alarm calls2,41–43 (but see44), its significance for the evolution of alarm cues in fishes has received little attention30. Indirect benefits can be gained when kin particularly benefit from alarm cue production. This might be reached when individuals are able to discriminate between the alarm signals of different senders45. Higher sensitivity to kin-alarm cues may result in an improved response to predation, and thus higher survival of individuals related to the sender which in turn may increase the indirect fitness of the sender.
The aim of the present experiment was to test whether the cichlid fish Pelvicachromis taeniatus discriminates between alarm cues produced by kin and non-kin. P. taeniatus is a socially monogamous small cave-breeder with biparental brood care46 which inhabits streams in Western Africa47. Previous studies revealed that this species possesses alarm cues, recognizes conspecific alarm cues and adjusts its behavior in the presence of alarm cues (Meuthen et al., submitted, unpublished data). Furthermore, P. taeniatus is capable of kin recognition48–50 which is most likely based on chemical cues51–53. In the experiment we measured the change in activity in individual P. taeniatus after the addition of alarm cues derived from kin and non-kin, respectively.
Material and methods
Ethics statement
This study conforms to the Association for the Study of Animal Behaviour’s Guidelines for the Use of Animals in Research and was carried out according to the German laws for experimentation with animals (§ 8 Abs. 1 TierSchG, V.m. § 2 Abs. 1.1 TierSchZustV NW 26.9.1989). No additional licences were required.
Animal collection and maintenance
We conducted an experiment using female F2 progeny of the cichlid species Pelvicachromis taeniatus, whose ancestors (F0) were collected from the Moliwe river near Limbe, Cameroon (04°04’ N, 09°16’ E). Female P. taeniatus were used exclusively due to their consistent activity levels54. Prior to experiments, fish were kept in mixed-sex 50 × 30 × 30 cm (L × W × H) stock tanks at densities up to 20 individuals and were fed daily with frozen invertebrate larvae ad libitum. These tanks were illuminated in a 12:12 h light:dark cycle; water temperature was kept at 25 ± 1°C.
Experimental setup
During experiments, we manipulated predation risk in three treatments by exposing individual fish to one of the following extracts: (1) Alarm cues derived from kin (from familiar and unfamiliar siblings); (2) Alarm cues derived from unfamiliar, unrelated conspecifics (non-kin); (3) Distilled water to control for disturbance effects upon introduction. We produced alarm cues from 26 donor cichlids which were previously starved for two days to exclude any effects caused by the individual’s selective diet. Each alarm cue consisted of a male and a female cichlid to control for sex effects. Fish were anaesthetized with a blow to the head and afterwards euthanized by cervical dislocation in accordance to § 4 of the German animal welfare act (BGB l. I S. 1207, 1313). They were then placed into a mortar and grinded with a pestle. This procedure, imitating a lethal predation event, ruptured cells and thus allowed alarm pheromones to be released. The homogenate was diluted with distilled water, passed through filter floss and frozen in 1 ml aliquots at -20°C until use. The final concentration each fish was exposed to during trials was 3.6 mg/l donor wet fish weight. Likewise, we prepared 1 ml aliquots of pure distilled water for control experiments.
Trials were run in 30 × 20 × 20 cm tanks which were supplemented with a 0.5 × 0.5 cm grey plastic tube leading below the water level at the middle of their short side. This duct allowed the direct addition of chemical cues into the tanks while minimizing fish disturbance. Furthermore, experimental tanks were surrounded on all sides (except the top) with white polystyrene to prevent fish agitation by neighboring fish or the experimenter. A video camera (QuickCam 9000, Logitech, China) viewing the tanks from the top enabled recording of fish behavior for evaluation. Tanks were filled with substrate-treated water54; individual fish were then introduced and acclimatized for 1 h, this period is referred to as the pre-stimulus phase from now on. Experimental stimuli were thereafter temperature-adjusted to tank conditions and introduced at the point of 1 h 15min. Subsequently, fish behavior was recorded for another hour. Afterwards, the experimental subjects were sized accurate to the nearest millimeter and weighed accurate to one milligram on an electrical precision scale (LC 2215, Sartorius, Germany). Between trials, tanks were cleaned with 3% hydrogen peroxide and then rinsed with tap water to remove remaining olfactory traces55,56. Furthermore, experimental stimuli assigned to individual tanks were alternated between trials.
In total, we tested 51 individuals from 8 families; extracts from the same donor fish were used throughout different treatments and thus represented – based on the family identity of the focal fish – as either related or unrelated conspecifics. Also, individuals from the same family were evenly distributed among the three extracts. Because in two cases the fish exhibited no activity during the pre-stimulus phase, we excluded them from analysis. Hence, the final sample size consisted of 49 individuals; 12 received alarm cues from familiar siblings, 9 from unfamiliar siblings, 14 from unrelated fish and 14 individuals were exposed to the control stimulus. Because sibling familiarity did not significantly affect focal fish activity (familiar vs. unfamiliar kin: χ2 = 0.090, p = 0.764), their activity scores were pooled to represent 21 fish receiving alarm cues derived from kin.
Statistical analysis
We evaluated fish activity by tracking its movement during 1 h pre-stimulus phase and the following 1 h post-stimulus phase with animal tracking software (Biobserve Viewer2, St. Augustin, Germany). Subsequently, we assigned an activity index to each fish by calculating the difference between the distances covered during the two experimental phases. Activity indices did not deviate significantly from normal distribution according to the Kolmogorov-Smirnov test (Lillie.test, R library “nortest”), thus we applied linear-mixed effect models (LME, R library “nlme”) for analysis. All test fish were only used once but to account for the repeated use families, we entered “family identity” as a random factor. All results were based on likelihood ratio tests (LRT); hence degrees of freedom always differed by one.
Results
The change in activity of female P. taeniatus was significantly affected by the treatment (χ2 = 10.057, p = 0.007, Figure 1). Activity indices of both alarm-cue treatment groups (kin/non-kin) were significantly different from those of the water-control group (Kin vs. water: χ2 = 8.346, p = 0.004; Non-kin vs. water: χ2 = 8.693, p = 0.003, Figure 1). Whereas fish of the control group showed on average an increase of 0.67 m in covered distance during the post-stimulus period, fish of both alarm cue treatments showed reduced activity in the post-stimulus phase (on average 0.31 m less compared to the pre-stimulus phase). However, fish did not respond differentially to alarm cues derived from related and unrelated individuals (χ2 = 0.233, p = 0.630).
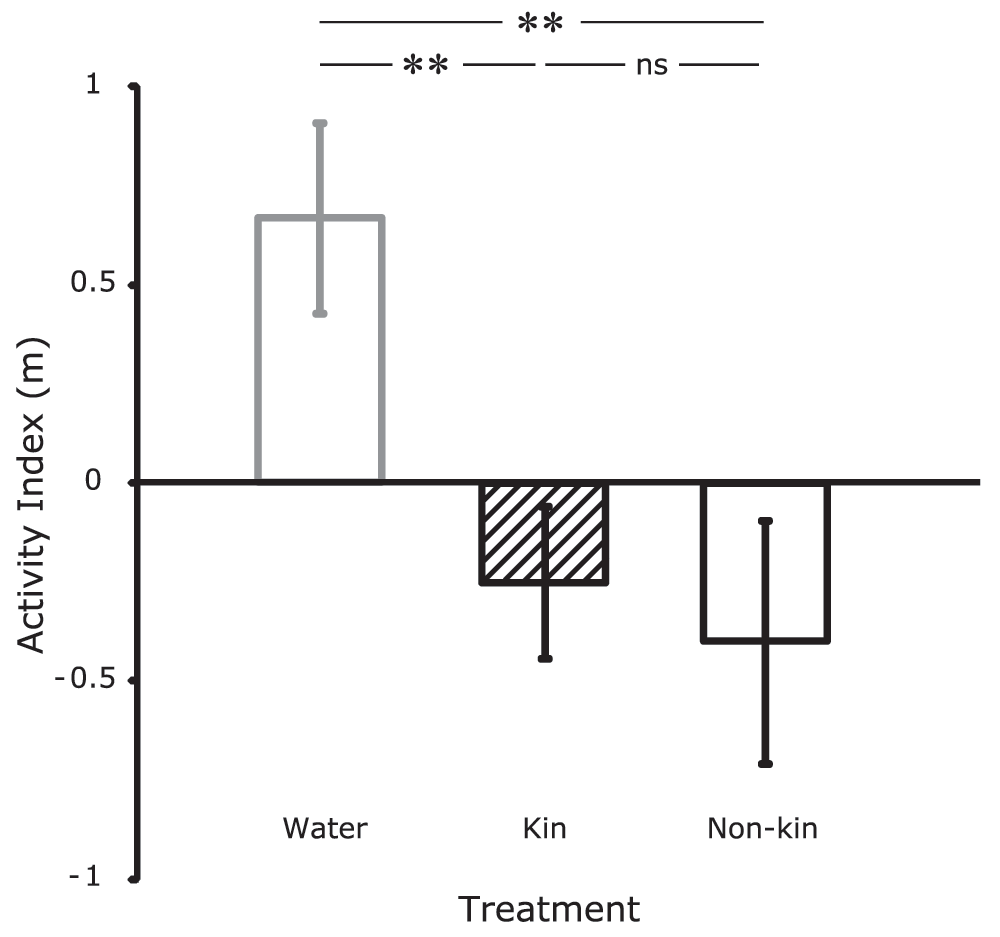
Figure 1. Activity indices of female P. taeniatus (mean±SD) exposed to distilled water (gray open bar) and alarm cues derived from related conspecifics (kin, black hatched bar) or unrelated conspecifics (non-kin, black open bar).
Activity indices were calculated by subtracting the distance covered during the 1 h prestimulus phase from the following 1 h poststimulus period. Asterisks above the bars indicate ** p < 0.01; ns p > 0.6.
Discussion
Generally, activity of female P. taeniatus was affected by the presence of conspecific alarm cues. Fish of the alarm cue treatment significantly decreased their activity relative to control fish. However, P. taeniatus did not discriminate between alarm cues derived from kin or non-kin in terms of activity changes.
These results are in accordance to numerous studies showing that the presence of conspecific alarm cues decreases prey activity in general57,58. Reduced activity concurrently decreases prey conspicuousness, which is an effective strategy against visual predators and enhances prey survival58. Furthermore, our results add to an earlier study, showing that males of P. taeniatus reduce territorial aggression in the presence of conspecific alarm cues (Meuthen et al., submitted, unpublished data). Thus both sexes of P. taeniatus are capable of recognizing alarm cues and adjust their behavior accordingly.
Against the expectations of the kin selection hypothesis, fish did not discriminate between kin and non-kin alarm cues. This is also surprising because theoretical models proposed that the evolution of communication in general is driven by the interaction between siblings40 and kin selection has been suggested to play an important role in the evolution of different alarm signals such as alarm calls2,41–43 but see44. Furthermore, individual discrimination of alarm signals is predicted to be highly beneficial45. However, lack of discrimination does not necessarily mean lack of recognition59. Thus, further studies in different contexts are required to confirm the present results. The results are in accordance with the “direct benefits” hypotheses32, including those hypotheses highlighting that alarm cues have primarily evolved as an immune enhancing mechanism and that the alarm signaling function is a by-product37. They are also in accordance with the theory that chemical signals have originally evolved from compounds without a communicative function8.
On a molecular level, the missing discrimination may result from a lack of kin-related information within alarm cues. Although to date, some studies were able to identify single chemical components of alarm cues such as Hypoxanthine-3-N-oxide60,61 or the glycosaminoglycan chondroitin62, the full suite of alarm cue components still remains to be identified62. The results of behavioral experiments have suggested that substantial individual variation in alarm cues exists. Sender body condition was determined to be an important factor affecting alarm cue response63. These results were, however, attributed to differences in club cell quantity30 because fish display graded responses based on alarm cue concentration64. Unidentified qualitative effects could, however, also be responsible for the altered response, especially because not all behaviors were specific to sender condition – whereas changes in activity and shoaling density were specific to sender condition, aggression remained unaffected65.
Kin discrimination in alarm cues might simply not have evolved in P. taeniatus due to the lack of necessity. The increase in fitness due to kin discrimination in alarm cues might not be sufficient to cause the necessary impact on natural selection, causing kin-related information to evolve in alarm cues. Many fishes are capable of identifying kin and live in kin-shaped groups66. This is also true for P. taeniatus which lives the first weeks of hatching in family group guarded by their parents46. Afterwards they live in shoals without defined territories throughout the juvenile stage47. Laboratory experiments showed that they prefer to shoal with familiar kin (Thünken et al., unpublished data). Furthermore, P. taeniatus preferentially mate with kin47 which is most likely also true for the natural population67. Accordingly, throughout their life the social environment of P. taeniatus is probably largely kin-structured in nature. In this case, individuals receiving the information transmitted by alarm cues are most likely kin. As a consequence, signaling individuals may increase their inclusive fitness.
In conclusion, our study found no evidence for differential response to kin derived alarm cues. However, under natural conditions behavioral mechanisms may lead to kin-biased alarm cue perception. Thus, kin selection potentially plays a role in alarm signaling in our model system. Still, further research is required determining the direct fitness benefits and costs for the signaling individual as well as the benefits for the receivers which are fundamental parameters to understand the evolution and maintenance of alarm cues.
Author contributions
TT and DM conceived the study. DM, SAB and TT designed the experiments. DM carried out the research. DM, SAB and TT analysed the data. DM and TT wrote the paper. All authors had read and improved the manuscript and agreed to the final content.
Competing interests
No competing interests were disclosed.
Grant information
This research was funded by the Deutsche Forschungsgemeinschaft (TH 1615/1-1).
Acknowledgements
We are grateful to the Bakker research group for discussion.
Faculty Opinions recommendedReferences
- 1.
Smith RJF:
Evolution of alarm signals: Role of benefits of retaining group members or territorial neighbors.
Am Nat.
1986; 128(4): 604–610. Reference Source
- 2.
Sherman PW:
Nepotism and the evolution of alarm calls.
Science.
1977; 197(4310): 1246–1253. PubMed Abstract
| Publisher Full Text
- 3.
Klump GM, Shalter MD:
Acoustic behavior of birds and mammals in the predator context. 1. Factors affecting the structure of alarm signals. 2. The functional significance and evolution of alarm signals.
Z Tierpsychol.
1984; 66(3): 189–226. Publisher Full Text
- 4.
Macedonia JM, Evans CS:
Essay on Contemporary Issues in Ethology: Variation among mammalian alarm call systems and the problem of meaning in animal signals.
Ethology.
1993; 93(3): 177–197. Publisher Full Text
- 5.
Blum MS:
Alarm pheromones.
Annu Rev Entomol.
1969; 14: 57–80. Publisher Full Text
- 6.
Kunert G, Otto S, Rose USR, et al.:
Alarm pheromone mediates production of winged dispersal morphs in aphids.
Ecol Lett.
2005; 8: 596–603. Publisher Full Text
- 7.
Rottman SJ, Snowdon CT:
Demonstration and analysis of an alarm pheromone in mice.
J Comp Physiol Psychol.
1972; 81(3): 483–490. PubMed Abstract
| Publisher Full Text
- 8.
Steiger S, Schmitt T, Schaefer HM, et al.:
The origin and dynamic evolution of chemical information transfer.
Proc Biol Sci.
2011; 278(1708): 970–979. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 9.
Sih A:
Antipredator responses and the perception of danger by mosquito larvae.
Ecology.
1986; 67(2): 434–441. Publisher Full Text
- 10.
Laforsch C, Beccara L, Tollrian R, et al.:
Inducible defenses: The relevance of chemical alarm cues in Daphnia.
Limnol Oceanogr.
2006; 51(3): 1466–1472. Publisher Full Text
- 11.
Parker DA, Shulman MJ:
Avoiding predation: Alarm responses of Caribbean sea-urchins to simulated predation on conspecific and heterospecific sea-urchins.
Mar Biol.
1986; 93(2): 201–208. Publisher Full Text
- 12.
Lawrence JM:
A chemical alarm response in Pycnopodia helianthoides (Echinodermata: Asteroidea).
Mar Behav Physiol.
1991; 19(1): 39–44. Publisher Full Text
- 13.
Sleeper HL, Paul VJ, Fenical W, et al.:
Alarm pheromones from the marine opisthobranch Navanax inermis.
J Chem Ecol.
1980; 6(1): 57–70. Publisher Full Text
- 14.
Kempendorff W:
Über das Fluchtphänomen und die Chemoreception von Helisoma (Taphius) nigricans.
Arch Molluskenkd.
1942.
- 15.
Hews DK, Blaustein AR:
An investigation of the alarm response in Bufo boreas and Rana cascadae tadpoles.
Behav Neural Biol.
1985; 43(1): 47–57. PubMed Abstract
| Publisher Full Text
- 16.
Kats LB, Petranka JW, Sih A, et al.:
Antipredator defenses and the persistence of amphibian larvae with fishes.
Ecology.
1988; 69(6): 1865–1870. Publisher Full Text
- 17.
Wisenden BD:
Olfactory assessment of predation risk in the aquatic environment.
Philos Trans R Soc Lond B Biol Sci.
2000; 355(1401): 1205–1208. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 18.
Hughes AL:
Evolution of adaptive phenotypic traits without positive Darwinian selection.
Heredity (Edinb).
2012; 108(4): 347–353. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 19.
Williams GC:
Measurement of consociation among fishes and comments on the evolution of schooling. Publications of the Museum, Michigan State University Biological Series. 1964; 2: 349–384. Reference Source
- 20.
Williams GC:
Natural selection: domains, levels, and challenges. (Oxford University Press, 1992). Reference Source
- 21.
Chivers DP, Brown GE, Ferrari MCO, et al.:
Chemical ecology in aquatic systems. eds C. Brönmark & L. A. Hansson (Oxford University Press) 2012; 312. Reference Source
- 22.
von Frisch K:
Zur Psychologie des Fisch-Schwarmes.
Naturwissenschaften.
1938; 26(37): 601–606. Publisher Full Text
- 23.
von Frisch K:
Über einen Schreckstoff der Fischhaut und seine biologische Bedeutung.
Z Vgl Physiol.
1942; 29(1–2): 46–145. Publisher Full Text
- 24.
Barreto RE, Barbosa A, Giassi ACC, et al.:
The ‘club’ cell and behavioural and physiological responses to chemical alarm cues in the Nile tilapia.
Mar Freshw Behav Physiol.
2010; 43(1): 75–81. Publisher Full Text
- 25.
Pfeiffer W:
The distribution of fright reaction and alarm substance cells in fishes.
Copeia.
1977; 1977(4): 653–665. Reference Source
- 26.
Kristensen EA, Closs GP:
Anti-predator response of naive and experienced common bully to chemical alarm cues.
J Fish Biol.
2004; 64(3): 643–652. Publisher Full Text
- 27.
Mathis A, Perriere C, Zaccone G, et al.:
In Fish defenses Volume 2: Pathogens, parasites and predators. eds G. Zaccone, C. Perriére, A. Mathis, & B. G. Kapoor. 2009; 2: 323–386 (Science Publishers). Reference Source
- 28.
Mathis A, Smith RJF:
Chemical alarm signals increase the survival time of fathead minnows (Pimephales promelas) during encounters with northern pike (Esox lucius).
Behav Ecol.
1993; 4(3): 260–265. Publisher Full Text
- 29.
Stabell OB, Lwin MS:
Predator-induced phenotypic changes in crucian carp are caused by chemical signals from conspecifics.
Environ Biol Fishes.
1997; 49(1): 139–144. Publisher Full Text
- 30.
Wisenden BD, Smith RJF:
The effect of physical condition and shoalmate familiarity on proliferation of alarm substance cells in the epidermis of fathead minnows.
J Fish Biol.
1997; 50(4): 799–808. Publisher Full Text
- 31.
Chivers DP, Smith RJF:
Chemical alarm signalling in aquatic predator-prey systems: A review and prospectus (fait parti du numéro thématique sur l’écologie chimique/part of the special feature on chemical ecology).
Ecoscience.
1998; 5(3): 338–352. Reference Source
- 32.
Smith RJF:
Alarm signals in fishes.
Rev Fish Biol Fish.
1992; 2(1): 33–63. Publisher Full Text
- 33.
Mathis A, Chivers DP, Smith RJF, et al.:
Chemical alarm signals: Predator deterrents or predator attractants?
Am Nat.
1995; 145(6): 994–1005. Publisher Full Text
- 34.
Chivers DP, Brown GE, Smith RJF, et al.:
The evolution of chemical alarm signals: Attracting predators benefits alarm signal senders.
Am Nat.
1996; 148(4): 649–659. Publisher Full Text
- 35.
Cameron AM, Endean R:
Epidermal secretions and the evolution of venom glands in fishes.
Toxicon.
1973; 11(5): 401–410. PubMed Abstract
- 36.
Al-Hassan JM, Thompson M, Criddle RS, et al.:
Composition of the proteinacous gel secretion from the skin of the Arabian Gulf catfish (Arius thallasinus).
Mar Biol.
1982; 70(1): 27–33. Publisher Full Text
- 37.
Chivers DP, Wisenden BD, Hindman CJ, et al.:
Epidermal ‘alarm substance’ cells of fishes maintained by non-alarm functions: possible defence against pathogens, parasites and UVB radiation.
Proc Biol Sci.
2007; 274(1625): 2611–2619. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 38.
Hamilton WD:
The genetical evolution of social behaviour I.
J Theor Biol.
1964; 7(1): 1–16. PubMed Abstract
| Publisher Full Text
- 39.
Maynard-Smith J:
Group selection and kin selection.
Nature.
1964; 201(4924): 1145–1147. Publisher Full Text
- 40.
Tamura K, Ihara Y:
Classes of communication and the conditions for their evolution.
Theor Popul Biol.
2011; 79(4): 174–183. PubMed Abstract
| Publisher Full Text
- 41.
Charnov EL, Krebs JR:
The evolution of alarm calls: Altruism or manipulation?
Am Nat.
1975; 109(965): 102–107. Publisher Full Text
- 42.
Sherman PW:
Alarm calls of Belding ground squirrels to aerial predators: nepotism or self-preservation.
Behav Ecol Sociobiol.
1985; 17(4): 313–323. Publisher Full Text
- 43.
da Silva KB, Mahan C, da Silva J, et al.:
The trill of the chase: Eastern chipmunks call to warn kin.
J Mammal.
2002; 83(2): 546–552. Publisher Full Text
- 44.
Shelley EL, Blumstein DT:
The evolution of vocal alarm communication in rodents.
Behav Ecol.
2005; 16(1): 169–177. Publisher Full Text
- 45.
Pollard KA:
Making the most of alarm signals: the adaptive value of individual discrimination in an alarm context.
Behav Ecol.
2010; 22(1): 93–100. Publisher Full Text
- 46.
Thünken T, Meuthen D, Bakker TCM, et al.:
Parental investment in relation to offspring quality in the biparental cichlid fish Pelvicachromis taeniatus.
Anim Behav.
2010; 80(1): 69–74. Publisher Full Text
- 47.
Lamboj A:
Die Cichliden des westlichen Afrikas. (Birgit Schmettkamp Verlag) 2004. Reference Source
- 48.
Thünken T, Bakker TC, Baldauf SA, et al.:
Active inbreeding in a cichlid fish and its adaptive significance.
Curr Biol.
2007; 17(3): 225–229. PubMed Abstract
| Publisher Full Text
- 49.
Thünken T, Bakker TCM, Baldauf SA, et al.:
Direct familiarity does not alter mating preference for sisters in male Pelvicachromis taeniatus (Cichlidae).
Ethology.
2007; 113(11): 1107–1112. Publisher Full Text
- 50.
Thünken T, Meuthen D, Bakker TC, et al.:
A sex-specific trade-off between mating preferences for genetic compatibility and body size in a cichlid fish with mutual mate choice.
Proc Biol Sci.
2012; 279(1740): 2959–2964. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 51.
Thünken T, Waltschyk N, Bakker TC, et al.:
Olfactory self-recognition in a cichlid fish.
Anim Cogn.
2009; 12(5): 717–724. PubMed Abstract
| Publisher Full Text
- 52.
Thünken T, Baldauf SA, Kullmann H, et al.:
Size-related inbreeding preference and competitiveness in male Pelvicachromis taeniatus (Cichlidae).
Behav Ecol.
2011; 22(2): 358–362. Publisher Full Text
- 53.
Hesse S, Bakker TCM, Baldauf SA, et al.:
Kin recognition by phenotype matching is family-rather than self-referential in juvenile cichlid fish.
Anim Behav.
2012; 84(2): 451–457. Publisher Full Text
- 54.
Meuthen D, Baldauf SA, Bakker TCM, et al.:
Substrate-treated water: a method to enhance fish activity in laboratory experiments.
Aquat Biol.
2011; 13(1): 35–40. Publisher Full Text
- 55.
McLennan DA:
Male brook sticklebacks’ (Culaea inconstans) response to olfactory cues.
Behaviour.
2004; 141(11): 1411–1424. Publisher Full Text
- 56.
Mehlis M, Bakker TC, Frommen JG, et al.:
Smells like sib spirit: kin recognition in three-spined sticklebacks (Gasterosteus aculeatus) is mediated by olfactory cues.
Anim Cogn.
2008; 11(4): 643–650. PubMed Abstract
| Publisher Full Text
- 57.
Bourdeau PE, Johansson F:
Predator-induced morphological defences as by-products of prey behaviour: a review and prospectus.
Oikos.
2012; 121(8): 1175–1190. Publisher Full Text
- 58.
Kats LB, Dill LM:
The scent of death: Chemosensory assessment of predation risk by prey animals.
Ecoscience.
1998; 5(3): 361–394. Reference Source
- 59.
Mateo JM:
Recognition systems and biological organization: The perception component of social recognition.
Ann Zool Fenn.
2004; 41(6): 729–745. Reference Source
- 60.
Brown GE, Adrian JC, Smyth E, et al.:
Ostariophysan alarm pheromones: Laboratory and field tests of the functional significance of nitrogen oxides.
J Chem Ecol.
2000; 26(1): 139–154. Publisher Full Text
- 61.
Pfeiffer W, Riegelbauer G, Meier G, et al.:
Effect of hypoxanthine-3(N)-oxide and hypoxanthine-1(N)-oxide on central nervous excitation of the black tetra Gymnocorymbus ternetzi (Characidae, Ostariophysi, Pisces) indicated by dorsal light response.
J Chem Ecol.
1985; 11(4): 507–523. Publisher Full Text
- 62.
Mathuru AS, Kibat C, Cheong WF, et al.:
Chondroitin fragments are odorants that trigger fear behavior in fish.
Curr Biol.
2012; 22(6): 538–544. PubMed Abstract
| Publisher Full Text
- 63.
Roh E, Mirza RS, Brown GE, et al.:
Quality or quantity? The role of donor condition in the production of chemical alarm cues in juvenile convict cichlids.
Behaviour.
2004; 141(10): 1235–1248. Publisher Full Text
- 64.
Brown GE, Bongiorno T, DiCapua DM, et al.:
Effects of group size on the threat-sensitive response to varying concentrations of chemical alarm cues by juvenile convict cichlids.
Can J Zool.
2006; 84(1): 1–8. Publisher Full Text
- 65.
Brown GE, Foam PE, Cowell HE, et al.:
Production of chemical alarm cues in convict cichlids: the effects of diet, body condition and ontogeny.
Ann Zool Fenn.
2004; 41(3): 487–499. Reference Source
- 66.
Ward AJW, Hart PJB:
The effects of kin and familiarity on interactions between fish.
Fish and Fisheries.
2003; 4(4): 348–358. Publisher Full Text
- 67.
Langen K, Schwarzer J, Kullmann H, et al.:
Microsatellite support for active inbreeding in a cichlid fish.
PLoS One.
2011; 6(9): e24689. PubMed Abstract
| Publisher Full Text
| Free Full Text

Comments on this article Comments (0)