Keywords
drug safety, efficacy, hemophilia A, human and medicine, immunogenicity, prophylaxis, rurioctocog alfa pegol
drug safety, efficacy, hemophilia A, human and medicine, immunogenicity, prophylaxis, rurioctocog alfa pegol
We have added an additional information regarding the serious adverse event occured related to the treatment, the dose regimens and pharmacokinetic. We have also added a reference from Stidl et aI. (2018), because the article explained about the safety and potential adverse effects that related to rurioctocog alfa pegol.
See the authors' detailed response to the review by Claudia Djambas Khayat
See the authors' detailed response to the review by Yelvi Levani
Hemophilia A is a rare, X-linked recessive, congenital bleeding disorder caused by mutations or defects in the factor VIII (FVIII)-producing genes.1 Those mutations manifest as a congenitally absence or decrease of the FVIII, an important pro-coagulant cofactor in the bleeding hemostasis.2 Hemophilia A occurs more commonly than hemophilia B (in 1 out of 5,000 male live births) and accounts for 80% of overall hemophilia cases.2,3 Hemophilia A may be further classified into mild, moderate, and severe based on the FVIII levels.1 The severe form of hemophilia A is defined as having FVIII levels <1% of normal, while the mild and moderate forms have higher FVIII levels that are approximately 5–50% and 1–5%, respectively.3 Patients with severe hemophilia often present with internal bleeding, especially in the joints and soft tissues. Joint and soft tissue bleeding, along with painful feelings, may restrict patients from their daily activities due to the restriction on their range of motions.3,4 If this bleeding continues without being treated adequately, hemophilic patients could suffer from more advanced complications, including hemophilic arthropathy. This is important since hemophilic arthropathy could negatively affect their quality of life due to the severe joint immobility.3
The current management of hemophilia A relies on two options: (1) episodic or on-demand FVIII replacement if the patients present with any bleedings to prevent further bleeding or (2) prophylactic FVIII treatment to maintain the FVIII levels and prevent any future bleedings.5 However, the first option was no longer recommended as primary long-term management due to no alteration found in its natural disease course.6 To date, the standard of care for hemophilia A, especially the severe form, still relies on a regular prophylactic intravenous FVIII replacement therapy. Additionally, more than 30% of patients with hemophilia A may develop ‘inhibitors’ or refer to as neutralizing anti-drug antibodies to the standard prophylactic treatment which has high immunogenicity in inducing its formation.3 Thus, extended half-life and safer prophylactic agents may be beneficial in reducing the daily treatment burden, and at the same time, those agents could maintain better clinical presentations and improve the treatment efficacy.7
Recently, rurioctocog alfa pegol (i.e., BAX 855), a third-generation recombinant FVIII (rFVIII) with a modification in its polyethylene glycol (PEG) component, has been developed.8 This modification prolongs the half-life of rFVIII by 1.4–1.5 folds the original rFVIII, thereby reducing the administration frequency and maintaining better bleeding hemostasis of the hemophilic patients.8,9 Yet, to the best of our knowledge, there are no pooled studies assessing the efficacy, safety, and immunogenicity of rurioctocog alfa pegol. Therefore, here, we aim to evaluate the efficacy, safety, and immunogenicity of rurioctocog alfa pegol, a newly-developed prophylactic agent, in previously treated patients with severe hemophilia A.
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 guidelines.10 A computerized and systematic data searching of relevant studies was conducted in PubMed, Scopus, Cochrane Library, Wiley Online Library, and CINAHL (via EBSCOhost) from inception to 16 February 2021. Keywords were constructed based on Medical Subject Headings (MeSH) terms and other additional terms listed as follows: (“rurioctocog alfa pegol” OR “bax 855” OR “TAK-660” OR “SHP660” OR “adynovate” OR “adynovi”) AND (“hemophilia A” OR “haemophilia A” OR “factor VIII deficiency” OR “factor 8 deficiency” OR “classic hemophilia” OR “classic haemophilia”). Two reviewers searched the literatures independently. Any disagreements were resolved in a consensus involving a third investigator.
Studies were included if the following criteria were met: (1) study design of clinical trial; (2) study population consists of previously treated severe hemophilia A patients with or without healthy subjects as control; (3) rurioctocog alfa pegol as a prophylactic treatment intervention; and (4) the reported outcomes related to the efficacy, safety, and immunogenicity of rurioctocog alfa pegol (annualized bleeding rate [ABR], patients with zero-bleeding during treatment, hemostatic efficacy, adverse events [AEs], number of deaths, development of FVIII ‘inhibitors’, and/or binding antibodies). The exclusion criteria were as follows: (1) irrelevant titles and abstracts; (2) review articles, systematic reviews, meta-analyses, case reports, case series, letter to editors, and conference abstracts; (3) non-English studies; or (4) irretrievable full-text articles.
The following relevant data were extracted from the included studies: (1) author and year of publication; (2) study location; (3) clinical trial number; (4) study design; (5) total patients included for prophylactic treatment, gender, and age; (6) definition of target joint (TJ); (7) regimen type; (8) patient characteristics (with or without target joints); (9) total patients in per-protocol analysis set (PPAS) or analyzed for ABR based on regimen type and target joints; (10) outcomes related to efficacy (types of ABR, number of patients with zero-bleeding during treatment, and/or hemostatic efficacy); (11) outcomes related to safety (number of patients with AEs, total AEs, AEs considered related to treatment, and/or number of deaths); and/or (12) outcomes related to immunogenicity (development of FVIII ‘inhibitors’ and/or binding antibodies). The quality assessment of the included studies was performed using the Methodological Index for Non-Randomized Studies (MINORS) scale11 for non-randomized studies and Modified Jadad scale12 for randomized studies. Studies with a MINORS score of ≥ 12 or a Jadad score of ≥ 4 were considered high-quality studies, and the rest were considered low-quality studies. The data extraction and quality assessment were conducted by three reviewers collaboratively through a group discussion and a final decision was taken based on the agreement of all reviewers.
Statistical analyses were performed using the latest version of OpenMeta [Analyst] from the Brown University Evidence-Based Practice Center13 and MetaXL ver. 5.3 (EpiGear International, Sunrise Beach, Australia). Single-arm meta-analysis of mean and standard deviation values was performed for four different efficacy outcomes: (1) total ABR; (2) spontaneous ABR; (3) injury ABR; and (4) joint ABR. Whilst, a meta-analysis of proportions was performed for two different efficacy outcomes: (1) zero-bleeding prevalence and (2) hemostatic efficacy with the rating of excellent or good. Subgroup analysis based on target joints (TJs) for total ABR was also performed. For the purpose of meta-analyses, 95% confidence intervals were transformed into standard deviation values based on a method suggested by the Cochrane Handbook Chapter 6.14
Heterogeneity between studies was assessed with a chi-square test (Cochran’s Q statistic) and quantified with the Higgins’ I2 statistic. P-value < 0.1 from the chi-square test indicated statistical heterogeneity, whereas the level of heterogeneity was determined using I2 values. I2 < 25% was considered a low heterogeneity, 25–75% a moderate heterogeneity, and I2 > 75% a high heterogeneity. If the I2 value was greater than 50%, a random-effects model was used for the meta-analysis. Otherwise, a fixed-effects model was applied. Publication bias was explored qualitatively using a funnel plot if the number of studies was adequate (n ≥ 10).
The initial search of this study yielded a total of 232 articles identified from PubMed, Scopus, Cochrane Library, Wiley Online Library, and CINAHL (via EBSCOhost). Of those, 174 studies were screened by titles and abstracts after duplicates removal. Twenty-three were fully reviewed based on the eligibility criteria and 19 of these were excluded due to: (1) studies with a sub-analysis of other included studies (n = 2); (2) not reporting the outcome of interest (n = 7); or (3) conference abstracts (n = 10). Finally, four clinical trials5,7,9,15 were included in the qualitative and quantitative synthesis. The overall study selection process is illustrated in Figure 1.
Table 1 provides a summary of the studies included in the systematic review. The four uncontrolled clinical trials5,7,9,15 included a total of 517 previously treated severe hemophilia A patients for prophylactic treatment, with the overall mean ± SD age of 23.9 ± 14.8. Only two studies by Mullins et al.9 and Chowdary et al.7 included a female patient. The trials were published between 2015 and 2021 and were all multicentered with a range number of 11 to 23 countries. Three7,9,15 out of four studies were in phase 3 clinical trial, whereas the study by Konkle et al.5 was in a phase 2/3 trial. All studies were non-randomized with the exception of Klamroth et al.15 Definition of target joint was the same across all studies, except for Klamroth et al.15 There were two different prophylactic regiment types used between studies: twice-weekly and pharmacokinetic (PK)-guided. The “excellent” hemostatic efficacy rating was defined as a complete resolution of pain and sign of bleedings after a single infusion without the requirement of additional infusion to control the bleeding, while the “good” rating was defined when there was a definite improvement in pain and/or signs of bleeding after a single infusion with a possible requirement of more than one infusion to complete the resolution. The “fair” rating was defined as a slight improvement in pain and/or signs of bleeding after a single infusion with definite requirement of more than one infusion to complete the resolution. If there was no improvement or the condition worsen, the hemostatic efficacy was rated “none”.5
Characteristics of the included studies.
| Author, year | Study location | Clinical trial number | Study design | Total patients included for prophylactic treatment (F) | Age* | Definition of target joint |
|---|---|---|---|---|---|---|
| Mullins et al., 20179 | Multicenter (11 countries) | NCT02210091 | Phase 3, open-label, non-randomized, uncontrolled clinical trial | 66 (1) | 6.0 ± 2.7 | A joint (ankles, knees, hips or elbows) with ≥ 3 spontaneous bleeding episodes in any consecutive 6-month period |
| Chowdary et al., 20207 | Multicenter (23 countries) | NCT01945593 (CONTINUATION study) | Phase 3b, open-label, non-randomized, uncontrolled clinical trial | 216 (1) | 22.8 ± 15.7 | A joint with ≥ 3 spontaneous bleeding episodes in any consecutive 6-month period |
| Konkle et al., 20155 | Multicenter (20 countries) | NCT01736475 (PROLONG-ATE study) | Phase 2/3, open-label, non-randomized, uncontrolled clinical trial | 120 (0) | 28.7 ± 9.0 | A joint with ≥ 3 spontaneous bleeding episodes in any consecutive 6-month period |
| Klamroth et al., 202015 | Multicenter (22 countries) | NCT02585960 (PROPEL study) | Phase 3, open-label, randomized, uncontrolled clinical trial | 57 (0) | 31.0 ± 13.6 | A joint with ≥ 4 spontaneous bleeding episodes in any consecutive 6-month period |
| 58 (0) | 31.6 ± 12.9 |
| Author, year | Regimen type | Patient characteristics | Total patients in PPAS or analyzed for ABR based on regimen Type & TJ | Total ABR | Spontaneous ABR | Injury ABR | Joint ABR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) | SD | Mean (95% CI) | SD | Mean (95% CI) | SD | Mean (95% CI) | SD | ||||
| Mullins et al., 20179 | Twice-weekly prophylaxis | With TJs | 14 | 3.54 (1.89–6.64) | 4.11 | 1.20 (0.92–1.56) | 2.22 | 2.09 (1.49–2.93) | 2.93 | 1.10 (0.64–1.91) | 2.58 |
| Without TJs | 52 | 2.92 (2.02–4.24) | 3.99 | ||||||||
| Chowdary et al., 20207 | Twice-weekly prophylaxis | With and without TJs | 186 | 2.23 (1.85–2.69) | 3.06 | 1.20 (0.92–1.56) | 2.33 | N/A | N/A | 1.23 (0.96–1.58) | 2.26 |
| PK-guided prophylaxis | With and without TJs | 25 | 2.64 (1.70–4.08) | 1.87 | 0.96 (0.54–1.71) | 0.92 | 1.40 (0.91–2.17) | 0.99 | |||
| Konkle et al., 20155 | Twice-weekly prophylaxis | With TJs | 32 | 3 | 4.9 | 2.2 | 3.7 | N/A | N/A | 2.2 | 3.2 |
| Without TJs | 69 | 3.7 | 4.4 | 1.9 | 2.9 | 1.2 | 2.4 | ||||
| Klamroth et al., 202015 | PK-guided prophylaxis (1–3%) | With and without TJs | 52 | 2.8 | 3 | 1.7 | 2.5 | 1.1 | 1.9 | 1.8 | 2.2 |
| PK-guided prophylaxis (8–12%) | With and without TJs | 43 | 1.2 | 2.4 | 0.6 | 1.5 | 0.7 | 1.7 | 0.8 | 2.3 | |
| Author, year | Patients with zero-bleeding during treatment | Hemostatic efficacy | Adverse events | Number of deaths | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rating | Events | Total number of bleedings | Number of patients with any AEs (non-SAEs and SAEs) | Total non-SAEs | Non-SAEs considered related to treatment | Number of patients with SAEs | Total SAEs | SAEs considered related to treatment | |||
| Mullins et al., 20179 | 25 | Excellent Good Fair None Not reported | 34 29 4 0 3 | 70 | 43 | 152 | 0 | 3 | 4 | 0 | 0 |
| Chowdary et al., 20207 | 51 | Excellent Good Fair None Not reported | 438 368 48 4 52 | 910 | 174 | 786 | 20 | 33 | 52 | 0 | 1 (considered unrelated to treatment) |
| Konkle et al., 20155 | 40 | Excellent/Good Fair/ None/Not reported | 498 20 | 518 | 73 | 166 | 7 | 5 | 5 | 0 | 0 |
| Klamroth et al., 202015 | 24 | N/A | N/A | N/A | 34 | 97 | 2 | 3 | 4 | 0 | 0 |
| 36 | 36 | 98 | 1 | 4 | 5 | 1 | 0 | ||||
| Author, year | Development of FVIII inhibitory antibodies | Development of binding antibodies to FVIII / PEG-FVIII/PEG during study |
|---|---|---|
| Mullins et al., 20179 | No subjects developed inhibitory antibodies |
|
| Chowdary et al., 20207 | No subjects developed inhibitory antibodies | |
| Konkle et al., 20155 | No subjects developed inhibitory antibodies | |
| Klamroth et al., 202015 | No subjects developed inhibitory antibodies | |
| 1 subject (resolved at the study end) |
Total ABR
A total of 473 hemophilia A patients from the four studies5,7,9,15 were included in this subgroup single-arm meta-analysis (Figure 2) to calculate the pooled mean of total ABR after rurioctocog alfa pegol treatment. A random-effects model was used for the analysis since heterogeneity among studies was greater than 50% (I2 = 67%). The overall pooled mean of total ABR was 2.59 (95% CI = 2.04–3.14).
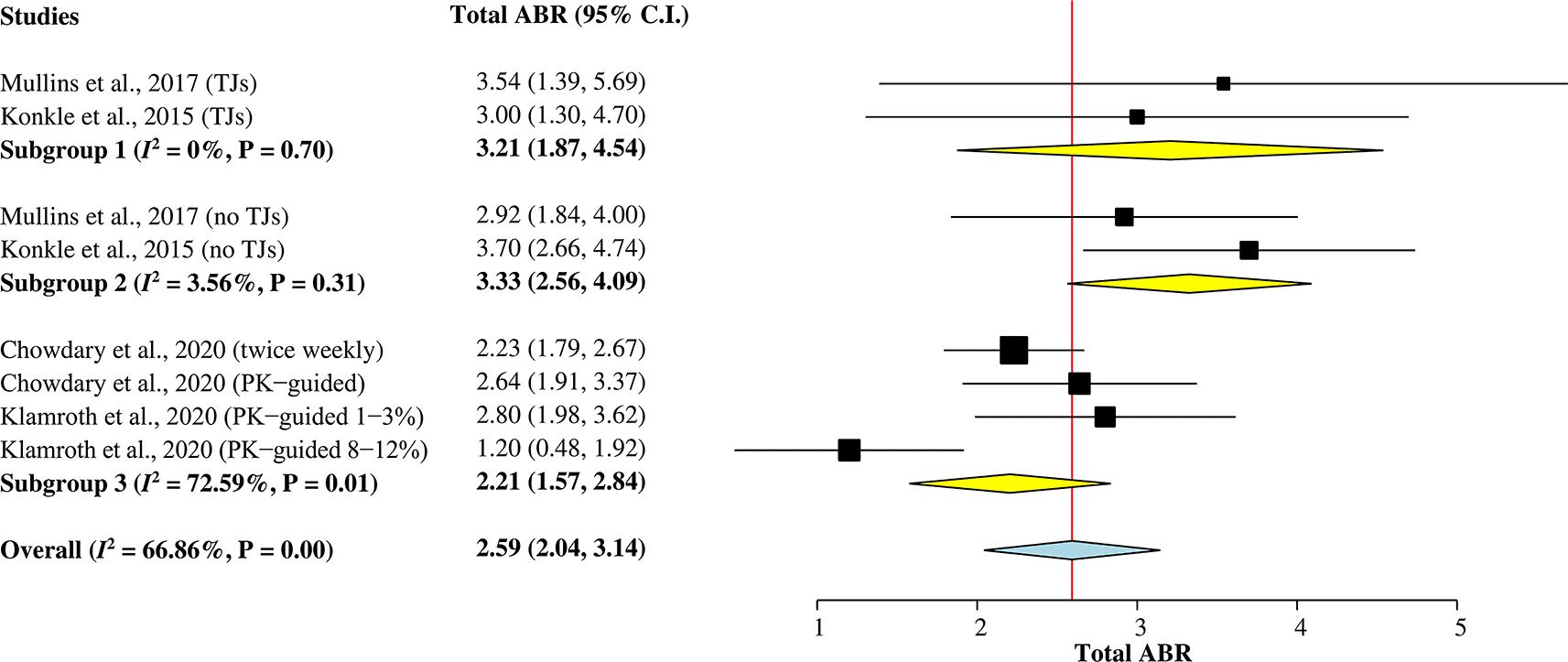
ABR, annualized bleeding rate; CI, confidence interval; PK, pharmacokinetic; TJ, target joint.
Two studies5,9 reporting mean of total ABR individually for patients with target joints (TJs) and without target joints were included in Subgroup 1 and Subgroup 2, respectively. The pooled mean of total ABR in patients with TJs was 3.21 (95% CI = 1.87–4.54), whilst the pooled mean of total ABR in patients without TJs was 3.33 (95% CI = 2.56–4.09). Subgroup 3 included other two studies7,15 with a combined mean of total ABR for patients with and without TJs. The pooled value was 2.21 (95% CI = 1.57–2.84).
Spontaneous ABR
The four studies5,7,9,15 with a total of 473 hemophilia A patients were included in this meta-analysis (Figure 3A). Heterogeneity between studies was greater than 50% (I2 = 64%); therefore, a random-effects model was used for the analysis. The result of the pooled mean of spontaneous ABR was 1.24 (95% CI = 0.91–1.58).
Injury ABR
A total of 161 hemophilia A patients from two studies9,15 that reported mean of injury ABR were included in this meta-analysis (Figure 3B). A random-effects model was used for the analysis since heterogeneity was greater than 50% (I2 = 80%). The pooled mean of injury ABR was 1.26 (95% CI = 0.53–1.99).
Joint ABR
A total of 473 hemophilia A patients from the four studies5,7,9,15 were evaluated in this subgroup analysis of joint ABR (Figure 3C). The heterogeneity across studies was low (I2 = 0%); therefore, a fixed-effects model was used for the analysis. The pooled mean of joint ABR was 1.31 (95% CI = 1.12–1.50).
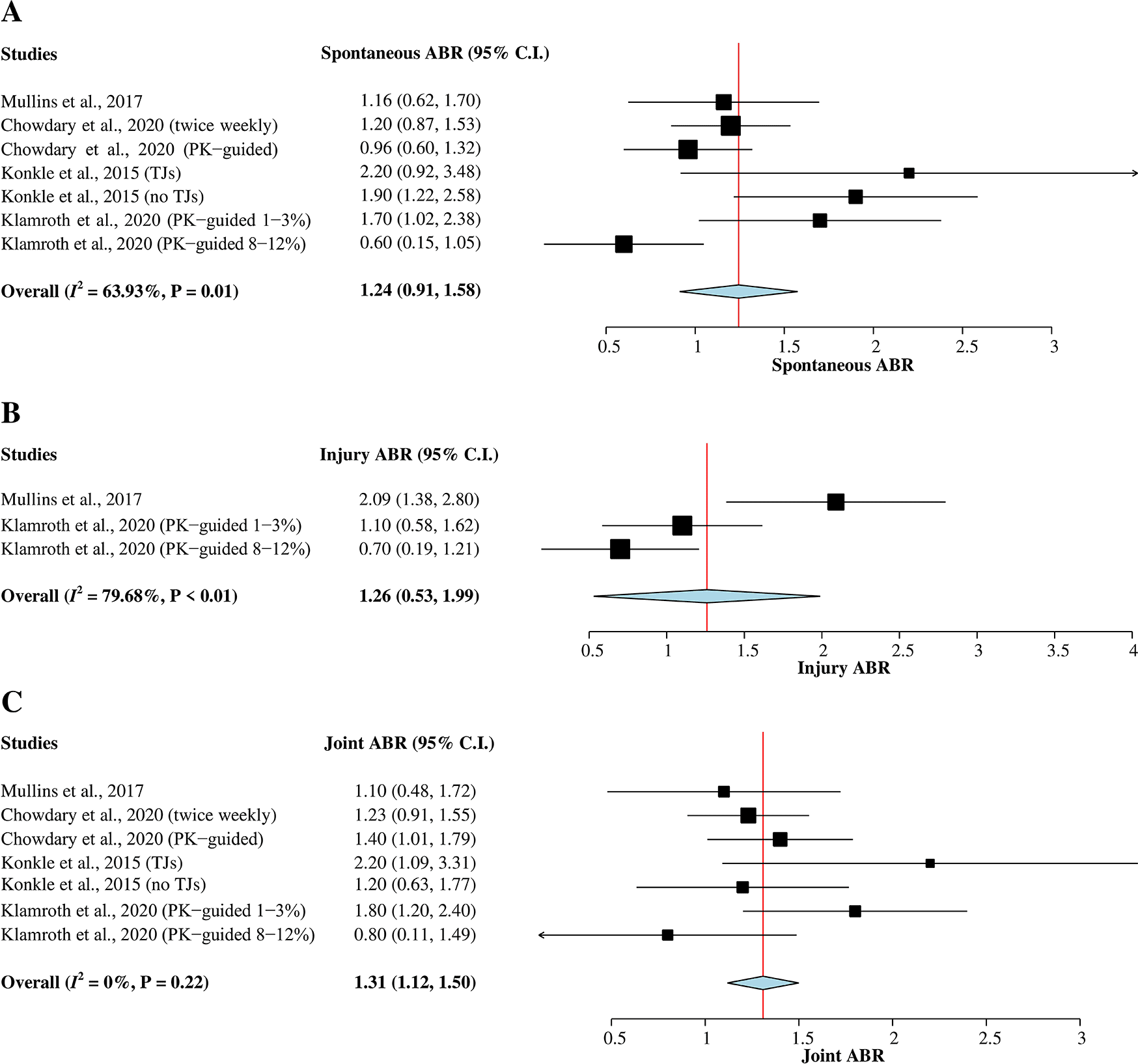
ABR, annualized bleeding rate; CI, confidence interval; PK, pharmacokinetic; TJ, target joint.
Zero-bleeding prevalence
All four studies5,7,9,15 were included in this meta-analysis of zero-bleeding prevalence (Figure 4A). A random-effects model was used due to the heterogeneity of the data (I2 = 88%). The pooled prevalence result was 40% (95% CI = 27%–54%).
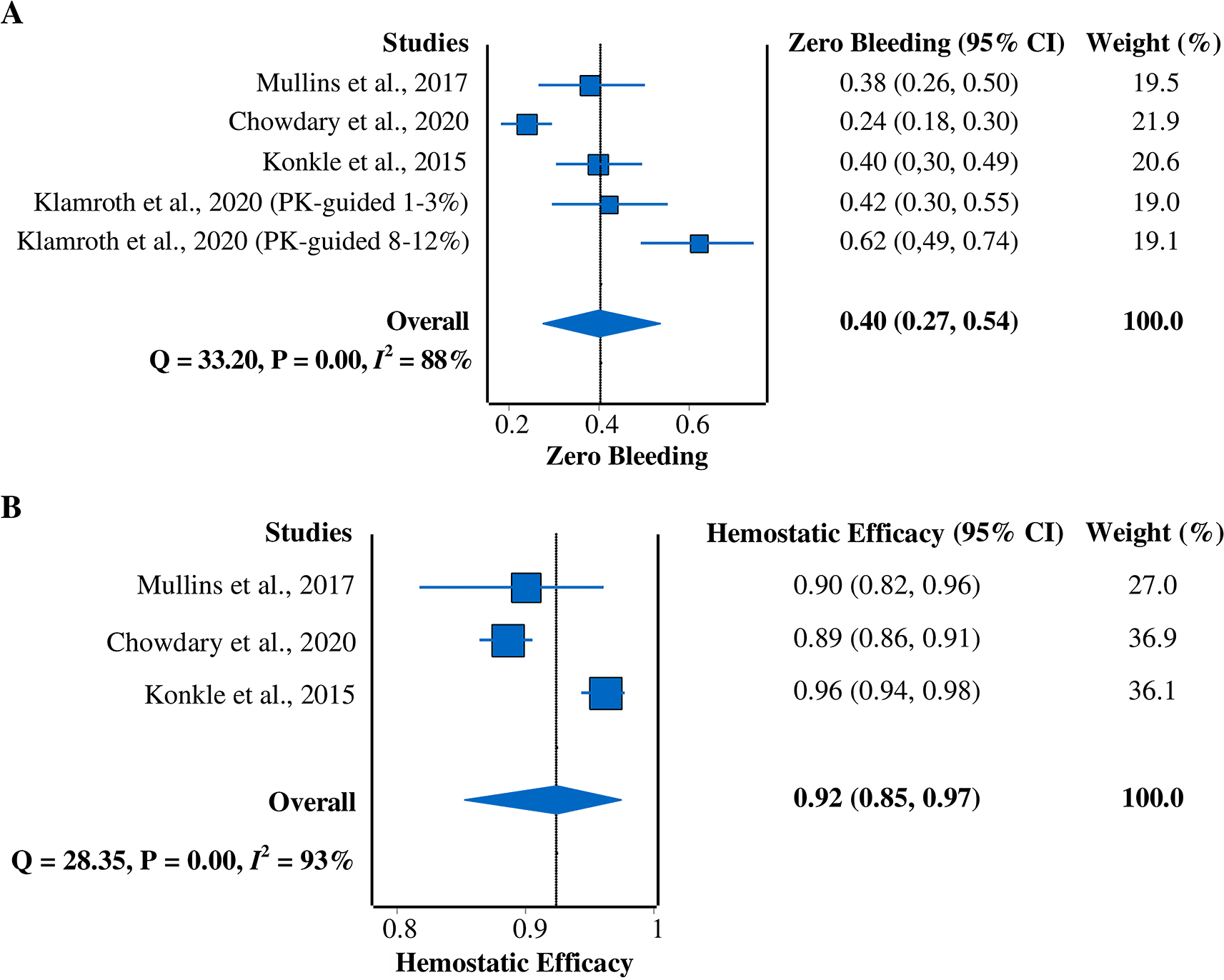
CI, confidence interval; PK, pharmacokinetic.
Hemostatic efficacy
Three studies5,7,9 that reported hemostatic efficacy with the rating of excellent or good were included in this meta-analysis (Figure 4B). A random-effects model was used due to the heterogeneity across studies (I2 = 93%). The pooled hemostatic efficacy was 92% (95% CI = 85%–97%).
A total of 1,299 non-serious adverse events (non-SAEs) occurred during the four studies.5,7,9,15 However, only 30 (2.3%) of them were considered related to rurioctocog alfa pegol treatment. Whilst, a total of 70 serious adverse events (SAEs) were observed in the four studies and only one (1.4%; a transient development of FVIII inhibitory antibodies) of them – as reported by Klamroth et al.15 – were considered related to treatment. Among all studies, only one death case was reported by Chowdary et al.7 and was not considered to be related to rurioctocog alfa pegol treatment.
Three studies5,7,9 reported no development of FVIII inhibitory antibodies among all patients. Klamroth et al.15 reported one patient with development of FVIII inhibitory antibodies and was resolved at the end of the study. Development of binding antibodies to either FVIII, PEG-FVIII, or PEG among patients was detected in 52 patients from the four studies. However, none of them was correlated to impaired rurioctocog alfa pegol treatment efficacy and AEs.
Publication bias using funnel plot was not performed due to the low number of the included studies. Details of the quality assessment using MINORS and Modified Jadad scale are provided in Table 2. All non-randomized studies5,7,9 were considered high in quality, whereas the randomized study by Klamroth et al.15 was considered low in quality.
| MINORS Scale | Modified Jadad Scale | ||||
|---|---|---|---|---|---|
| Items | Mullins et al., 20179 | Chowdary et al., 20207 | Konkle et al., 20155 | Items | Klamroth et al., 202015 |
| A clearly stated aim | 2 | 2 | 2 | Randomization | 1 |
| Inclusion of consecutive patients | 2 | 2 | 2 | ||
| Prospective collection of data | 2 | 2 | 2 | Concealment | 0 |
| Endpoints appropriate to the aim of the study | 2 | 2 | 2 | ||
| Unbiased assessment of the study endpoint | 0 | 0 | 0 | Blinding | 0 |
| Follow-up period appropriate to the aim of the study | 2 | 2 | 2 | ||
| Loss to follow up less than 5% | 2 | 2 | 2 | Withdrawal or drop-out | 1 |
| Prospective calculation of the study size | 1 | 1 | 1 | ||
| Results | Results | ||||
| Total score | 13 | 13 | 13 | Total score | 2 |
| Study quality | High | High | High | Study quality | Low |
This study was the first far-reaching, single-arm meta-analysis that evaluates the efficacy, safety, and immunogenicity of rurioctocog alfa pegol, a newly developed rFVIII product with a prolonged half-life, as a prophylactic treatment for previously treated patients with severe hemophilia A. Rurioctocog alfa pegol (BAX 855) is a pegylated full-length rFVIII product designed to reduce the frequency of prophylactic infusions while maintaining hemostatic efficacy in patients with hemophilia.16,17 This study indicated the long-term safety and efficacy of the pharmacological agent, which were consistent with the study of rurioctocog alfa pegol for perioperative hemostasis in hemophilia A patients,18,19 also with the previous parent studies.20–22
The overall pooled mean of total ABR of rurioctocog alfa pegol is lower compared to the several conventional rFVIIIs (Advate®, Xyntha®, Novoeight®, REFACTO®) with their total ABR ranged from 3.3 to 6.5.23 This could indicate that rurioctocog alfa pegol has advantages over conventional recombinant antihemophilic FVIII. The ABRs were also similar for spontaneous and injury-related bleeding. Any reduction in joint bleeds is considered an improvement in quality of life for hemophilia patients.24 Decreased bleeding in joints thereby shows better joint health, activity, and satisfaction for the patients.25 The mean ABR for patients with target joints was similar to those without target joints, indicating that rurioctocog alfa pegol had an equal efficacy for both groups of patients. Moreover, all studies reported that rurioctocog alfa pegol had higher good and excellent hemostatic efficacy events. This data was comparable with results reported for other rFVIII preparations.26–29 The efficacy of rurioctocog alfa pegol was also supported by the finding on the pooled zero-bleeding prevalence.
Our study also demonstrated the safety of rurioctocog alfa pegol in patients by assessing the non-SAEs and SAEs. Rurioctocog alfa pegol was also proven to be acceptable and safe for perioperative hemostasis in patients with hemophilia A, with minor findings in both non-SAEs and SAEs.18 Our data showed that most of the adverse reactions were mild. Additionally, rFVIII usage decreased the risk of blood-borne infections and restored longer life expectancies.30 This extended half-life recombinant also improved adherence to prophylactic regimen and reduced the burden of treatment.31,32 However, there are some concerns regarding the safety of PEG component, particularly when it is used for lifelong prophylaxis. Nevertheless, the PEG levels found in rurioctocogalfa pegol were minimal (less than 1 mg/dose) and evidence has showed no specific side effects to central nervous system, liver, or kidney.42
The development of FVIII ‘inhibitors’ is a major issue in patients treated with blood coagulation factor products. The development of neutralizing alloantibodies against FVIII can reduce the treatment benefits.33,34 Currently available studies revealed some predictors of ‘inhibitor’ development, but the predictive power remained low.35,36 Some studies also reported either transient or persistent ‘inhibitor’ development in patients treated with plasma-derived FVIII.37,38 However, our findings showed no development of persistent FVIII inhibitory antibodies, and this was consistent with the US Food and Drug Administration's approval of rurioctocog alfa pegol for the treatment of hemophilia A patients.39 There was some development of binding antibodies observed. However, this development did not interfere with rurioctocog alfa pegol treatment safety and efficacy until the end of the study.
Overall, our study successfully demonstrated the pooled efficacy, safety, and immunogenicity of rurioctocog alfa pegol as a treatment for hemophilia A. These results can be used to plan an alternative treatment for hemophilia A patients. Nevertheless, high heterogeneity existed between the included studies. We used the random-effects model to minimize this issue. Substantial efforts were made to explore the possible source for heterogeneity, revealing that different dose regimens and prior prophylactic drugs for treatment could be responsible for the high heterogeneity. Regarding the zero-bleeding prevalence (Figure 4A), a difference in the administered dose regimens was observed among studies. Different dose regimens were considered because pharmacokinetic profiles, targets of FVIII level, and age group varied among patients. The PK-guided dosing was applied to provide more individualized prophylaxis according to each patient’s PK profiles (e.g., plasma half-life), targets of FVIII level, and body weight. Age is also a determining factor since it influences the PK profiles.6
Several other limitations exist in this meta-analysis. First, our study only included single-arm clinical trials. The highest possible quality cannot be ensured due to the lack of control arms. However, since hemophilia is a rare genetic disease, comparison with a control arm receiving prophylaxis with other conventional FVIII products was not recommended, as stated by the regulatory guide.40 Second, diverse prior prophylactic strategies in the patients before switching to rurioctocog alfa pegol may affect the treatment outcomes. Finally, only a few published studies were evaluated in this meta-analysis since rurioctocog alfa pegol is a newly-developed drug. However, these limitations were partly compensated by the multicentered settings of the included studies.
Our study suggests that rurioctocog alfa pegol is effective, safe, and has low immunogenicity for previously treated patients with severe hemophilia A. Despite the lack of direct comparison studies, rurioctocog alfa pegol could serve as an alternative bleeding prophylaxis in hemophilia A. A network meta-analysis with a multi-arm approach on hemophilia A treatment is warranted to corroborate the current evidence.
All data underlying the results are available as part of the article and no additional source data are required.
Open Science Framework: PRISMA Checklist for “Efficacy, Safety, and Immunogenicity of Rurioctocog Alfa Pegol for Prophylactic Treatment in Previously Treated Patients with Severe Hemophilia A: A Systematic Review and Meta-Analysis of Clinical Trials”. http://doi.org/10.17605/OSF.IO/7MNRP.41
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Hemophilia and rare Bleeding disorders
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
References
1. Stidl R, Denne M, Goldstine J, Kadish B, et al.: Polyethylene Glycol Exposure with Antihemophilic Factor (Recombinant), PEGylated (rurioctocog alfa pegol) and Other Therapies Indicated for the Pediatric Population: History and Safety.Pharmaceuticals (Basel). 2018; 11 (3). PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 13 Jan 22 |
read | |
|
Version 2 (revision) 11 Nov 21 |
read | read |
|
Version 1 15 Oct 21 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)