Keywords
ZnO nanoparticles, green synthesis, bush tea, reducing agent, natural extract.
This article is included in the Nanoscience & Nanotechnology gateway.
ZnO nanoparticles, green synthesis, bush tea, reducing agent, natural extract.
This new version includes some modification on figures 4,5 and 6. The JCPDS standard pattern of ZnO nanoparticles has been inserted in Figure 4. Details of FTIR spectra have been inserted in Figures 5 and 6. Minor grammatical errors reviewed in the introduction.
See the authors' detailed response to the review by Mohammod Aminuzzaman
See the authors' detailed response to the review by Saeid Taghavi Fardood
Nanomaterials with a size less than 100 nm are globally synthesized owing to their various properties such as, antimicrobial, anti-inflammatory, wound healing, catalytic, magnetic, optical, and electronic properties, that have put them at the forefront of a wide variety of studies (Gunalan et al., 2012; Jamdagni et al., 2018). Compared to their counterpart bulk materials, they present a reduced surface-to-volume ratio that increases as the size is reduced (Ohno et al., 2010), thus placing them on the borderline between single molecules and bulk materials (Mansoori and Soelaiman, 2005).
The introduction of nanoparticles in the consumer industry, health, food, space, chemical, and cosmetics, has called for a green and environmentally responsible strategy for their production (Rao and Gautam, 2016). Metal oxides and dioxides such as zinc oxide, silver, gold and titanium dioxide have received copious consideration because of their multiple properties and applications (Dobrucka and Długaszewska, 2016). However, their synthesis has been done through numerous physicochemical methods. Laser ablation, microwave irradiation and vapour deposition have been reported to date (Satyanarayana and Reddy, 2018), they involve forces of condensation, dispersion, or fragmentation of bulk particles into nanoparticles, as well as some toxic chemicals, harmful to the environment (Dhandapani et al., 2014; Krupa and Vimala, 2016).
Synthesis of nanomaterials through biological systems assisted by some biotechnological tools is an emerging asset of nanotechnology that provides a safe, cost-effective and eco-friendly synthesis process (Shinwari and Maaza, 2017). Plants, diatoms, fungi, yeast, algae, bacteria, and human cells have been used. Their proteins and other metabolites have been well reported to have a reductive capacity that can transform metal ions into metal nanoparticles (Dobrucka and Długaszewska, 2016; Parveen et al., 2016). The biological synthesis of nanoparticles provides more advantages than chemical and physical ones (Kharissova et al., 2013). Numerous metal oxide nanoparticles, such as TiO2, CuO, and ZnO have been produced by total green chemistry. Among them, ZnO, an n-type semiconductor, has gained interest owing to its easy production, cost-effectiveness, and safety of synthesis and usage (Agarwal et al., 2017). Several studies have successfully been led to synthesize ZnO nanoparticles using different organisms such as bacteria, fungi, algae, and plants (Agarwal et al., 2017).
Among all biological systems, phytosynthesis of nanoparticles using plants has shown great potential. Plant-mediated nanoparticle synthesis is simple, eco-friendly, and provides antibacterial assets (Gunalan et al., 2012; Iravani et al., 2015; Thema et al., 2015). A variety of metabolites such as terpenoids, polyphenols, sugars, alkaloids, phenolic acids and proteins have been reported to have metal ion reduction assets (Parveen et al., 2016). Several studies dedicated to the green synthesis of ZnO nanoparticles using plant extracts as capping or reducing agents have shown the use of different plant aerial parts, such as leaves and fruits of different species such as Aloe vera, Hibiscus sabdariffa, Allium sativum, Allium cepa, Petroselinum crispum, Moringa oleifera and Camellia sinensis, for the synthesis of nanoparticles (Mahendiran et al., 2017; Matinise et al., 2017; Senthilkumar and Sivakumar, 2014; Stan et al., 2015). Bush tea, mostly known as a medicinal tea plant in southern Africa where it originates has high concentrations of phenolic compounds such as tannins and flavonoids (Lerotholi et al., 2017). However, data explaining the synthesis processes of nanoparticles using this plant are lacking. Hence, the objective of this study was to contribute to the explanation of the compounds induced in the synthesis process of ZnO nanoparticles using Athrixia phylicoides leaf extract.
Leaves of bush tea (A. phylicoides DC) were used to reduce zinc nitrate hexahydrate. Analytical grade Zn (NO3)2.6H2O of 99% purity was purchased from Sigma-Aldrich, South Africa. Bush tea leaves were harvested from the wild in Thohoyandou (22.8785°S; 30.4818°E) in the Limpopo province, South Africa. Following the harvest, the leaves were washed with deionized water and freeze-dried for 72 hours at -50°C at a pressure of 0.32 Kpa, hereafter they were ground and kept for further usage.
Extract preparation
Ten grams of ground bush tea leaves were weighed and mixed with 300 ml of deionized water. The mixture was heated at 60°C for 30 minutes until the water changed to a dark green colour. After centrifugation using a Hermle Labortecnik GmbH Z 216-M benchmark centrifuge at 4000 rpm for 10 minutes, the mixture was filtered twice using Whatman filter paper number 1, and the extract was kept in an airtight container in a fridge at ≈4°C for analysis and ZnO nanoparticles synthesis.
Synthesis of ZnO nanoparticles
In this study, zinc nitrate hexahydrate [Zn (NO3)2.6H2O] was used as the precursor. One gram of the precursor was mixed with 25 ml of A. phylicoides extract. The mixture was kept on a magnetic stirrer at 300 rpm at 60°C for 30 minutes and then left to cool down at room temperature for 12 hours, a precipitate was observed. The mixture was centrifuged for 15 minutes at 4000 rpm. The supernatant was collected and transferred to LC-MS vials for analysis and the precipitate was dried at 60°C for one hour and then annealed at 800°C for two hours. The obtained powder was then kept for characterization.
Bush tea compounds profiling and identification
The determination and profiling of different compounds present in the extract before the synthesis as well as the supernatant after synthesis were performed using liquid chromatography quadrupole time-of-flight mass spectrometry (LC-Q-TOF-MS) using a Bruker impact II (Germany). After peak integration and Pareto scaling, the liquid chromatography-mass spectrometry (LC-MS) data were transformed into buckets using the Bruker Compass data analysis programme version 4.3.110 (https://www.bruker.com/en/). Peaks were determined using real mass, MS/MS, and retention time (RT). The accuracy of the mass and MS/MS spectral data was compared to the Kyoto standard Encyclopaedia of Genes and Genomes (KEGG) and ChemSpider databases using the MetFrag 2.2 online software (Tete et al., 2020). Principal component analysis (PCA) and T-tests were performed using MetaboAnalyst 4.0.
ZnO nanoparticle characterization
The characterization of the obtained ZnO nanopowders was done using X-ray diffraction (XRD), Ultraviolet-visible spectroscopy (UV-Vis), Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS). The crystallite size of ZnO nanoparticles was estimated using the modified Scherrer equation:
where is the X-ray wavelength, β the peak width at half maximum weight, K = 0.9, and the Scherrer constant (Monshi et al., 2012).Evaluation of extract composition relative to the synthesis of ZnO nanoparticles using LC-Q-TOF-MS
The crude extract from bush tea leaves and the supernatant after the synthesis of ZnO nanoparticles were investigated. The differences between the composition of the crude extract and the supernatant after synthesis are represented in Figure 1. The results from the PCA co-variance of data show that two distinct groups were observed from the three principal components with 85.1%, 8.6% and 2.1% respectively for principal components 1, 2 and 3. The compounds (represented in red) resulting from the supernatant after synthesis of ZnO nanoparticles clustered together following the Y-axis of the PCA while the compounds of crude extract were on the Z-axis. The differences observed are due to the reaction between the plant extract and the precursor to form ZnO nanoparticles. The synthesis of ZnO nanoparticles involves a reaction between the plant extract and the precursor resulting in the reduction of Zn+2 ions into ZnO nanoparticles (Hussain et al., 2019).

Figure 2 shows the different compound peaks observed using LC-Q-TOF-MS analysis. The dissection of observed spectra into compounds produced 100 different peaks for the crude extract (Figure 2a) and 84 peaks for the supernatant after synthesis (Figure 2b). The reduction in the number of compounds confirms that the synthesis took place and secondary metabolites from A. phylicoides DC. The extract has reacted with the precursor reducing Zn2+ ions into ZnO nanoparticles.

Bush tea extract compounds identification before and after synthesis
Different peaks identified, after chromatogram dissection (Figure 2), from LC-Q-TOF-MS revealed the presence of several compounds in both the crude extract and the supernatant after ZnO nanoparticle synthesis, with a reduction of the compound’s amount in the supernatant collected after synthesis. Thus, revealing the presence of an interaction between the precursor and the extract mainly by the oxidation, reduction or degradation of the phytochemical compounds that occur during nanoparticle formation (Jeevanandam et al., 2016). Table 1 presents the secondary metabolites investigated for both the crude bush tea extract and the supernatant solution after synthesis, respectively.
Table 2 present the compounds identified from the supernatant after the synthesis of ZnO nanoparticles. The secondary metabolites investigated present a reduced number compared to the ones from the crude extract, thus revealing that a reaction has taken place between bush tea natural extract metabolites and the precursor resulting in the formation of ZnO nanoparticles.
Assessment of the implication of bush tea compounds in ZnO nanoparticles synthesis
In this study, compound identification was carried out using Bruker data analysis and data profiling tools. The KEGG and ChemSpider databases were consulted to find the name and the chemical formula of each identified compound. The different compounds with mass to ratio (m/z) values as well as their retention time (in seconds) were shown with variable importance in the progression (VIP) score plot (Figure 3). The concentration of eight compounds was found to be high in the crude extract compared to the supernatant after the synthesis of ZnO nanoparticles where their concentrations were low.
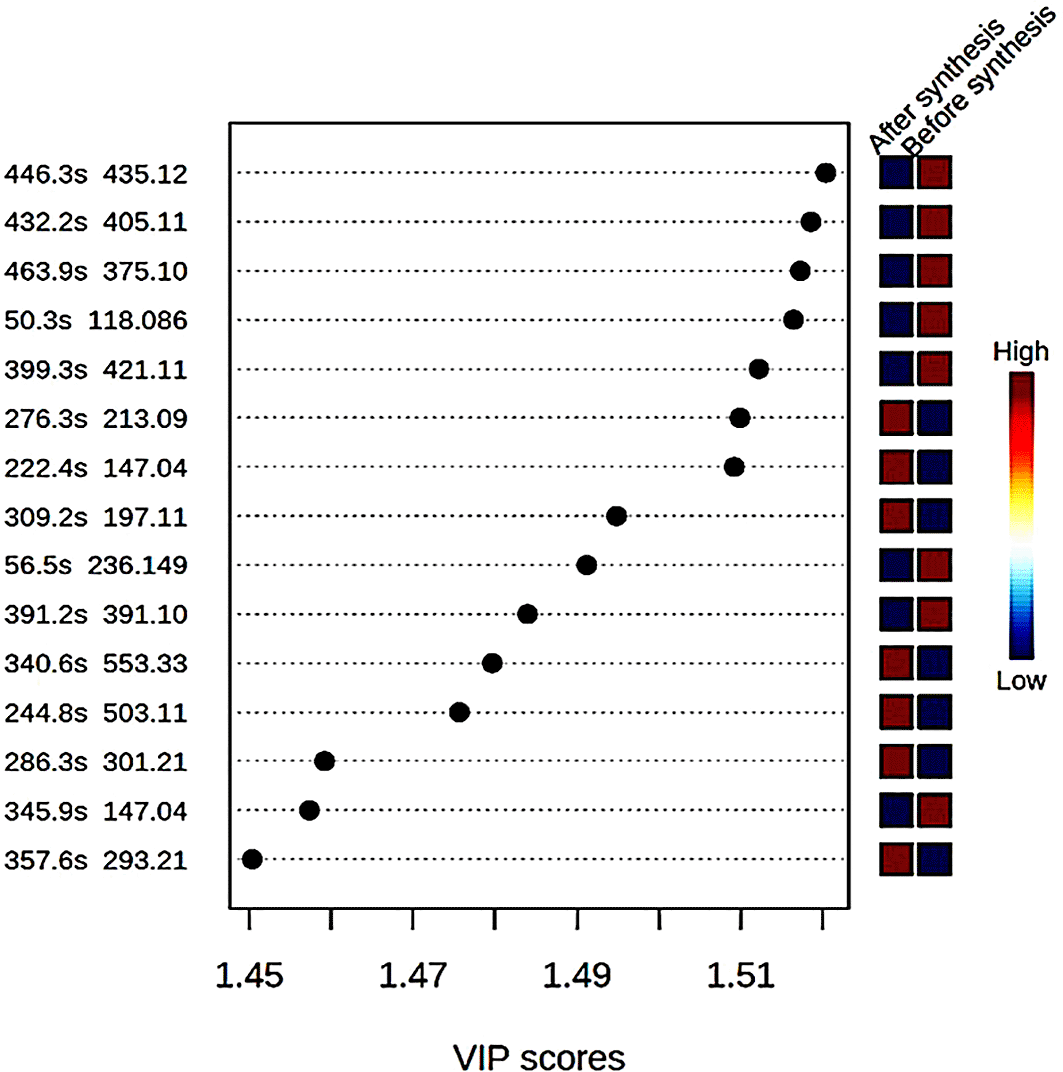
Table 3 present the various compounds that were involved in the synthesis process of ZnO nanoparticles including five flavonoids and two polyphenol compounds, as well as one aromatic compound, which highly reacted with the precursor to form ZnO nanoparticles. Studies have shown that the synthesis of nanoparticles using plant extracts involves terpenoids, flavonoids, alkaloids and phenolic acid, which act as reducing, capping, and stabilizing agents (Kuppusamy et al., 2016).
XRD analysis
The XRD analysis was done to confirm the crystallinity of the synthesized ZnO nanoparticles using a Bruker AXS (Germany) D8 advance X-ray diffractometer. Figure 4 presents the XRD pattern of the ZnO nanoparticles. The crystallinity of the powder resulting from the synthesis using A. phylicoides DC extract. The peaks (100), (002), (101), (102), (110), (103), (200), (112), (201), (004) and (202) are lattice planes. The diffraction peaks reveal that the synthesized ZnO nanoparticles are essentially crystalline, in accord with the ICDD #897102 in the wurtzite structure (Noman et al., 2020). The same results have been observed by the green synthesis of ZnO nanoparticles using Ocimum basilicum (Salam et al., 2014) and Agathosma betulina (Thema et al., 2015). The average crystallite size of obtained ZnO nanoparticles calculated using the modified Scherrer equation was approximately 24.53 nm.
Fourier-transform infrared spectroscopy
The PerkinElmer Frontier FTIR spectrometer was used to perform FTIR analyses using Potassium bromide (KBr) (Potassium bromide) optics. The presence of ZnO nanoparticles was confirmed by the peak at 479 cm−1 as shown in Figure 5. The other observed peaks are attributed to the phytochemical components present in the extract solution. The peak at 1113 cm−1 is attributed to the C-O stretching of primary alcohols. The peak at 1427 cm−1 corresponds to the O-H bending of the carboxylic acid. The peak observed at 2351 cm−1 is attributed to the O=C=O stretching of carbon dioxide. The FTIR spectra of bush tea extract, presented in Figure 6, show the presence of carboxylic acid bonding, primary alcohol stretching as well as the intramolecular hydrogen bond.
UV-Vis analysis
UV-Vis analyses were performed at a resolution of 1 nm at a 250–800 nm wavelength range using a PerkinElmer Lambda 650S UV-Vis spectrometer. The absorption of ZnO nanoparticles is observed in the wavelength range of 250–400 nm (Kolekar et al., 2011). The measured peak at 380 nm (as shown in Figure 7) reveals the presence of ZnO nanoparticles with a band gap energy of 3.11 eV, smaller than the bulk ZnO of 3.37 eV. Thus, the presence of hexagonal wurtzite structures in the analysed samples is indicated, in accordance with the XRD results.
SEM and EDS analyses
A JEOL JSM-7500F field-emission scanning electron microscope (FE-SEM) coupled with a JXA-8230/SXEDS/EDS/WDS energy-dispersive X-ray spectrometer (EDS) was used to get the morphology and the purity of the ZnO nanoparticles. SEM results are represented in Figure 8. The image shows quasi-spherical shaped ZnO nanoparticles agglomerated together. The EDS confirmed the presence of Zn and O. These findings are supported by Nethavhanani (2017) using natural extracts of Aspalathus linearis as a reducing agent (Nethavhanani, 2017).
Understanding the process of nanoparticle synthesis using the green route is key to the efficiency of the process and the outcome. Following the lack of data on chemical interactions of plant extracts with different metals to form nanoparticles, this study aimed to investigate the interaction of compounds with zinc nitrate to form ZnO nanoparticles. The identification of plant metabolites was performed using LC-MS tools employing different databases such as KEGG, ChemSpider or Metfrag (Cecilia et al., 2020). Henceforth, the differences in the extracts resulting from the synthesis of ZnO nanoparticles were shown utilizing PCA and the VIP score plot. Bush tea leaves contain a high percentage of flavonoids and tannins, apart from non-structural carbohydrates, proteins, fatty acids, and minerals, such as calcium, magnesium, phosphorus, potassium, sodium, iron, manganese, zinc, copper, aluminium, sulphur and fluoride (Lerotholi et al., 2017). Hence, the synthesis process resulted in the complete use of some metabolites as shown in Figure 2. The supernatant recorded low quantities of 8-C-Glucosylnaringenin/Naringenin 7-O-beta-D-glucoside, Scullcapflavone II, Mallotophenone, 6-Methoxyaromadendrin 3-O-acetate, 2-Phenylacetamide, 7-Hydroxy-2″,4″,5″-trimethoxyisoflavone, Coumarin, Malonyldaidzin (Figure 3). A variety of metabolites, such as terpenoids, polyphenols, sugars, alkaloids, phenolic acids, and proteins can reduce metal ions into nanoparticles (Marslin et al., 2018). Flavonoids, polyphenols as well as an aromatic compound interacted most with the precursor to form ZnO nanoparticles (Table 2). UV-Vis is a wonderful tool for the examination of the size and the shape of nanoparticles (Raut Rajesh et al., 2009). The analysed samples show the presence of a wurtzite structure at 380 nm. These findings are supported by (Krupa and Vimala, 2016) who reported the synthesis of ZnO nanoparticles absorbing light at 368 nm. The wavelength of 380 nm corresponds to the bulk band-edge of 3.2 eV for ZnO (Kolekar et al., 2011).
In this study, bush tea metabolites were screened to understand their interaction with metal ions to form nanoparticles. The LC-MMS peaks in both the crude extract before ZnO nanoparticles synthesis and the supernatant after synthesis revealed a significant difference, shown by the PCAs. Different flavonoids, polyphenols and an aromatic compound were found to react with zinc nitrate to form zinc nanoparticles. The FTIR as well as the XRD and UV-Vis analyses confirmed the formation of ZnO nanoparticles with a hexagonal wurtzite structure.
All data underlying the results are available as part of the article and no additional source data are required.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Green synthesis and materials characterization
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Green-NPs synthesis, Cancer research, antioxidant
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Green synthesis and materials characterization
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Nanoparticles; green synthesis, photocatalysis, dye degradation; metals oxide.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Taghavi Fardood S, Ramazani A, Moradi S, Azimzadeh Asiabi P: Green synthesis of zinc oxide nanoparticles using arabic gum and photocatalytic degradation of direct blue 129 dye under visible light. Journal of Materials Science: Materials in Electronics. 2017; 28 (18): 13596-13601 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Nanoparticles; green synthesis, photocatalysis, dye degradation; metals oxide.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Green synthesis of Nanomaterials, Solar cells, Photocatalyst
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 4 (revision) 23 Aug 22 |
read | read | ||
|
Version 3 (revision) 11 Apr 22 |
read | read | ||
|
Version 2 (revision) 14 Jan 22 |
read | |||
|
Version 1 25 Oct 21 |
read | |||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)