Keywords
Aquaculture, fatty acids profile, coconut water, palm sap sugar, giant gourami, growth performance, feed efficiency
This article is included in the Agriculture, Food and Nutrition gateway.
Aquaculture, fatty acids profile, coconut water, palm sap sugar, giant gourami, growth performance, feed efficiency
In Indonesia, giant gourami (Osphronemus goramy Lacepede, 1801) is one of the most important freshwater fish species because it has a high market price1–4. However, the giant gurami contributes less to the total production of freshwater aquaculture than tilapia, African catfish, and Pangasius catfish5. For this reason, the Ministry of Maritime Affairs and Fisheries of the Republic of Indonesia drives fish farmers to increase the annual production of giant gurami from various local strains, namely Tambago, Palapa, Bastar, Galunggung, Blusafir, and Sago4,6–8.
Efforts have been made to increase the production of giant gurami globally using aquaculture activities such as different feeding rates in floating cages9, differences of stocking density in concrete freshwater ponds4, and increased variety in the aquaculture systems, such as in an earthen freshwater pond, concrete freshwater pond and floating cages7,8,10. These aquaculture operations use commercial pellet fish feed, but the feed conversion ratio is higher, ranging between 1.43 and 1.65 . Conversely, the feed efficiency ratio is still low. In this context, between 30 to 40% of fish feed is released as a waste load to water bodies4,8,11. This is a particular problem, as the cost of fish feed is a significant challenge for aquaculture operations12–18.
Different strategies have been developed to improve aquafeed nutrition, including enriching feed with fish oil9,19, soybean oil20, iodine and selenium21, EPA and DHA22, and the use of probiotics23. The goal is to make the diet rich in nutrients such as amino acids, fatty acids, minerals, and vitamins because the animal cannot synthesize them in sufficient quantities to meet their needs17,24,25.
Currently, it is essential to evaluate the supplementation of feed with natural ingredients that are cost-effective and sustainable from plant resources, without compromising the growth of cultured fish and aquafeed quality. Among these ingredients are coconut water and palm sap sugar; both contain health-friendly nutrients such as minerals, amino acids, enzymes, organic acids, fatty acids, vitamins, and phenolic compounds26–28. Water coconut has been used successfully to treat diseases in humans, such as throat infections, tapeworms, gonorrhea, digestive problems, influenza, lice, giardia, bronchitis, and cholera29–31. Palm sap sugar is also claimed to have health benefits due to its low glycemic index and possesses antioxidants, vitamins, and minerals32–34. On the other hand, some researchers have reported that fungi also improves aquafeed's nutritional value15,35,36. Based on previous research, supplementation of commercial feed with product formulas consisting of coconut water, palm sugar, and fermented with various fungi (Aspergillus niger, Rhizopus oligosporus, and Saccharomyces cerevisiae) is very important to evaluate. In addition, fish has a dominant function on consumer health because it is rich in amino acids, fatty acids, vitamins, and minerals19,21,25. Therefore, we also analyzed gurami sago's health lipid indices by analyzing several carcass fatty acids types.
We hypothesized that commercial feed supplemented with various formulated products could improve the nutritional quality of the feed and carcass, feed efficiency, and juvenile gurami sago growth rate. The current study evaluated the fatty acid composition and proximate composition of commercial pellet fish feed supplemented with various formulation products and their effect on growth performance, feed use efficiency, and fatty acid composition in the carcass of gurami sago juvenile.
The study conducted by Hafrijal Syandri and his colleagues under the project entitled Optimization of New Formula Products Based on Local Materials To Strengthen Food Independence In The Aquaculture Sector In The New Normal Era of Coronavirus disease (Covid-19). The Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia funded this research under grant number:170/E4.1/AK.04.PT/2021. The Ethics Committee for Research and Community Service at Bung Hatta University has approved this research (110/LPPM/Hatta/III-2021 which followed the ARRIVE guidelines. Approval was given to collect and rear juvenile gurami sago in the Aquaculture Laboratory, Faculty Fisheries, and Marine Science Universitas Bung Hatta. All efforts have been made to relieve the suffering of experimental animals. Therefore, gurami sago didn't suffer for this study, and they were still in good condition when they returned to the pond after research was completed. Where some fish were euthanized, this was carried out by piercing part of the fish's brain. Gurami sago fish were not classified as a protected animal according to Indonesian legislation.
A total of 300 g of palm sap sugar (Arenga pinnate M.), purchased from local palm sugar farmers, was cooked in 3,000 ml freshwater at 60 °C for 15 minutes, and then cooled for 20 minutes in an open space. Next, we mixed 6,000 ml of mature coconut water (Cocos nucifera L.) with 3,000 ml of the palm sap sugar solution. The total product formulated is 9,000 ml and was divided into three containers of 3,000 ml. The first part added 6 g of Aspergillus niger (called P1), the second part added 6 g of Rhizopus oligosporus (called P2), and the third part added 6 g of Saccharomyces cerevisiae (called P3). Each portion (3,000 ml) was fermented for 48 hours in a plastic jerry can with a capacity of 5 liters. The aeration process was carried out continuously using Aerasi Fujimac MAC-40K-40L/min made in Japan.
Floating commercial feed (781-2 PT. Japfa Comfeed Indonesia Ltd) was 2 mm with a proximate composition (dry weight %) of 10.66% moisture content, 30.10% crude protein, 4.09% crude fat, and 45.35% carbohydrate total with 2.5% ash, and 9.18% crude fiber. The feed is supplemented with the products P1, P2, P3, up to 300 ml/1 kg of feed. Commercial feed with only added freshwater (P4) was used as a control. These are referred to as P1, P2, P3 and P4 feeds. The products P1, P2, P3, and P4 were each sprayed evenly to 1 kg of the commercial feed, and then dried in the open air for 30 minutes. After that, we gave feed to the experimental fish.
A total of 360 juvenile gurami sago of local strains were obtained from the Aquaculture Laboratory Faculty of Fisheries and Marine Science at Universitas Bung Hatta Padang of Indonesia. Exclusion criteria were if fish were found to be in poor health or would not eat the commercial food initially provided. Gurami sago juveniles were acclimatized for 30 days before the experiment, which commenced during January 2021. Juvenile fish were placed in the concrete freshwater pond 24-m3 (6×4×1 m) with a capacity of 5,600 L. During the acclimation, juveniles were fed commercial feed (781-2 PT. Japfa Comfeed Indonesia Ltd) with 30.10% crude protein content, 4.09% crude fat, 2.5% crude ash, and 9.18% crude fiber). Feeding was done three times daily (09.00 AM, 1.00 PM and 5.00 PM), and fish were fed the equivalent of 3% of their body weight per day.
The 360 juvenile gurami sago weighed on average 50±2.5 g and had an average length of 13.2±0.4 cm, and had not previously been used in earlier research. The juveniles' weight was measured using AD-600i scales with 0.001 g accuracy (ACIS model number AD-600i , China) and its use was approved by the Indonesian Directorate of Metrology, and body length was measured using a meter ruler with 1 mm accuracy. The fish were distributed in 12 nets framed with size 2-m3 (2×1×1 m) PVC pipe (1200 L capacity) placed inside two freshwater concrete ponds of size 18-m3 (6×2×1.5 m). The experimental groups were P1, P2, P3, and one control group (P4) with three replications each (12 total). Each net contained 30 fish. Fish were randomized to groups using a lottery method by Hafrijal Syandri and Azrita undefined. The water temperature varied between 27 °C and 30 °C (mean 28.5 °C). The dissolved oxygen (DO) level ranged from 5.8 to 6.2 mg L-1 (mean 5.72). The pH varied between 6.5 and 6.8 (mean 6.67). Temperature, DO level, and pH measurements were recorded weekly throughout the experiment. The water samples were collected at 10.00 AM at depth of 20 cm from each concrete pond for the determination of the water temperature, dissolved oxygen, and pH. Water temperature was measured using a thermometer (Celcius scale). An oxygen meter (YSI model 52, Yellow Spring Instrument Co, Yellow Spring, OH, USA) was used in-situ, pH values were determined with a pH meter (Digital Mini- pH meter, 0-14pH, IQ Scientific, Cemo- Science Thailand).
Fish were given a feed of 2 mm floating type pellets supplemented with product formulations P1, P2, P3, and control (P4) three times a day at 9.00 AM, 1.00 PM, and 6.00 PM. Fish were hand-fed at a 3% body weight rate per day until study termination, which was after rearing them for 90 days from February to April 2021. Fish samples were collected every 30 days to evaluate length and weight, and fish were fasted for 24 hours before sampling to empty the intestinal contents. Nine fish per net were randomly sampled every 30 days and euthanized with tricaine methanesulfonate (MS 222, Sigma Aldrich Co, USA MO, 50 mg L-1), then length and weight were measured. The amount of MS 222 used is 2,000 mg at each measurement of the sample (0, 30, 60, and 90 days).
This study used standard methods from the Association of Authorized Analytical Chemists37 to analyze the proximate composition of experimental diets and fish carcasses. One fish (mean weight 100 g) from each experiment replicates (P1, P2, P3), and control (P4) were collected from the rearing nets. We then euthanized the 12 fish by injecting their brains to examine the carcasses. Samples of the diet and wet fish were dried at 135 °C for two hours. The crude protein was analyzed using an automatically processed Kjeldahl (Buchi 430/323) using a Kjeltec methods (6.25), automatic Kjeldahl system (Buchi/430/323) model 1625, Moline IL USA. The fat content was analyzed using a Soxhlet Apparatus with the Soxhlet system 1046 (Foss, Hoganas Sweden). The ash content was analyzed using a muffle furnace (600 °C for four hours). We have calculated the total carbohydrates by subtracting the sum of % crude protein, % crude fat, % crude ash, and % moisture contents from 100%37. The proximate compositions of diets and carcasses were calculated by P.T. Saraswanti Indo Genetech Bogor Indonesia (SIG Laboratory, Accredited Testing Laboratory-LP-184-IDN).
Diets and carcasses were analyzed using a fatty acid composition by the gas chromatography-mass spectrometry (GC-MS) method. The total lipid extraction was carried out according to modified Folch et al. (1957) as described by Rajion, 198538 using a chloroform: methanol (2.1. v/v) solvent system. Transmethylation was performed using 14% methanolic boron trifluoride. Diets and carcasses were analyzed for fatty acids composition by PT. Saraswanti Indo Genetech Bogor Indonesia (SIG Laboratory, Accredited Testing Laboratory-LP-184-IDN).
Nine fish sampled from each net were weighed and accounted for separately during the final sampling. Weight gain (WG, %), specific growth rate (SGR, %/day), feed conversion ratio (FCR), and feed conversion efficiency (FCE) were analyzed based on formulas9,10,39:
The nutritional quality of lipids AI and TI was calculated based on the equations40.
Where
AI = Atherogenic index
TI = Trombogenic index
C12:0 = lauric acid
C14:0 = myristic acid
C16:0 = palmitic acid
C18:0 = stearic acid
ΣMUFA = sum concentrations of all monosaturated fatty acid
Σn-6 = sum concentrations of n-6 polyansaturated fatty acid
Σn-3 = sum concentrations of n-3 polyunsaturated fatty acid
SPSS 16.0 software package (SPSS; Chicago IL) was used for data analysis. Levine's test was used for determining homogeneity of data. One-way ANOVA was used for the determined treatment effect, followed by a post hoc Duncan's multiple range test41. Data are reported as mean value ± standard deviation for each treatment42. Microsoft Office Professional Plus 2019 was used for plotting the figures.
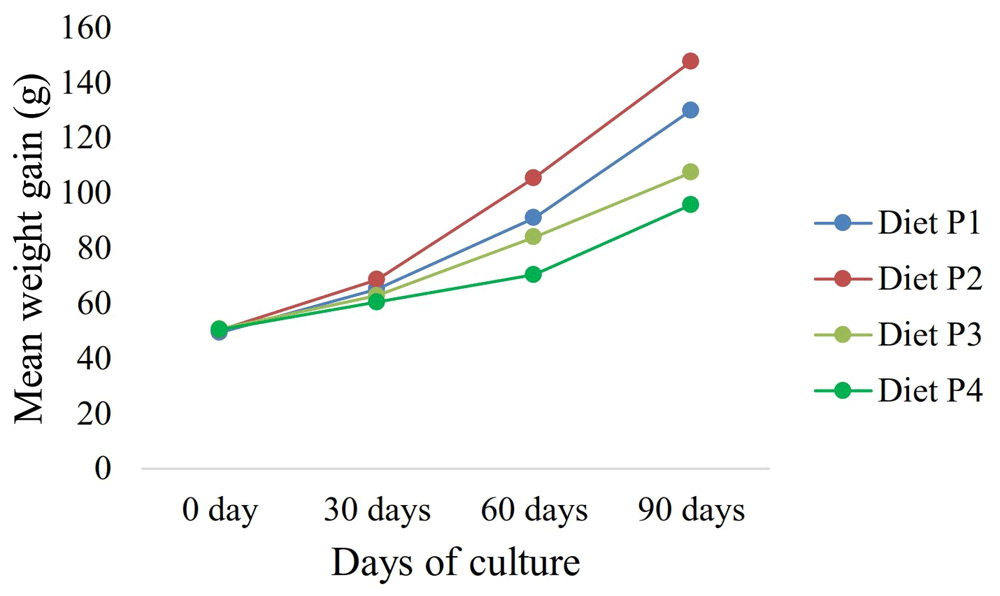
No samples were excluded from analysis. Figure 1 shows the growth performance of gurami sago at 30, 60, and 90 days for all groups during the experimental phase. No death was observed with any diets, so survival remained at 100% for all treatments during the 90 days of experimentation. Juvenile gurami sago fed feed P2 presented significantly higher growth performance with increased weight gain percent, lower feed conversion ratio (FCR), and improved feed conversion efficiency (FCE), compared to animals fed feed P1, P3, and P4 (Table 1). On the other hand, the P2 diet also led to a significant increase in fat and carbohydrates but not a significance difference in protein (Table 2).
| Diet P1 | Diet P2 | Diet P3 | Diet P4 | P-value | |
|---|---|---|---|---|---|
| Biometric measurements | |||||
| Initial body weight (g) | 49.40±3.80 | 50.26±0.47 | 50.69±2.48 | 50.80±0.68 | |
| Final body weight (g) | 132±2.08a | 147.74±1.02b | 118.74±1.22c | 109.73±1.89d | 0.435 |
| Weight gain (%) | 167.24±13.97a | 193.99±4.46b | 134.22±12.59c | 115.98±2.88d | 0.011 |
| Specific growth rate (%/day) | 1.09±0.10a | 1.15±0.01b | 0.95±0.06c | 0.98±0.05d | 0.033 |
| Feed conversion ratio | 1.50±0.08a | 1.36±0.04b | 1.53±0.03c | 1.55±0.02d | 0.069 |
| Feed conversion efficiency | 0.65±0.01a | 0.73±0.02b | 0.65±0.01a | 0.64±0.01a | 0.394 |
| Diet P1 | Diet P2 | Diet P3 | Diet P4 | P-value | |
|---|---|---|---|---|---|
| Proximate composition (g/100 g) | |||||
| Moisture | 62.49±0.36a | 62.77±0.26b | 64.23±0.12c | 65.55±0.39d | 0.265 |
| Protein | 30.29±0.45a | 28.98±0.37a | 29.30±0.16a | 28.69±0.28a | 0.363 |
| Lipid | 2.88±0.02a | 4.67±0.04b | 2.99±0.03c | 2.79±0.03d | 0.505 |
| Carbohydrates total | 1.98±0.07a | 2.04±0.07b | 1.30±0.08c | 1.29±0.06c | 0.834 |
| Crude fibre | 0.68±0.01a | 0.82±0.01b | 0.95±0.04c | 0.37±0.01d | 0.096 |
| Ash | 2.16±0.02a | 1.54±0.01b | 2.11±0.04c | 1.63±0.02d | 0.223 |
The fat content differed significantly among the four diets. Diet P2 showed substantially higher protein and fat contents than diets P1, P3, and P4. Carbohydrate content was similar in diets P1, P2, and P3 but significantly higher than diet P4. The energy content differed considerably among the four diets (Table 3).
| Proximate composition (g/100 g) | Diet P1 | Diet P2 | Diet P3 | Diet P4 | Initial composition | P-value |
|---|---|---|---|---|---|---|
| Moisture | 36.95±0.79a | 37.28±0.01b | 35.60±0.16c | 38.46±0.18d | 10.66 | 0.005 |
| Protein | 20.44±0.10a | 21.27±0.12a | 20.28±0.03a | 19.36±0.41d | 30.10 | 0.029 |
| Crude lipids | 3.48±0.04a | 3.65±0.11b | 3.49±0.04c | 3.08±0.05d | 4.09 | 0.067 |
| Carbohydrates | 29.60±0.74a | 28.78±0.30ab | 30.36±0.14ac | 26.21±0.36d | 45.35 | 0.101 |
| Crude fibre | 6.74±0.06a | 6.67±0.06b | 6.38±0.22c | 9.19±0.01d | 9.18 | 0.105 |
| Ash | 2.80±0.07a | 2.36±0.01b | 2.45±0.06c | 2.75±0.30d | 2.50 | 0.007 |
| Energy (kcal/100g) | 234.4±0.29a | 237.49±0.66b | 240.55±0.81c | 230.05±0.26d | 340.99±0.40 |
The four diets demonstrate a high level of saturated fatty acids (SFAs), with palmitic acid (C16:0) and stearic acid (C18:0) as the abounding. The concentration of monosaturated fatty acids (MUFAs) was similar in diets P1, P2, and P3 and very low in diet P4, with the highest values recorded for oleic acid (C18:1 n-9) in all diets. We did not detect myristoleic acid (C14-1 n-9) or heptadecenoic acid (C17:1 n-8) in the P4 died and initial feed compositions. Regarding polyunsaturated fatty acids (PUFAs), diet P2 had the highest value, with linolenic acid (C18:3 n-3) dominant. Additionally, we recorded EPA (C20:5 n-3) and DHA (C22:6 n-3) in diets P1, P2, and P3, whereas, in diet P4, they were not detected (Table 4).
| Type FA | Diet P1 | Diet P2 | Diet P3 | Diet P4 | Initial composition | P-value |
|---|---|---|---|---|---|---|
| C12:0 (Lauric acid) | 0.79±0.01a | 0.65±0.01b | 0.57±0.01c | 0.35±0.01d | 0.34 | 1.000 |
| C13:0 (Tridecanoic acid) | n.d | n.d | n.d | n.d | n.d | - |
| C14:0 (Meristic acid) | 0.75±0.01a | 0.74±0.0b | 0.75±0.01ac | 0.51±0.01d | 0.13 | 0.446 |
| C15:0 (pentadecanoic acid) | 0.95±0.01a | 1.01±0.0b | 1.03±0.01c | n.d | n.d | 0.094 |
| C16:0 (Palmitic acid) | 27.4±0.15a | 27.55±0.0b | 23.45±0.01c | 19.07±d | 21.05 | 0.014 |
| C17:0 (Heptadecanoic acid) | 0.22±0.01a | 0.24±0.0b | 0.17±0.0c | 0.12±0.0d | 0.45 | 0.931 |
| C18:0 (Stearic acid) | 5.25±0.01a | 5.27±0.0b | 5.24±0.01c | 4.24±0.01d | 5.31 | 0.532 |
| C20:0 (Arachidic acid) | 0.14±0.0a | 0.53±0.01b | 0.28±0.0c | n.d | n.d | 0.067 |
| C24:0 (Lignoceric acid) | n.d | n.d | n.d | n.d | n.d | |
| ∑ SFAs | 35.5±0.17a | 35.99±0.02b | 31.49±0.03c | 24.59±0.04d | 27.28 | 0.117 |
| C14-1 n-9 (Myristoleic acid) | 0.48±0.01a | 0.46±0.01b | 0.38±0.01c | n.d | n.d | 0.167 |
| C16:1 n-7 (Palmitoleic acid) | 2.08±0.01a | 2.89±0.01b | 2.83±0.01c | 1.97±0.01d | 1.99 | 0.940 |
| C17:1 n-8 (heptadecenoic acid) | 0.12±0.01a | 0.13±0.01b | 0.11±0.01c | n.d | n.d | 0.167 |
| C18:1 n-9 (Oleic acid) | 26.88±0.01a | 27.68±0.01b | 23.35±0.02c | 19.76±0.01d | 22.98 | 1.000 |
| C20:1 n=7 (Eicocyanic acid) | 0.15±0.01a | 0.14±0.01b | 0.15±0.00ac | 0.06±0.01d | 0.06 | 0.441 |
| ∑ MUFAs | 29.71±0.04a | 31.33±0.04b | 26.82±0.03c | 21.79±0.02d | 25.03 | 0.849 |
| C18:2 n-6 (Linoleic acid) | 27.81±0.01a | 28.86±0.01b | 27.21±0.01c | 22.66±0.01d | 25.21 | 0.702 |
| C18:3 n-3 (Linolenic acid) | 1.07±0.01a | 1.08±0.01b | 1.07±0.01c | n.d | n.d | 0.167 |
| C20:2 n-6 (Eicosadienoic acid) | n.d | n.d | n.d | n.d | n.d | |
| C20:4 n-6 (Arachidonic acid) | 0.21±0.01a | 0.20±0.01b | 0.21±0.01ac | 0.07±0.01d | 0.09 | 0.702 |
| C20:5 n -3 (EPA) | 0.56±0.01a | 0.59±0.34b | 0.62±0.01c | n.d | n.d | 0.001 |
| C22:6 n-3 (DHA) | 2.09±0.01a | 2.52±0.01b | 2.10±0.01c | n.d | n.d | 0.330 |
| ∑ PUFAs | 31.74±0.02a | 32.66±0.03b | 31.21±0.05c | 22.78±0.02d | 25.3±0.02 | 0.002 |
| ∑n.dFA | 2.80±0.01 | 0.02±0.01 | 10.48±0.03 | 30.84±0.03 | 22.39±0.01 | |
| ∑FA (%) | 100 | 100 | 100 | 100 | 100 | |
| Σ n-3 | 3.74±0.03a | 4.19±0.02b | 3.82±0.02c | n.d | n.d | 0.066 |
| Σ n-6 | 28.02±0.02a | 29.06±0.01b | 27.42±0.02c | 27.42±0.02d | 22.72±0.01 | 0.382 |
| Σn-6/ Σn-3 | 7.53±0.06a | 6.93±0.03b | 7.17±0.04c | 0.0 | 0.0 | 0.036 |
| PUFA/SFA | 0.85±0.03a | 0.85±0.01b | 1.04±0.08c | 0.92±0.02c | 0.96±0.006 | 0.004 |
| DHA/EPA | 3.66±0.05a | 4.21±0.10b | 3.34±0.02c | 0.0 | 0.0 | 0.030 |
| Lipid content (%) | 3.47±0.01a | 3.68±0.02b | 3.49±0.00c | 3.09±0.00d | 3.12±0.03 | 0.494 |
Gurami fish fed diets P1, P2, P3, and P4 showed high levels of saturated fatty acids (SFA) in their body carcasses, with palmitic acid (C16:0) and stearic acid (C18:0) being the most abundant. The oleic acid (C18:1 n-9) in all body carcasses of gurami sago fish contained higher values. For gurami sago fed P1 diet, their carcasses also had the highest palmitoleic acid content (C16:1) compared to other feeds. Polyunsaturated fatty acids (PUFA) had the highest value in animal carcasses fed the P2 diet. For the four carcasses, linolenic acid (C18:2n-6) was dominant. We noted that EPA and AA levels were lacking in all four carcass bodies, whereas DHA was high (Table 5).
| Type FA | Diet P1 | Diet P2 | Diet P3 | Diet P4 | P value |
|---|---|---|---|---|---|
| C12:0 (Lauric acid) | 0.41±0.01a | 0.56±0.01b | 0.60±0.01c | 0.40±0.01d | 0.757 |
| C13:0 (Tridecanoic acid) | n.d | n.d | n.d | n.d | |
| C14:0 (Meristic acid) | 0.85±0.02a | 0.81±0.01b | 0.51±0.02c | 0.49±0.02d | 0.802 |
| C15:0 (pentadecanoic acid) | 0.57±0.01a | 0.88±0.02b | 0.65±0.02c | 0.60±0.02d | 0.802 |
| C16:0 (Palmitic acid) | 22.83±0.02a | 24.03±0.01b | 23.04±0.02c | 20.70±0.02d | 0.482 |
| C17:0 (Heptadecanoic acid) | 0.11±0.01a | 0.14±0.02b | 0.12±0.02c | 0.11±0.02d | 0.786 |
| C18:0 (Stearic acid) | 5.24±0.01a | 5.23±0.01b | 5.26±0.01c | 5.22±0.02d | 0.702 |
| C20:0 (Arachidic acid) | 0.59±0.01a | 0.76±0.01b | 0.48±0.02c | 0.56±0.02d | 0.702 |
| C24:0 (Lignoceric acid) | 0.07±0.01a | 0.09±0.01b | 0.05±0.02c | 0.03±0.01d | 0.757 |
| ∑ SFA | 30.70±0.30a | 32.51±0.01b | 30.72±0.03ac | 30.51±1.64d | 0.612 |
| C14-1 n-9 (Myristoleic acid) | n.d | n.d | n.d | n.d | |
| C16:1 n-7 (Palmitoleic acid) | 2.48±0.01a | 2.42±0.01b | 2.43±0.02c | 2.08±0.02d | 0.797 |
| C17:1 n-8 (heptadecenoic acid) | 0.22±0.01a | 0.68±0.02b | 0.75±0.02c | 0.58±0.02d | 0.797 |
| C18:1 n-9 (Oleic acid) | 26.19±0.01a | 26.46±0.01b | 24.18±0.02c | 21.98±0.02d | 0.702 |
| C20:1 n=7 (Eicocyanic acid) | 0.26±0.01a | 0.39±0.02b | 0.29±0.02c | 0.25±0.02d | 0.786 |
| ∑ MUFA | 29.15±0.0a | 29.95±0.02b | 27.65±0.0c | 24.89±0.03d | 0.098 |
| C18:2 n-6 (Linoleic acid) | 27.39±0.01a | 29.48±0.01b | 24.11±0.02c | 20.15±0.02d | 0.670 |
| C18:3 n-3 (Linolenic acid) | 1.72±0.01a | 1.79±0.01b | 3.19±0.02c | 3..03±0.02d | 0.629 |
| C20:2 n-6 (Eicosadienoic acid) | 0.36±0.0a | 0.42±0.01b | 0.28±0.01c | 0.27±0.01d | 0.532 |
| C20:4 n-6 (Arachidonic acid) | 0.31±0.01a | 0.46±0.02b | 0.38±0.01c | 0.36±0.02d | 1.000 |
| C20:5 n -3 (EPA) | 0.22±0.02a | 0.50±0.01b | 0.25±0.02c | 0.11±0.01ad | 0.983 |
| C22:6 n-3 (DHA) | 2.08±0.01a | 2.11±0.02b | 2.14±0.01c | 2.08±0.01d | 0.546 |
| ∑ PUFAs | 32.08±0.02a | 34.76±0.02b | 30.35±0.01c | 26.00±0.02d | 0.467 |
| ∑ ndFA | 8.07±0.0 | 2.79±0.0 | 11.25±00 | 21.01±00 | |
| ∑ FA (%) | 100 | 100 | 100 | 100 | |
| Σ n-3 | 4.02±0.01a | 4.4±0.03b | 5.59±0.3c | 5.22±0.02d | 0.482 |
| Σ n-6 | 28.06±0.96a | 30.36±0.02b | 24.77±0.44c | 20.73±0.03d | 0.001 |
| Σn-6/ Σn-3 | 7.12±0.21a | 6.74±0.31b | 4.43±0.03d | 3.97±0.03d | 0.007 |
| PUFA/SFA | 1.03±0.01a | 1.07±0.01b | 0.99±0.01c | 0.92±0.0d | 0.067 |
| DHA/ EPA | 9.35±0.65a | 4.08±0.11b | 8.63±0.69c | 18.54±2.54d | 0.063 |
| AI | 1.78±0.16a | 1.75±0.0b | 1.87±0.01c | 1.79±0.02d | 0.009 |
| TI | 0.60±0.0a | 0.56±0.05b | 0.43±0.02c | 0.72±0.02d | 0.006 |
| Lipid content (%) | 2.90±0.02a | 4.42±0.01b | 2.98±0.01c | 2.76±0.03d | 0.786 |
a b c d - significant differences in rows
Values are % total fatty acid expressed as mean ± SD. of three separate determinations.
n.d= Unidentified fatty acids, SFA= Saturated fatty acids; MUFA= Monounsaturated fatty acids; PUFA= Polyunsaturated fatty acid; FA= Fatty acids
Initial commercial pellet feed
Commercial feed equipped with formulated products directly impacts the growth performance; in this study, the highest increases in final body weight (g), body weight gain (%), and the highest specific growth rate (%/day) were shown in the P2 diet at 147.74±1.02 g, 193.99±4.46%, and 1.15±0.01, respectively. In this experiment, supplementing the feed with formula products caused the feed protein level to decrease from 30% (initial composition) to 19.36±0.41% and 21.27±0.12% of all the diets experimental. Considering this result, the moisture of the feed increased from 10.66% to between 35.60% and 37.28%. However, while this does not cause a difference in body protein levels, it does affect the bodyweight of livestock. In this context, the experimental phase did not significantly affect the carcass protein of the body. Although the protein content is not different for each diet, there was a difference between the gurami sago's final growth and feed conversion ratio. We calculated that the FCR at diet P2 was 1.36, while at P4, it was 1.55. However, commercial fish feed resulted in a higher FCR when fish were cultured in earthen freshwater ponds (FCR = 1.87) than concrete freshwater ponds (FCR = 1.45)4,8. On the other hand, the weight gain and growth of the animal decreased with increasing fiber content in the diet43. The P4 diet has a high fiber content because it does not contain formulated products; as a result, the growth and feed conversion efficiency on the P4 diet are lower than other diets.
Furthermore, carbohydrate levels in all experimental diets are lower than the initial composition; nevertheless, they did not decrease the growth of the gurami sago. This species belongs to the group of herbivorous fish10. Herbivorous and omnivorous fishes can increase amylase activity higher than carnivorous fish44. However, the poor overall growth of organisms is caused by low carbohydrate digestibility45. Several scientists have reported that fish growth and feed efficiency can increase by providing feeds supplemented with varying levels of carbohydrates46–48, regardless of the content of feed protein, fat, crude fiber, and carbohydrates in the experimental diet. We recommend using products formulated from natural sources of coconut water, palm sugar, and fungus in commercial fish feeds, thereby increasing the production value of the net yield and bringing more significant financial benefits.
The aquaculture industry needs to use rich nutritional feed, i.e., high in protein, fatty acids, minerals, and vitamins16,17,49,50. Therefore, increasing feeding nutrition and maximizing digestive enzyme activity in farmed fish can be done by providing raw feed ingredients fermented15,51–53. In this context, the new approach we are developing to improve feed quality, feed efficiency, and growth rate in gurami sago is to supplement commercial feed with formulated P1, P2, and P3 products. This strategy has been successful in increasing the nutrient quality of commercial feed. Fish feed supplemented with P2 products had the highest total fat content with 35.99% SFA, 31.33% MUFA, and 32.66% PUFA. In contrast, the P4 diet had the lowest total fat content, having 24.59% SFA, 21.79% MUFA, and 22.78% PUFA, respectively.
This study showed that the commercial fish feed equipped with products P1, P2, and P3 contained more complete fatty acids than the diet P4; the feeds' fat content was 3.47%, 3.68%, 3.49%, and 3.09%, respectively. Diet P4 did not contain linolenic acid (C18:3 n-3), EPA (C20:5n-3), and DHA (C22:6n-3). Accordingly, the commercial feed must be supplied with linolenic acid, EPA, and DHA because these are crucial to meet physiological needs, production performance, and health for fish22,54–56. In this experiment, EPA + DHA levels were 2.65%, 3.11%, and 2.72% of P1, P2, and P3 diets, while in the P4 diet (control), EPA + DHA was not recorded. For Atlantic salmon (Salmo salar L.) juveniles, the EPA and DHA levels in their diet are recommended to range from 0.50 to 1.0%57,58. Regardless of not recordeding EPA and DHA in the P4 diet however, freshwater fish, including gurami sago, are estimated to be able to synthesize unsaturated fatty acids (HUFAs), such as C20 and C22, from C18 PUFAs in the feeds through series reactions of chain elongation and desaturation. Thus, adequate amounts of C18:3-n3 and C18:2n-6 would meet their EFA requirements. The amount of DHA and EPA varies significantly between the P1, P2, and P3 diets, while DHA/EPA ratios in the diet have increased due to supplementing with formulation products. In this context, the differences in the DHA/EPA ratio in each experimental diet are due to differences in the use of fermenters, namely Aspergillus niger, Rhizopus oligosporus, and Saccharomyces cerevisiae. Other researchers state that to develop better feed formulations, the calculated ratio of DHA/EPA in the diet must be precise59. For example, the DHA/EPA ratios in the diet for maximum growth of Golden pompano, Trochinotus ovatus juvenile was 1.4617, 1.30 for gilthead seabream, Sparus aurata60, 0.53 for Atlantic salmon, Salmo salar in freshwater58 and 1.02 for Nile tilapia, Oreochromis niloticus61.
This study has estimated that the DHA/EPA ratios of 1.70 in the P2 diet can optimally increase the fish growth rate and feed efficiency. However, farmed fish's growth rate and feed efficiency depend on physiological, environmental, and farming factors62. The Aspergillus niger, Rhizopus oligosporus, and Saccharomyces cerevisiae used successfully in this experiment are also reported by other scientists to ferment fish feed raw materials such as corn-cob, soybean meal, and sunflower cake63,64. Furthermore, Rhizopus oligosporus is the main microorganism used in the fermentation process because it produces a wide range of enzymes, such as carbohydrase, proteases, lipases, and phosphatase65–67. Nevertheless, coconut water also plays a vital role in enriching the nutritional of feed in this experiment because coconut water contains minerals, amino acids, enzymes, organic acids, fatty acids, vitamins, and a few phenolic compounds26–28. Moreover, palm sap sugar also has an essential role in increased feed quality because palm sap sugar contains minerals, vitamins, and antioxidants33–34.
Many freshwater fish species have low values of PUFA and higher levels of MUFA and PUFA19,67–69; however, the gurami sago found a high level of PUFA and a lower presence of MUFA. Some of the fatty acids in the animal body are affected by diet. Palmitic acid (C16:0) in the group SFA is most abundant in all treatments, however lower in the P4 group, with the highest body carcass level attained in diets P2 and P3 were 24.03%, and 23.04%, respectively. The C:16 and C18:2 n-6 fatty acids accumulate in giant gurami-fed high fat content diets, as demonstrated for common carp (Cyprinus carpio)70 and Atlantic salmon (Salmo solar)69. In experiments carried out with Oreochromis niloticus fed diets, rich lipids, linolenic and oleic acids have been accumulating in significant concentrations19,70,71. Other fish species such as Silver barb (Puntius gonionotous)72 and Asian red-tailed catfish (Hemibagrus wyckioides)73 can synthesize 20C PUFA of n-3 and n-6 series from 18C PUFA by desaturation and elongation. This capacity highlights the essential role of 20C PUFA in this group, serving as potential precursors of prostanoids. This potent ability has been observed in other freshwater fish, such as the zebrafish (Danio rerio)74.
In the present study, eicosatrienoic acid (C20:2 n-6) was not detected in all experiment diets; however, this fatty acid was present in the body carcass of giant gurami in all treatments ranging from 0.27% to 0.42% of total fatty acids composition. This data showed that this fatty acid type has an essential role in this species. The gurami sago fish synthesized it from other precursors ranging from 0.095% and 0.097% of total body lipids composition. In addition, AA, EPA, and DHA fatty acids, which were restricted in the diets, remained in the experimental fish in different proportions between feeds; this difference is due to the variation of added product formulation to each diet.
The results of our study show that the giant gurami sago strain is a strain capable of preserving its normal PUFA levels (C18:2 n-6, C18:3 n-3, C20:2 n-6, C20:4 n-6, C20:5 n -3 and C22:6 n-3). However, these fatty acids were at very low or undetectable levels in the diets, like eicosadienoic acid type. This fact demonstrates that gurami sago, similar to other freshwater fish species, such as Oreochromis niloticus61 and Cyprinus carpio21, can elongate and desaturate fatty acids from precursors present in the diet, including 18C:0 fatty acids and possibly from linoleic fatty acid in large quantities from the diets.
In the present study, the atherogenic index (AI) ranged from 1.68 and 2.19, and the thrombogenic index (TI) between 0.43 and 0.72 in all diets. This finding is related to a significant discrepancy of SFA values between experimental diets. AI and TI indexes are directly related to the levels of C14:0, C16:0, and C18:0, all of which are thrombogenic promoters19. AI and TI levels in gurami sago fed feed P1, P2, and P3 diets were lower than P4 (control); AI and TI indices show potential to stimulate platelet aggregation. The smaller the AI and TI values, the greater the protective potential of coronary artery disease75. The AI and TI values that the Food and Agriculture Organization and the World Health Organization76 recommend for human health range from 0.4 to 0.5. Although the AI and TI values of the gurami sago were higher than 0.5, we have hypothesized that consuming gurami sago meat does not harm consumers' health. In aquaculture, AI and TI indices, among others, depend upon levels of fish oil supplement in the diet19, different sources of oils in diet9,55, the effect of fish farming activities and handling methods after harvest77. In addition, feed quality used, age, gender, species, and environmental conditions also affect AI and TI values67,68,78,79.
This study shows that feed products made from natural and sustainable sources of coconut water, palm sap sugar, and fungus combined with commercial feeds can enhance nutrient-rich diets in gurami sago. The main factor that improved was the fatty acid composition of the PUFA group (i.e., linolenic acid, DHA, and EPA). The increase is related to growth rate, feed conversion ratio, and feed conversion efficiency. The P2 formulation was optimal for feed quality, fish growth, feed efficiency, and carcass fatty acids. This feed also contributes to improving the nutritional quality of giant gourami lipids, which is beneficial to consumers' health. These findings provide basic knowledge about efforts to improve the quality of nutrient-rich feeds and the basis for efficient use of feeds in future fish farming operations.
figshare: The utilization of new products formulated from water coconut, palm sap sugar, and fungus to increase nutritional feed quality, feed efficiency, growth, and carcass of gurami sago (Osphronemus goramy Lacepède, 1801) juvenile. https://doi.org/10.6084/m9.figshare.1664107380
This project contains the following underlying data:
- Table 1a. Raw data growth for 0, 30, 60, 90 days.
- Table 1b. Raw Data growth performance, FCR, FCE_90 days_giant gurami
- Table 2. Raw data proximate composition carcass of gurami sago after 90-days
- Table 3. Raw data proximate composition of diets
- Table 4. Raw data Composition of fatty acids and total lipid in the diets enriched
- Table 5. Raw data Composition of fatty acids of the carcass
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: aquaculture
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Food Science.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 2 (revision) 29 Dec 23 |
read | read | ||
|
Version 1 08 Nov 21 |
read | read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)