Keywords
Sleep apnea, therapy, airway pressure
Sleep apnea, therapy, airway pressure
AHI: apnea hypopnea index
BMI: body mass index
NAP: negative airway pressure
OSA: Obstructive Sleep Apnea
OMT: orofacial myofunctional therapy
PAP: positive airway pressure (could be continuous or auto-titrated)
1RM: one-repetition maximum
REM: rapid eye movement
Obstructive sleep apnea (OSA) is caused by upper airway collapse during sleep resulting in a disruption of regular periodic breathing. The phenotypes of OSA pathology are variable and a particular patient can have the disorder because of anatomical and/or non-anatomical reasons.1 Continuous or auto-titrated positive airway pressure (PAP) is the current standard treatment; however, other therapies have emerged as alternatives. Mandibular advancement devices (or oral appliances) are currently recommended as a second line therapy2; though the ability to properly track compliance (and thus efficacy) is a challenge. Surgical interventions have targeted anatomical or morphological problems in those unresponsive to standard therapies. However, the evidence supporting its widespread application is lacking.3
We have been studying the collapsibility of the upper airway during sedation-induced sleep by provoking its occurrence through the use of negative airway pressure (NAP).4,5 People who are awake can breathe against this NAP (it feels like a vacuum on the back of the throat) down to negative pressures that would readily collapse the airway during sleep or sedation. Awake and healthy OSA patients have been able to tolerate breathing with the application of up to -40 cm of water.6 The ability to tolerate these pressures is the result of increased airway muscle tone (both tonic and reflex phasic activation of the airway dilator muscles).7
Subjects in our laboratory have commonly described their breathing against this vacuum as a “workout” on their airway. This idea sparked our curiosity to test the ability of NAP application as a therapeutic intervention, exercising the oropharynx, increasing muscle tone and thus decreasing upper airway collapsibility during sleep. Over the last decade, orofacial myofunctional therapy (OMT) has been applied to treat this disorder.8 Both as a stand-alone therapy and in conjunction with PAP, OMT has been shown to reduce the apnea hypopnea index (AHI), reduce snoring, and improve quality of sleep and life.9 While other OMT protocols involve methods that require specific training,8,10,11 we considered that OSA sufferers already familiar with PAP would more easily adapt to our protocol which involves the use of a similar device. The key to our proposed therapy is the use of NAP when awake so that the increased reflex phasic drive to the muscles will result in muscle conditioning. Thus, we developed a regimen similar to OMT, with a primary hypothesis that AHI measured via attended polysomnography after treatment intervention would be %50 reduced compared to baseline AHI.
This study was approved by the University of Rochester Research Subject Review Board (RSRB#24149) and conducted between December 2008 and January 2012. At the time the study was approved, it was not standard practice or mandatory to publicly register trials. This trial’s information was first submitted in 2012, posted in 2014, and results posted in 2015 in ClinicalTrials.gov (NCT02109731) after the study was completed, but in line with our understanding of what was evolving as a research standard at that time.
All subjects provided written informed consent to participate in this non-randomized, non-blinded feasibility study. The estimated sample size for a one sample comparison of means of AHI utilizing the s.d. of the delta AHI between two measurements was 11 subjects to have a 90% power to detect a 50% difference or a reduction in the AHI of 20 at alpha = 0.05. Taking into account possible dropout of subjects over time, we planned to enroll 15 subjects in the study. For the primary analysis, differences between baseline and follow-up were analyzed using two sample t-tests.
Twenty subjects (>18 years of age) were recruited from local sleep medicine laboratories via flyers or mailing from their treating sleep physicians. Once consented, subjects were additionally screened for inclusion and exclusion criteria and 15 recruited for the study. All subjects had a diagnosis of OSA (apnea hypopnea index, AHI >10) but were not currently using their previously prescribed PAP. Each participant received a targeted history and physical exam to assess for stability of medical conditions, including having a normal electrocardiogram and a negative urine drug test. Measurements of body mass index (BMI), neck circumference, and an airway exam were collected, and the Epworth sleepiness scale survey was administered.
Exclusion criteria included: major upper airway morphologic abnormality (such as profound micrognathia), history of airway surgery, regular use of central nervous system depressants or alcohol (e.g., > 14 alcoholic drinks per week or >2 per day), morbid obesity (BMI > 40 kg/m2), or unstable medical or psychiatric illness. Participants were also excluded if they were undergoing or planning to undergo an intervention for weight loss, or were pregnant or lactating.
Participants were initially evaluated by the University of Rochester Strong Sleep Disorders Center with an overnight polysomnographic study incorporating 16 electrophysiologic signals: 2-channel electro-oculogram, 8-channel electroencephalogram (Fz, C3, CZ, C4, Pz, Oz, T3, and T4), bipolar mentalis electromyogram, 2 lead electrocardiogram, nasal/oral airflow thermocouple, two respiratory effort sensors, a pulse oximeter, and a channel representing A1/A2. The resulting data were analyzed in 30-second epochs by an independent and certified sleep scorer (LL; see Acknowledgements).
The NAP myofunctional therapy protocol was designed to minimize the development of muscle hypertrophy and optimize strength and/or endurance.12 In muscle conditioning therapy, one-repetition maximum (1RM) is the unit designated to describe a maximum weight or resistance that a muscle group can move. For this study, the 1RM was defined as the maximum negative pressure the participant could comfortably tolerate for one breath, not less than −25 cm H2O. As is conventional in exercise physiology, ideal strength training involves targeting each subject’s 1RM repetitiously for 1-5 breaths per set and repeating this set 4-7 times with rests of 1-2 minutes between sets.
Training visits
The initial visit to the University of Rochester outpatient clinical research center was used to familiarize subjects with the apparatus, which appears similar to a typical PAP machine. Subjects were acclimated to both the mask fit (over the mouth and nose) and the feeling of breathing against a negative pressure. Airflow was measured with a pneumotachograph (Hans Rudolph, Shawnee, KS) inserted into the mask, which was calibrated with a rotameter. Mask pressure was measured by connecting a port to a pressure transducer (Validyne, Northridge, CA) calibrated with a water manometer.
Subjects were instructed to breathe through the nose with a closed mouth while sitting in order to minimize any tendency for the upper airway to collapse. At the first visit, the participants were exposed to a variety of negative pressures in an increasing-decreasing manner (from −5 cm H2O for five breaths and down) and also in random sequence in order to determine each subject’s 1RM.
Experiment
Thereafter, the subjects would schedule three observed sessions per week for three months in our clinical research center. As described above, each session entailed the subject breathing NAP at the predefined 1RM for five breaths per set (reps) and repeating this set 4-7 times with rests of 1-2 minutes between sets. Constant observation by study personnel allowed for verification of wakefulness (eyes open) and breathing with the mouth closed during the entire procedure. Each session lasted approximately 30 minutes. After the first and second month, the 1RM was re-evaluated and adjusted, as appropriate. For the following month, the new 1RM of NAP was used for the training exercises. Each subject’s BMI and neck circumference were re-measured after the first and second months.
Participants completed a post-intervention overnight sleep study within a week of finishing the prescribed course of airway physical therapy. Data from this study were collected and analyzed in the same manner as the initial overnight sleep study.
Participants were also instructed to maintain nightly sleep diaries. This included recording and rating aspects of sleep quality and sleep-related quality of life on a 1-5 scale (1 = never; 5 = frequently) upon waking, and recording bedtimes, time out of bed, time required to fall asleep, number of awakenings during the night, time awake after falling asleep, time awake before alarm or intended wake time, and time out of bed at night.
Since this was a pilot study without a control group, the primary outcome was a difference in AHI (as measured by overnight attended polysomnography) between the initial sleep study and the final study in each subject. Specifically, the study was sized to detect a 50% reduction in AHI or an AHI reduction of more than 10. Other secondary outcomes include nadir saturation, non-REM AHI, and protocol compliance. A pre-planned subgroup analysis of the subjects with a mild to moderate AHI (AHI < 30) was performed.
For the primary analysis, the differences between baseline and post-intervention data were analyzed using paired Student’s t-tests. Symptoms scores were analyzed nonparametrically.
Data are reported as mean ± standard deviation and 95% confidence intervals. p < 0.05 was considered significant. All statistical analyses were performed using STATA/IC 13.1, (Stata Corp LP, College Station, TX).
Fifteen subjects completed the study and Table 1 provides their demographics, which demonstrates no significant changes in BMI, weight or neck circumference after the training session (see Figure 1 for CONSORT flow chart). The initial airway training pressures ranged from -8 to -18 cm H2O and all airway training pressures became more negative except for one subject who stayed at -18 cm H2O (Figure 2), which was the most extreme pressure utilized for the study.
BMI = body mass index. Data is mean ± standard deviation.
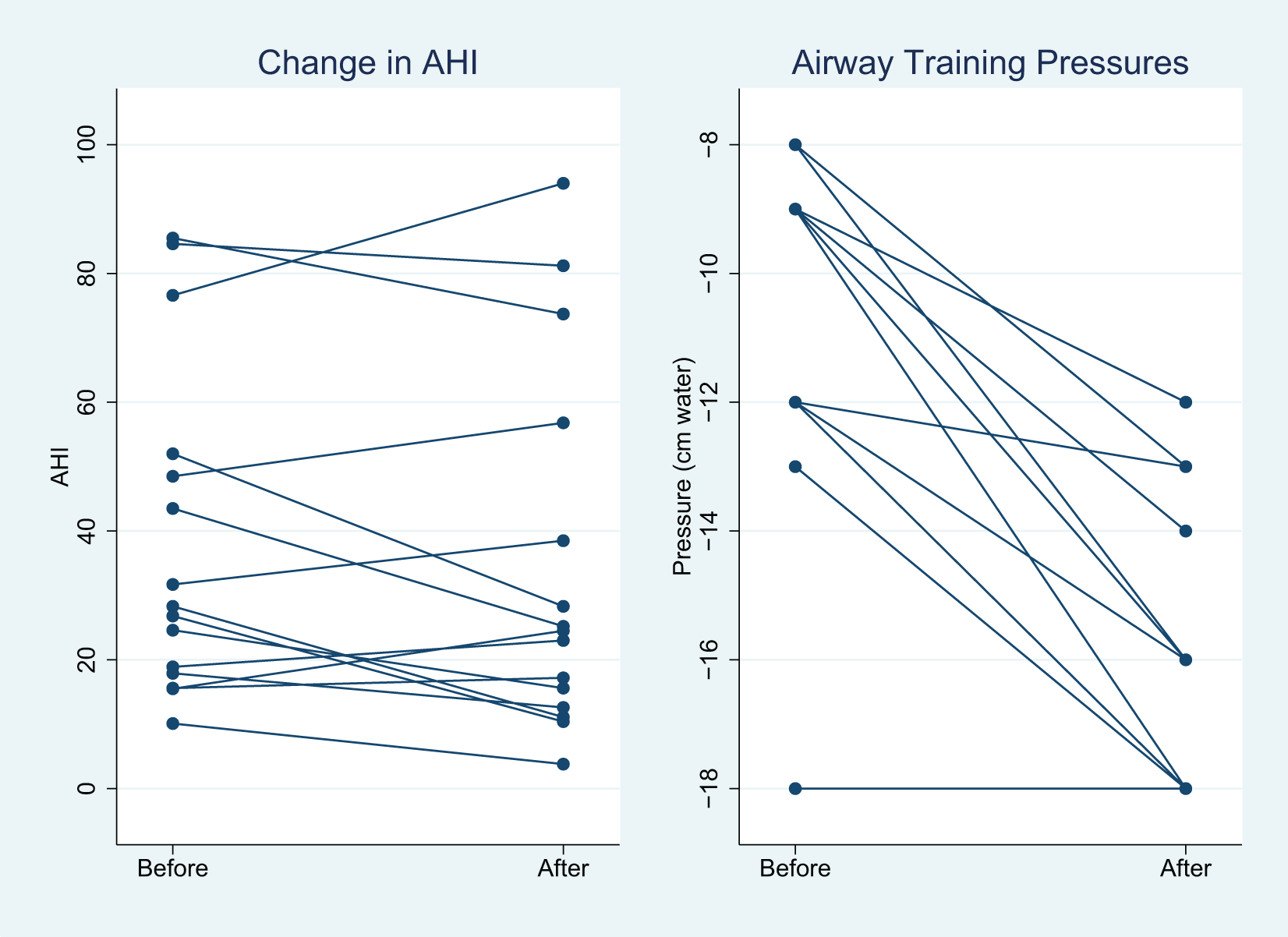
The left panel shows the change in the AHI from before and after the training for all subjects. The right panel shows the change in the initial to the final training pressure.
While the mean post study AHI was slightly improved (−4.3 ± 12.0 [−10.9, 2.3]), it was not statistically significant (Table 2). Six subjects actually increased their AHI, only one subject decreased to below 10 and this was the subject with the lowest pre-study AHI of 10.1 (Figure 2). The results were similar when the AHI was categorized by the sleep state (REM vs. non-REM). The nadir saturation was also essentially unchanged. The eight subjects with mild or moderate OSA (AHI < 30) showed similar results.
AHI – apnea hypopnea index. The AHI was calculated during rapid eye movement (REM) and non-REM sleep. Data given as mean ± stand deviation, except for difference which is mean and 95% confidence interval.
Of the 15 subjects, only 10 completed their sleep diaries. There were no differences noted in sleep quality or feeling refreshed from the intervention.
This study demonstrates the novel use of a modified standard PAP machine to provide NAP as a form of quantifiable myofunctional therapy for OSAS. In this small cohort, the intervention did not cause significant changes in AHI. The small size of this pilot study makes it difficult to definitively exclude a clinically helpful effect, but we can exclude a clinically significant increase in the AHI, thus indicating its safety. However, we believe that sharing the methodology and results may provide clues for further study.
Though PAP therapy remains the gold standard approach for effective treatment of OSA,13 compliance remains a challenge.14 OMT has emerged as a promising alternative or adjunct to standard OSA therapy.8 In a recent review of the 11 studies published to date, de Felicio et al. provide an analysis of comparative methodology and ideas for further study.15 In most OMT studies, the intervention exercises target a variety of oral and facial muscles including the tongue, palate, pharynx, mouth, and cheeks. In our study, the NAP therapy was hypothetically the reverse of PAP and may have exercised similar structures. However, we do not know whether NAP targets specific muscles whose tone (or lack thereof) contributes to OSA. With increasing attention being paid to OSA phenotypes, it has been suggested that most would benefit from anatomic intervention.1 In addition, standardized classification of location, directionality and severity of pharyngeal collapse as determined during drug-induced sedation endoscopy16 could be explored as a method to target appropriate candidates who might benefit from myofunctional therapy, as it has been used to evaluate success with other OSA interventions such as surgery and electrical stimulation.
We considered that prior familiarity with the PAP machine but failure to adhere to nighttime usage would be a reasonable approach for recruitment of subjects for our study, which included daytime and more limited use of the machine. In hindsight, non-use of PAP may have actually been an inappropriate criterion for inclusion as this may have predicted a phenotype á priori which would not benefit from our approach of myofunctional therapy.1 Most PAP machines can now be interrogated to ensure patient compliance. Future investigations may benefit from recruiting PAP users and assessing devices for sleep-related pressure changes over the course of an exercise intervention during wakefulness.
As was done in our study, OMT is typically utilized during wakefulness as a method to decrease muscle hypotonia during sleep. Theoretically, the frequent repetitions at low levels of resistance may target Type 1 (slow-resistant to fatigue) muscles, such as those found in the tongue.17 These muscles and Type 2A (fast-intermediate fatigue) muscles have been stimulated electrically.18,19 and trained11,20 In patients with OSA there are even more type 2A fibers than in normal controls, which could contribute to the increase fatigability.21
Our protocol lasted for three months, a time period that is frequently used in muscle training.15 Unlike other protocols, we chose to have subjects observed in a laboratory and reasoned that visiting three times per week would not be too onerous. Many of the training protocols describe daily exercises, sometimes three8,22 to five times23 per day. It is possible that our training regimen was not sufficient to see an effect.
We asked our subjects to maintain a mouth closed position while performing exercises, mostly as a means to standardize their positions. It has also been shown that mouth breathing exacerbates upper airway obstruction with advocates for mouth closure training to promote airway patency maintenance during sleep.24,25
Despite the limitations of our study, there were no reported adverse events. In contrast, participants shared that they appreciated doing this therapy, and could envision doing it at home were it to become available. We have been contacted by former subjects and a few interested citizens who have located this study on ClinicalTrials.gov to inquire about inclusion in ongoing investigations.
In conclusion, subjects with OSAS were able to tolerate a three-month exercise protocol using the novel application of NAP as orofacial myofunctional therapy. Though was no significant reduction in AHI in this small cohort, the methods elucidate a new daytime use for a machine with which many OSAS sufferers are familiar. Future studies utilizing this protocol may target current PAP users or those whose drug-induced sedation endoscopies indicate this approach as an alternative therapy.
Figshare: AirwayPT Data Original 100121.csv, https://doi.org/10.6084/m9.figshare.16727488.v1.26
Figshare: pt_sleep_diaries_042412.xls, https://doi.org/10.6084/m9.figshare.16798138.v1.27
Figshare: CONSORT checklist for ‘Myofunctional training with negative airway pressure for obstructive sleep apnea: a prospective non-randomized cohort feasibility study’, https://doi.org/10.6084/m9.figshare.16818352.v2.28
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors thank Dr. Lynn Liu for help in reading the sleep studies, Dr. Michael Perlis and Michael Leonard for input into the study design, and Dr. Elia Rackovsky for assistance in equipment setup for the experiment.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Respiratory sleep medicine
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
References
1. O'Connor-Reina C, Ignacio Garcia J, Rodriguez Ruiz E, Morillo Dominguez M, et al.: Myofunctional Therapy App for Severe Apnea–Hypopnea Sleep Obstructive Syndrome: Pilot Randomized Controlled Trial. JMIR mHealth and uHealth. 2020; 8 (11). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: I am expertise in sleep medicine and myofunctional therapy
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 27 Oct 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)