Keywords
CNS lymphoma, imaging, response assessment.
This article is included in the Oncology gateway.
CNS lymphoma, imaging, response assessment.
Primary central nervous system lymphoma (PCNSL) is a rare type of B cell lymphoma, accounting for 1–2% of all lymphomas1 and approximately 4% of newly diagnosed CNS tumours.2 Secondary CNS involvement occurs in 2.3–10% of patients with systemic diffuse large B-cell lymphoma (DLBCL)3 but can also occur in other lymphoma subtypes.
Despite recent advances in treatment, with the addition of thiotepa to the methotrexate-cytarabine backbone,4 prognosis for PCNSL remains poor with 2-year overall survival rates of between 42 and 69%, and only a fifth of patients being alive at 5 years.5 For secondary CNSL, the prognosis is even more dire, with median OS of 10 months.6 It is challenging to decipher which patient will do poorly, as only age and performance status having an impact on prognosis. Unlike other lymphoma types, most noticeably Hodgkin’s lymphoma,7 metabolic imaging has no role in response assessment and the associated prognostic impact and is confined to diagnostic staging to exclude concurrent systemic disease.8
The gold standard for CNS imaging is with gadolinium-enhanced magnetic resonance imaging (MRI).9 However, MRI has its limitations. When used in end-of-treatment (EOT) imaging, some patients will have small persistent abnormalities from scarring due to focal haemorrhage or from a previous biopsy, which be difficult to discern from residual tumour and is currently classified as unconfirmed complete response (CRu).9 There have also been recent safety concerns over exposure to gadolinium-based contrast agents (GBCAs) with case reports of the development of nephrogenic systemic fibrosis,10 as well as gadolinium deposits in other organs.11 This led to the European Medicines Agency (EMA) restricting or suspending the use of linear gadolinium products in 2017.12
Choline positron emission tomography–computed tomography (PET/CT) uses analogues of choline as a radiotracer. Choline can be labelled with either [18F] fluoromethyl (18FCH) or [11C] carbon (11CH3). FCH was first developed as a radiotracer for PET imaging in 2000. Choline is a precursor of phospholipids, which upon entry to the cell, is phosphorylated by the enzyme choline kinase.13 The expression of choline kinase is upregulated in tumour cells and allows increased uptake of the choline tracer.14 There is evidence that MYC, which regulates lipid metabolism and can be overexpressed or translocated in high-grade lymphomas, plays a part in the increased uptake.15 MYC over-expression a poor prognosis in PCNSL with patients having a 5-fold higher 5-year risk of progression and/or death than those without.16 When Bcl-2 is also over-expressed (so called double expressor) the risk is 13-fold higher.
As there is minimal background grey matter uptake of the tracer, FCH-PET/CT can be used to detect tumours with a high lesion-to-CNS background ratio and has been used in diagnosis and follow up imaging in brain tumours, particularly high-grade gliomas.14 There are case reports of both systemic and CNS lymphoma showing FCH-PET/CT avidity, including incidental lymphoma picked up during imaging for prostate cancer17 and histology proven CNS lymphoma in brain tumour series.18 This contrasts with the lack of utility of the 2-deoxy-2-[18F]fluoro-D-glucose (FDG) PET/CT for CNS lymphoma analysis, due to high FDG physiological activity levels within the grey-white matter. Although high-grade CNS lymphomas do have a standard uptake value maximum (SUVmax) greater than background grey-white matter at diagnosis (average SUVmax 13.5±5.4 compared to background SUVmax of 5.3±1.2),19 for interim and EOT scanning the higher physiological background levels limit accurate detection of residual active disease.
Our centre has been using FCH-PET/CT alongside MRI for CNS lymphoma assessment since 201120 following approval by the Administration of Radioactive Substances Advisory Committee (ARSAC). Our primary objective to to assess concordance between FCH-PET/CT and MRI.
To assess concordance between FCH-PET/CT and gold standard MRI, our centre conducted a retrospective cohort analysis of patients who had EOT response assessment conducted using both modalities between 1st November 2011 and 10th October 2019 at the Royal Marsden Hospital, London. Approval for this study (approval number 782), including ethical approval was obtained from the Committee for Clinical Review (CCR). Patients with a histopathological or specialist neuroradiological (where biopsy was not feasible) diagnosis of primary or secondary CNS lymphoma who had an EOT MRI and FCH-PET/CT were identified from a radiology database. Patients who did not complete treatment, or had the imaging performed after consolidative therapy were excluded. Patient characteristics, clinical information, and survival data were collected from the electronic patient recorded (EPR). MRI and FCH-PET/CT reports were collected from EPR and PACS. EOT response was classified as either complete response (CR) for MRI/metabolic complete response (mCR) for FCH-PET/CT, partial response (PR), stable disease (SD) or disease progression (PD). MRI response was reported as per the International Primary CNS Lymphoma Collaborative Group (IPCG).4 For FCH-PET/CT, PR is defined as reduction in activity (SUVmax), PD is an increase in SUVmax, SD as no change in SUVmax, and mCR is defined as no activity. Statistical analysis was performed using IBM SPSS version 27 (RRID:SCR_019096), JASP (RRID:SCR_015823) is an open-access alternative. Survival analysis was evaluated using log-rank test.
A total of 40 patients met the inclusion criteria. Patient characteristics are shown in Table 1. PCNSL was the most common type of CNS lymphoma, and MATRix regimen the most common type of chemotherapy used. 16 patients (40%) were consolidated after EOT imaging, with autologous stem cell transplant (ASCT) being the preferred method.
14 patients (35%) had discordant results on EOT imaging. The majority of discordant cases (11 out of 14) were patients who had PR on MRI but showed CMR on FCH-PET/CT. In the remaining discordant three cases, two had PD on MRI with PR on FCH-PET/CT and one had CR on MRI and PR on FCH-PET/CT. Figure 1 shows examples of two concordant cases and one discordant case.
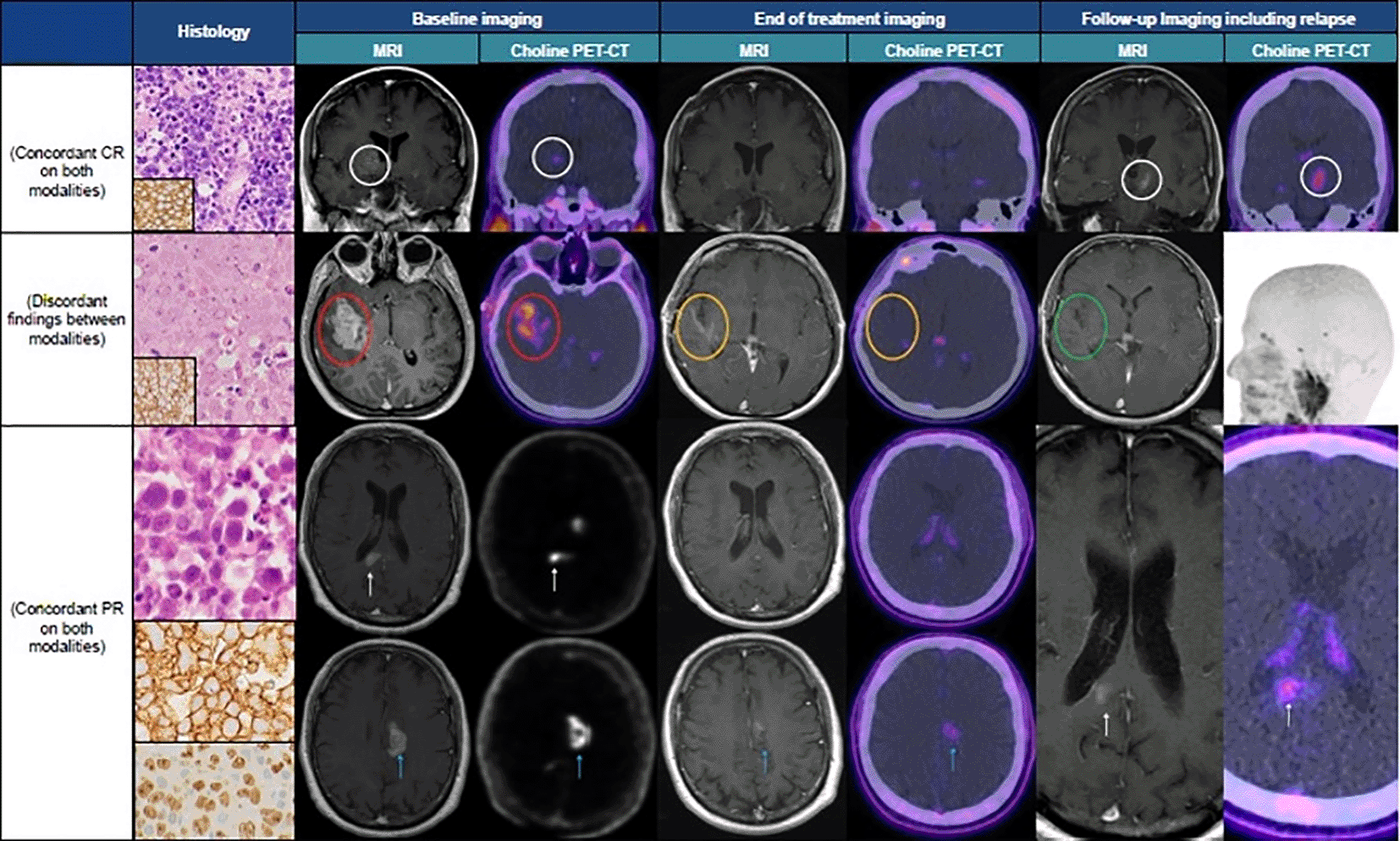
Top row. Histology – large B-cell lymphoma, composed of sheets of large atypical lymphoid cells, (haemtoxylin and eosin [H&E] stain) that are CD20 positive (inset). This patient had concordant MRI and FCH-PET/CT imaging findings throughout treatment. The baseline coronal CE T1W MRI demonstrates an enhancing lesion within the right thalamus and a focus of increased tracer uptake in the same region on the contemporaneous coronal FCH-PET/CT image (white circles). These areas of enhancement and tracer uptake had both resolved on the EOT imaging, consistent with radiological CR. Follow up imaging demonstrates a new focus of enhancement and corresponding tracer uptake within the left thalamus and cerebral peduncle on the coronal CE T1W MRI and FCH-PET/CT images, respectively (white circles). Middle row. Histology - large B-cell lymphoma, composed of sheets of large atypical lymphoid cells (H&E stain) that are CD20 positive (inset). This patient had discordant findings between imaging modalities. The baseline axial CE T1W MRI shows an enhancing mass within the right medial temporal lobe and posterior insula (red circle). The contemporaneous choline PET-CT is concordant, demonstrating increased uptake in the same anatomical location (red circle). Partial response is achieved on the EOT CE T1W MRI as illustrated by interval reduction in size of the mass although some pathological enhancement remains (amber circle, this area was not hyperintense on the pre-contrast T1W imaging - not shown). The EOT choline PET-CT is discordant, showing metabolic complete response (amber circle). Follow up imaging shows resolution of the mesial temporal and insular enhancement (green circle) and ongoing normal physiological FCH tracer uptake in the cranium on the maximum intensity projection FCH-PET/CT reconstruction, consistent with radiological CR. Bottom rows. Histology - large B-cell lymphoma, composed of sheets of large atypical lymphoid cells, (H&E stain, top panel) that are CD20 positive (middle panel) and display a high Ki 67 proliferation index (lower panel). This patient had a partial response which was concordant between modalities throughout treatment. The baseline axial CE T1W MRIs depict an enhancing lesion within the right side of the splenium of the corpus callosum (top, white arrows) and the left cingulate gyrus (bottom, blue arrows) with corresponding increased uptake on the axial FCH-PET/CT in the same regions. The splenial lesion has resolved on both the EOT MRI and FCH-PET/CT (top) but the left-sided lesion is still present on both modalities (bottom, blue arrows). Follow up imaging shows recurrence of the right-sided splenial lesion on both modalities (white arrows).
Abbreviations: CR = complete response, PR = partial response. CE T1W = contrast-enhanced T1 weighted. EOT = end of treatment.
For the whole cohort, the overall response rates (ORR) were similar between the two modalities. The ORR for MRI was 90%, with 16 patients (40%) achieving CR and 20 (50%) achieving PR. FCH-PET/CT had a slightly higher ORR of 95%, with 26 (65%) patients achieving mCR and 12 (30%) achieving PR.
A total of 17 patients subsequently progressed (47%). The progression-free survival (PFS) was 78% at 100 days and 51% at 2 years. Overall survival (OS) was 61%.
To our knowledge, this is the first published study for FCH-PET/CT imaging in CNS lymphoma.21 Our results showed a concordance rate of 65% between FCH-PET and MRI. 11 out of 14 discordant cases were in patients who achieved a PR by MRI, but were in mCR on FCH-PET/CT. This group had a clinical course similar to the patients who achieved a CR/mCR on both. One theory is that the residual enhancement seen on MRI was scarring or post-treatment changes and may have been better classified as CRu.
There are no studies with direct comparison. A similar study by Ahn et al.22 looked at the prognostic value of metabolic imaging, using 11C-methionine (11C-MET) rather than FCH as a tracer. Imaging was done after four cycles of methotrexate-containing chemotherapy and again after completion of chemotherapy. The concordance at interim imaging between 11C-MET PET/CT and MRI was 89%, with four cases achieving CMR on 11C-MET PET/CT who had residual lesions on MRI. There are limited studies looking at the effect of FDG-PET/CT on prognosis. Bursen et al.23 showed PET negativity (defined as SUV uptake below physiologic background uptake) after two cycles of methotrexate-containing chemotherapy is associated with improved PFS but not OS. This conflicts with results from Jo et al.24 who showed improved PFS only with EOT and not interim scanning. Again, there was no difference in OS.
Our study does have limitations. Our numbers are small and consist of a combination of primary and secondary CNSL as well as relapsed cases, which have different expected outcomes. There was also considerable variation in treatment and consolidation regimens, with the addition of thiotepa from 2016 onwards for young, fit patients. Therefore, the prognostic value of FCH-PET/CT could not be studied using this cohort. Further work is being done by our group to assess the prognostic value of FCH-PET/CT in patients with PCNSL who undergo ASCT.25
We conclude that FCH-PET/CT is an important a new tool for assessment of CNS lymphoma. At the current time, its use may be limited to patients who have a contraindication to MRI.26 Further studies are warranted evaluating the potential role for FCH-PET/CT in CNS lymphoma, including assessment of whether FCH PET/CT is superior to MRI in predicting persistent disease or identifying patients that need consolidation therapy.
OSF: Underlying data for ‘[18F] Fluoromethylcholine PET/CT for CNS lymphoma assessment: a new tool’ https://doi.org/10.17605/OSF.IO/KTNDG.27
The project contains the following underlying data:
Data file 1: Patient database.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Written informed consent for publication of the patients’ details was obtained from the patients.
KM wrote the initial draft of the manuscript. KM, SC, FS, EG, BS collected data. AA provided histopathology images. BS, SC, FS, EG provided radiology images. All authors contributed to design, review and redrafting of the manuscript.
The authors acknowledge National Health Service funding to the National Institute for Health Research Biomedical Research Centre (London, UK).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: radionuclide imaging, lymphoma imaging, PET/CT
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 10 Nov 21 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)