Keywords
Metabolic syndrome; green tea; green coffee; cardiac fibrosis; inflammation.
Background: Metabolic syndrome is a significant risk factor for cardiovascular diseases. Green tea and green coffee extracts, antioxidant and anti-inflammatory agents may participate in metabolic syndrome-induced cardiac fibrosis alleviation. However, the effect of combination of those extracts still needs exploration. Therefore, this study investigated the effect of green tea and decaffeinated light roasted green coffee extracts and their combination in metabolic syndrome-induced cardiac fibrosis rats.
Methods: Metabolic syndrome rat model was i1nduced through high-fat high sucrose diets feeding for 8 weeks and injection of low dose streptozotocin at the 2nd week. The metabolic syndrome rats were divided into 4 experimental groups metabolic syndrome rats (MS); metabolic syndrome rats treated with 300 mg/ kg b.w green tea extract (GT); metabolic syndrome rats treated with 200 mg/ kg b.w decaffeinated light roasted green coffee extract (GC); metabolic syndrome rats treated with the combination of the two extracts (CE); and a normal control (NC) group was added. Angiotensin 2 level was analyzed by ELISA method. Gene expression of NF-κB, TNF-α, IL-6, Tgf-β1, Rac-1, and α-sma were analyzed by touchdown polymerase chain reaction methods.
Results: Metabolic syndrome rats treated with green tea and decaffeinated light roasted green coffee significantly decreased angiotensin-2 serum level and cardiac inflammation and fibrosis gene expression level (NF-κB, TNF-α, IL-6, Tgf-β1, Rac-1, and α-sma). More significant alleviation was observed in the combination group.
Conclusion: This study suggested that combination of green tea and decaffeinated light roasted green coffee extracts showed better improvement in metabolic syndrome-induced cardiac fibrosis rat model compared to that of single extract administration through inflammation inhibition
Metabolic syndrome; green tea; green coffee; cardiac fibrosis; inflammation.
Editorial Note (6th December 2023): The F1000 Editorial Team has not yet received a new version of this article, as detailed in the Editorial Notes published on 16th June and 4th August 2023. The F1000 Editorial Team will no longer be requesting a new version from the authors. Readers should be aware that there is key information missing from the article regarding animal treatments and ethical approval; this information had been previously given to and verified by the F1000 Editorial Team, but the authors have not yet updated their article. Peer review activity remains suspended until the authors publish a new version of this article.
Editorial Note (4th August 2023): The F1000 Editorial Team has not yet received a new version of this article, as detailed in the Editorial Note published on 16th June 2023. The F1000 Editorial Team is actively contacting the authors to request the new version of the article. Peer review activity remains suspended until the authors publish a new version of this article.
Editorial Note (16th June 2023): Since publication, it has been brought to the attention of the Editorial Team that the article was missing key information regarding animal treatments and ethical approval. The Editorial Team requested further detail and an explanation from the authors in March 2023. The authors provided an adequate response and were requested by the Editorial Team to create a new version of the article to include the additional details. Peer review activity has been until the authors publish a new version of this article.
Metabolic syndrome (MetS), the presence of at least three of five manifestations, obesity, insulin resistance, dyslipidemia, and hypertension, has been considered a major risk factor for cardiovascular diseases (CVD) such as cardiac fibrosis.1–3 The risk of cardiovascular disease was seven times higher among individuals with MetS and diagnosed diabetes mellitus.4 Overnutrition and lack of physical activities leading to obesity and diabetes presented as the most common etiology of MetS.5
Obesity and diabetes caused reactive oxygen species (ROS) production that activated neurohumoral systems, including the renin-angiotensin system (RAS). ROS and RAS stimulated inflammation through inflammation mediators release and immune cell activation.6 Angiotensin-II and TGFβ-1, well known potent pro-fibrotic agents, lead to fibroblast differentiation and excessive ECM production.7,8 Previous studies suggested that rats or mice with a high-fat diet and low-dose streptozotocin showed cardiac morphology alteration with evidence of inflammation and oxidative stress, leading to cardiac fibrosis.9,10 It showed cardiac fibrosis features characterized by functional and structural cardiac changes through morphological, biochemical, and molecular alterations.11
Treatment strategies for MetS and its complication mostly used conventional drugs to solve one or some metabolic manifestation, but it failed due to its inability to reduce the cardiovascular complications especially heart damage.12–16 Recently, scientists have focused on bioactive phytochemicals due to their wide range of pharmacological activities. Epigallocatechin-gallate (EGCG), a bioactive phenolic from green tea leaves, is well known for its antioxidant and anti-inflammatory effects.17 Besides that, chlorogenic acid (CGA), a phenolic compound from plants such as green coffee beans, is also known for its antioxidant and anti-inflammation activities.18 Combining two antioxidants significantly attenuates MetS metabolic parameters rather than individual substrate administration, clinically and molecularly.19,20 Furthermore, EGCG signifies the internal mechanism of the anti-fibrosis effect by preventing NF-κB transfer to the nucleus and downregulating CTGF gene expression that reduces fibroblast proliferation and synthesis of extracellular matrix.21 Moreover, CGA from coffee administration improved the outcomes of diabetes and non-alcoholic fatty liver disease (NAFLD) patients through a TGF-β1 lowering effect,22 and worked as an anti-fibrosis effect on the heart.23 Nevertheless, previous studies reported a drawback of green coffee administration to CVD risk due to its caffeine content.24–26 Therefore, this study investigated the administration of green tea and decaffeinated light roasted green coffee extract combination on prevention of cardiac MetS complications, especially cardiac fibrosis, through inhibition of inflammation responses.
All experimental procedures were approved by the ethical committee of Faculty of Medicine, Brawijaya University with registration number 405/EC/KEP/10/2016. All efforts were made to ameliorate harm to the animals by using the standard protocol from the Indonesian Ministry of Health ethical research guidelines for animal experimental research.
This study was part of a larger study20 and was carried out on 25 male Sprague–Dawley rats 8–12 weeks old, maintained and acclimatized as in the previous study. The Ethics Committee approved all animal procedures of the Faculty of Medicine, Brawijaya University Malang. Animals were divided randomly into five groups of five rats in each group as in the previous publication.20 The list of groups is Group I: negative control group (Control); Group II: metabolic syndrome group (MetS); Group III: metabolic syndrome treated with 300 mg/kg b.w. green tea extract (GT); Group IV: metabolic syndrome treated with 200 mg/kg b.w. decaffeinated light roasted green coffee extract (GC); Group V: metabolic syndrome treated with the combination of 300 mg/kg b.w. green tea extract and 200 mg/kg b.w. decaffeinated light roasted green coffee extract (CE). The metabolic syndrome model was created by the same method as Rohman et al. (2017) with a high fat and sucrose diet (HFSD) for 8 weeks, and an intraperitoneal injection of low dose streptozotocin (STZ 30 mg/kg b.w.) on day 14 after diet start.27 Then, extracts were treated daily via oral gavage for 9 weeks after MetS were confirmed as NCEP ATP III criteria. After treatment, the animals were euthanized by diethyl ether with prior a 12-h fasting period.20
The method of measurement of body weight, biochemical analysis, and systolic blood pressure were presented in a previous study.20
The extraction protocol to get the final extract of young green tea leaf and green coffee bean (robusta) was described in the previous study.20
Caffeine, EGCG, and CGA content in green tea and decaffeinated light roasted green coffee extracts were analyzed using a Shimadzu HPLC System (model SCL10AVP, Osaka, Japan) following the previous study’s protocol.20
The dose was determined by the preliminary studies considering each extract's optimal single dose effect.19,20
Serum was collected at the end of the study and stored at −80oC. Analysis of ELISA was conducted for angiotensin-II serum level using a Rat Angiotensin-II ELISA Kit (Ref E-EL-R1430, Elabscience, USA) as written on the manufacturing protocol and read using ELx808 Absorbance Microplate Reader (BioTek, China) to get the result as ng/mL.
After the euthanasia process, the heart was collected immediately and preserved at RNA buffer solution to keep the RNA integrity. Total RNA Extraction Kit easy-BLUE (Intron Biotechnology, South Korea) reagent was used to extract the total RNA and then stored at −80°C until gene expression analysis. Reverse transcription reaction was performed using the ReverTra Ace-α kit (Ref FSK-101, Toyobo, Japan). A procedure of touchdown PCR was applied using polymerase chain reaction (PCR) LightCycler 96 system (Takara, Japan). Each tube of PCR mix contained cDNA, GoTaq Master Mix (Ref M7122, Promega, Madison, USA), and the primers (Integrated DNA Technologies, Singapore). All primers were designed using NCBI Primer-BLAST as follows.
Tgf-β1 Forward 5′-TCCTGTCCAAACTAAGGCTC-3′;
Reverse 5′-CAAGGTAACGCCAGGAATTG-3′;
Rac-1 Forward 5′-GCTCATCAGTTACACGACCA-3′;
Reverse 5′-TAAGAACACGTCTGTTTGCG-3′;
α-sma Forward 5′-AAAAGGACAGCTATGTGGGG-3′;
Reverse 5′-CTCCAGAGTCCAGCACAATA-3′;
NFkB Forward 5′-AACGCATCCCAAGGTGCTGGA-3′;
Reverse 5′-GCAGCTGGAAAAGCTCAAGCCA-3′;
Tnf-α Forward 5′-CGTCAGCCGATTTGCCATTTC-3′;
Reverse 5′-TGGGCTCATACCAGGGCTTG-3′;
Il-6 Forward 5′-CCCAACTTCCAATGCTCTCCTAAT-3′;
Reverse 5′-GCA CAC TAG GTT TGC CGA GTA GA-3′;
β-actin Forward 5′-CGAGTACAACCTTCTTGCAG-3′;
Reverse 5′-CATTGTAGAAAGTGTGGTGC-3′
The touchdown PCR amplification protocol consisted of 5 min at 95°C for pre-denaturation, 10 cycles of denaturing at 95°C for 30 sec, annealing at 60°C for 30 sec, and extension at 72°C for 30 sec. This was followed by a further 10 cycles of denaturing at 95°C for 30 sec, annealing at 57°C for 30 sec, and extension at 72°C for 30 sec, then continued with other 10 cycles of denaturing at 95°C for 30 sec, annealing at 55°C for 30 sec, and extension at 72°C for 30 sec. The reaction was held at 4°C. The product of PCR was electrophoresed using Mupid-exU Submarine Electrophoresis System (Advance, Japan) and was captured by using ImageQuant LAS 500 Chemiluminescence CCD Camera (Guangdong Denley Technology, China). All bands were semi-quantified using ImageJ as the relative expression level of each gene after normalization to the housekeeping gene (β-actin).
The result of the extraction process was analyzed by HPLC and represent of EGCG of green tea extract was 74,176 μg/g, CGA of the decaffeinated light roasted green coffee extract was 27,134 μg/g, and caffeine in the decaffeinated light roasted green coffee extract was 63,348 μg/g.20
Examination of food intake, body weight, fasting blood glucose, LDL, HDL, total cholesterol level, and systolic blood pressure already described by Rohman et al. (2021).20
This study revealed a significant difference in Ang-2 serum level between the MetS group and the extract group. The MetS group had a significantly higher angiotensin-2 serum level (p < 0.001) compared to that of the normal control group (NC). Treatment of green tea extract 200 mg/kg b.w. (GT), decaffeinated light roasted green coffee extract 300 mg/kg b.w. (GC), and their combination of those extract (CE) resulted in a significantly lower Ang-2 level (p ≤ 0.001) compared with that of MetS group. Moreover, a significant different in Ang-2 serum levels between NC and all extract-treated groups was observed in this study, which suggested that all doses of extract administration could not bring back the normal level of Ang-2 serum. Meanwhile, the data showed that the CE group had the lowest Ang-2 serum level compared with that of all extract treated groups (Figure 1).
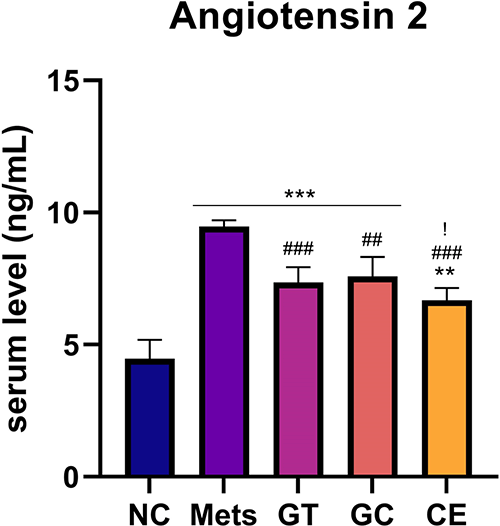
Data are Mean ± SD (n = 4-5). *p < 0.05, **p < 0.01, ***p < 0.001 versus NC. #p < 0.05, ##p < 0.01, ###p < 0.001 versus MetS. $p < 0.05, $$p < 0.01, $$$p < 0.001 versus GT. !p < 0.05, !!p < 0.01, !!!p < 0.001 versus GC.
Gene expression analysis conducted from rat heart tissue suggested higher inflammation markers in the MetS group compared to that of the NC group. Nevertheless, administration of green tea and decaffeinated light roasted green coffee extracts resulted in the lower mRNA expression levels of NF-κB, TNF-α, and IL-6 (p < 0.05) compared to that of the MetS group. However, the CE group had the lowest mRNA expression level compared with GT and GC (Figure 2).
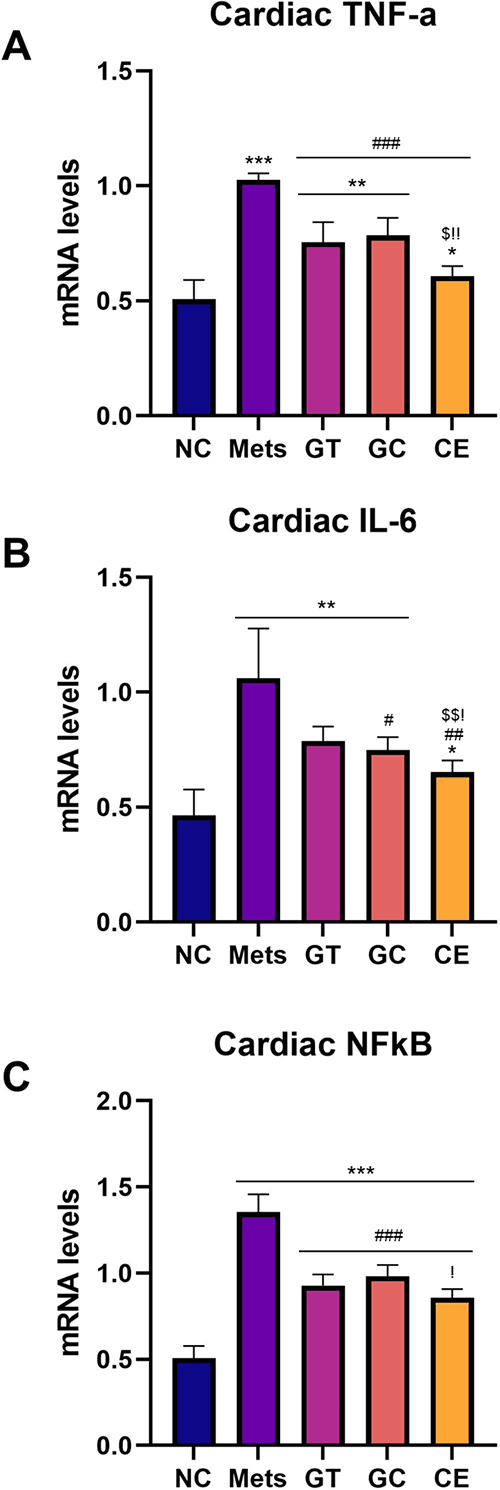
Relative mRNA expression level of (A) TNF-α, (B) IL-6, and (C) NFkB. Data are Mean ± SD (n = 4-5). *p < 0.05, **p < 0.01, ***p < 0.001 versus NC. #p < 0.05, ##p < 0.01, ###p < 0.001 versus MetS. $p < 0.05, $$p < 0.01, $$$p < 0.001 versus GT. !p < 0.05, !!p < 0.01, !!!p < 0.001 versus GC.
Similar to inflammation markers, the cardiac fibrosis gene expression analysis of rat heart tissue suggested higher inflammation markers in the MetS group compared to that of the NC group. Furthermore, all doses administration of green tea and decaffeinated light roasted green coffee extracts resulted in a significantly lower mRNA expression level of Tgf-β1, Rac-1, and α-sma compared to that of the MetS group (p < 0.05) (Figure 3). A significant difference of Tgf-β1, Rac-1 and α-sma mRNA expression was also observed between the NC group and GT or GC group (except Tgf-β1 mRNA expression level in the GC group), suggested that green tea and decaffeinated light roasted green coffee extracts could not fully alleviate the fibrosis to normal condition but they lower the markers as much as possible. However, similar Tgf-β1, Rac-1 and α-sma mRNA expression levels were observed between NC and CE groups, suggesting that extract combination administration could lower the NC group’s metabolic syndrome induced high cardiac fibrosis gene expression.
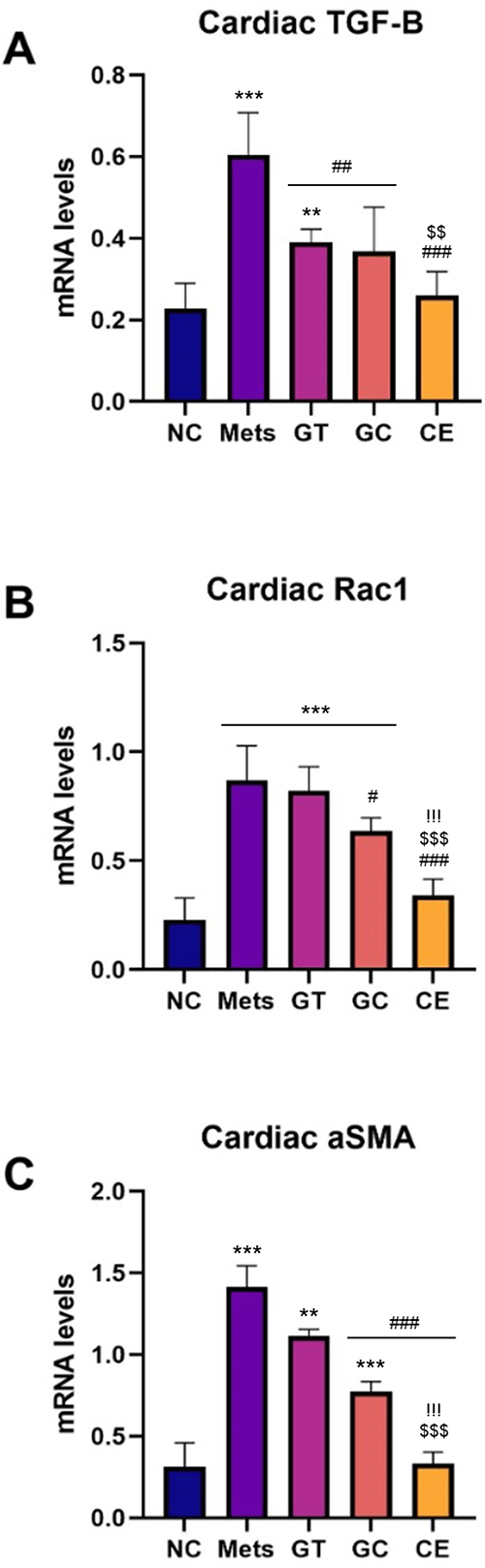
Relative mRNA expression level of (A) TGF-β1, (B) Rac1, and (C) α-SMA. Data are Mean ± SD (n = 4-5). *p < 0.05, **p < 0.01, ***p < 0.001 versus NC. #p < 0.05, ##p < 0.01, ###p < 0.001 versus MetS. $p < 0.05, $$p < 0.01, $$$p < 0.001 versus GT. !p < 0.05, !!p < 0.01, !!!p < 0.001 versus GC.
MetS remains a major risk factor for CVD and global health concerns.28 The mechanism underlies MetS are excessive ROS production and low-grade chronic inflammation.29–31 GT and GC extract both has antioxidant and anti-inflammatory effect with their major bioactive compounds, EGCG and CGA, which is proven for various disease treatments17,32–37 though the possible role of their combination extract against cardiac fibrosis induced by MetS remains to be elucidated. In this study, HFHS/LDSTZ-MetS rat model was treated with 300 mg/kg b.w. GT extract, 200 mg/kg b.w. GC extract and combination of those two extracts (300 mg/kg b.w. green tea extract and 200 mg/kg b.w. decaffeinated light roasted green coffee extract). Metabolic parameters of those extracts treated animals were already described in Rohman et al. (2021).20 The data revealed attenuation of body weight, fasting blood glucose level, HDL, LDL, total cholesterol level, and systolic blood pressure in the MetS rat model. Administration of a combination of GT and GC extracts showed better alleviation of metabolic parameters than that of single extract administration.20 This study is the first report that showed the effect of GT and GC extracts combination dose against MetS induced cardiac fibrosis through inflammation alleviation.
This study showed that rats with HFHS/LD-STZ-induced MetS increased cardiac fibrosis biomarkers by increasing angiotensin-2 serum and cardiac inflammation gene expression levels. A previous study also denoted cardiac fibrosis by increased cardiac galectin-3 mRNA and collagen deposition.10 High-fat and high-sucrose diet and low-dose STZ induction causes oxidative stress in many tissues, such as adipose, liver, pancreas, heart, and skeletal muscle.30 Increases in ROS production stimulate cells to produce angiotensinogen then converted to angiotensin-2 (Ang-2) throughout the body.8,38–40 Furthermore, ROS can activate signaling kinases and transcription factors such as Rac GTP-binding protein, associated with fibrosis and hypertrophy, and the NF-κB pathway, related to pro-inflammatory gene transcription.41 Moreover, visceral obesity is strongly correlated with epicardial adipose tissue, and it means a more significant amount of Ang-2 and pro-inflammatory adipokines production.42 Ang-2 also stimulates expression and activates NF-kB to promote more inflammation.8 All the induction and inflammation processes continuously develop, causing chronic inflammation and promoting cardiac fibrosis development.
Ang-2 serum levels were significantly lower in the GT, GC, and CE group than in the MetS group. The most significant lower level of Ang-2 was observed in the CE dose. Earlier data showed that TNF-α serum level was decreased most at combination doses of 300 mg/kg b.w. GT extract and 200 mg/kg b.w. GC extract.20 Ang-2 can express and activate NF-kB to promote inflammation with the release of TNF-α, IL-6, and TGF-β1, so reducing of its serum level impacts NF-kB and inflammatory mediators.8,43–46 Interleukin-6 denoted its role in collagen synthesis through STAT-3 signaling activation in cardiac fibroblast.47 Moreover, overexpression of cardiac TNF-α caused cardiac fibrosis with heart failure development by involved collagen synthesis and activating matrix-degrading proteases (MMP2 and MMP9), which activates latent TGF-β1.48 In this present study, NFkB, TNF-α, and IL-6 relative mRNA expression level in the heart of the metabolic syndrome rat model treated with GT and GC extracts showed a lower level compared to that of MetS group. The lowest inflammatory markers were observed in the CE groups. It suggested that a combination of GT and GC extract worked better in lowering inflammation.
TGF-β1 is a potent pro-fibrotic factor affected by increased Ang-2, inflammation mediators, and NF-kB. Lu et al. (2021) reported a rise of TGF-β1, α-SMA, and Col-1 relative mRNA levels on heart tissue and cardiac fibrotic histopathological finding in high fat and low-dose STZ-induced diabetic mice.9 Rac1 is a small GTPase molecule, one of TGF-β1 noncanonical signaling pathway molecules that function on the regulation of expression and activation of the actin cytoskeleton, including α-SMA as actin stress fiber myofibroblast.49–53 α-SMA is a hallmark marker of fibrosis that indicates fibroblast differentiation to mature myofibroblast. It can stretch more, attracting ECM and cells in the cardiac tissue environment that causes interstitial fibrosis and stiffness.54,55 The relative mRNA expression level of TGF-β1, Rac1, and α-SMA in the heart decreased in all extracts, but the most significant decrease was at combination dose. This study suggested that a combination of GT and GC extracts attenuated MetS-induced cardiac fibrosis better than the administration of a single extract. Nevertheless, Rac1 also plays a role in GLUT4 translocation in skeletal muscle by activating and modulating actin cytoskeleton to transfer GLUT4 vesicles from the cytoplasm to the membrane.56–58 It was contradictory with our recent study that the lower relative expression of Rac1 in the heart did not affect the fasting blood glucose. A possible explanation of this issue was that Rac1 was not involved in GLUT4 translocation signaling pathways in the rat heart. Hence, further study is needed to investigate GLUT4 mRNA expression level in rat hearts as well as the protein level of molecules involved in this pathway.
This study suggested that the HFHS/LDSTZ-MetS model showed sustained cardiac fibrosis through enhancement of RAS and inflammation. However, GT and GC extract were attenuated cardiac fibrosis markers with the most optimal dose at the combination of 300 mg/kg b.w. GT extract and 200 mg/kg b.w. GC extract. The combination administration lowered Ang-II serum level and relative mRNA expression level of NFkB, TNF-α, IL-6, TGF-β1, Rac1, and α-SMA on heart rat tissue. All these results provided a clear explanation regarding the effect of combination GT and GC extracts against MetS-induced cardiac fibrosis via inflammation inhibition.
Figshare: Underlying data for ‘Improvement of cardiac fibrosis biomarkers through inflammation inhibition by green tea and decaffeinated light roasted green coffee extract combination administration in metabolic syndrome rat model’ https://doi.org/10.6084/m9.figshare.13249163.v3
This project contains the following underlying data:
- Data of Metabolic Syndrome Rat Model.xlsx (This file contains the analyzed data)
- RAW data metabolic syndrome.xlsx (This file contains the actual observed values of the variables)
- Chlorogenic acid Coffee HPLC.pdf (This file contains the value of coffee chlorogenic acid levels as measured by HPLC)
- Caffeine coffee HPLC.pdf (This file contains the value of coffee caffeine levels as measured by HPLC)
- Green Tea Catechin HPLC.pdf (This file contains the value of the catechin levels in the tea)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
D.A.N: Conceptualization, Data Curation, Methodology, Resources
M.L: Conceptualization, Data Curation, Methodology, Writing – Review & Editing
M.S.R: Conceptualization, Funding acquisition, Supervision, Writing – Review & Editing
N.A.W: Data Curation, Investigation, Writing – Original Draft Preparation
M.N.R: Data Curation, Formal Analysis, Investigation, Writing – Original Draft Preparation
N.W: Project Administration, Supervision, Writing – Review & Editing
The authors acknowledge the Laboratory of Animal Physiology and Laboratory of Molecular Biology Department of Biology Mathematics and Natural Sciences of Brawijaya University. Ministry of Education, Culture, Research, and Technology.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)