Keywords
complex systems; immune memory; information transfer; immune response; randomness
No evidence of the possibility of altering a constituent of the immune system without directly affecting one of its associated components has yet been shown.
A schematic model was developed in which two triggers, fasting and splenectomy, were studied for their ability to affect the expression of cell membrane epitopes and the cytokine secretion of out-of-body autogeneic and syngeneic lymphocytes.
The effect of fasting and/or splenectomy on promoting correlations between immune systems was studied by determining the alterations in expressions of cell membrane epitopes and in cytokine secretion by out-of-body autogeneic and syngeneic lymphocytes. The effect of fasting as a trigger decreased expression of CD8 and CD25 and increased TNFα levels. The effect of splenectomy as a trigger was investigated in non-fasting mice by comparing splenectomized and non-splenectomized mice. An increase in the CD8 expression and in TNFα, IFNg, and IL10 secretion was noted. The effect of splenectomy as a trigger in fasting mice was determined by comparing splenectomized and non-splenectomized mice. Splenectomy significantly affected the expression of CD25 and CD4 CD25 and on secretion of TNFα, IFNg, and IL10. To determine the effect of keeping the cells in an out-of-body location on the expression of lymphocyte epitopes, tubes kept on top of the cages of the fasting mice were compared with tubes kept on top of empty cages. The results showed a significant change in the CD8 expression was noted. To determine the effect of keeping cells in an out-of-body location on cytokine secretion, tubes kept on cages were tested for cytokine levels significant decrease was noted in the secretion of TNFα and IFNg.
The study showed that a mouse could affect cells at a distance and alter the expression of surface markers and cytokine secretion following two types of triggers: fasting and/or splenectomy. The data characterized a system for the induction of correlations between two’s immune system components without a transfer of mediators. It suggests that an out-of-body correlation can be induced between two components of the immune system.
complex systems; immune memory; information transfer; immune response; randomness
Changes made to the new version:
See the authors' detailed response to the review by Suprabhat Mukherjee
See the authors' detailed response to the review by Rohit Malik
See the authors' detailed response to the review by Biswadeep Das
IFNγ: interferon gamma
TGFβ: transforming growth factor beta
TNFα: tumor necrosis alpha
IL10: interleukin 10
OL: an independent control laboratory
Living systems carry information, and the more complex a system is, the higher the likelihood it contains information. Like other biological systems, the immune system may not have a perfect structure or symmetry, but it optimizes functionality. The system’s irregular pathways offer an opportunity to apply physics concepts to the biological system.1
Correlations between immune system components always involve reactions mediated by a direct association between different system elements.2 Current paradigms of immune crosstalk between cells or at a subcellular molecular level are based on direct contact or the transfer of mediators in both health and disease.3–5 These elements serve as links for relocating associations between immune system constituents. No evidence for correlations between components of the immune system that alter a constituent of this system without directly affecting one of its associated parts has been reported.
This study aimed to develop a system where a correlation exists between two immune system components without either a direct interaction or a transfer of mediators. The data support the concept that out-of-body correlations can occur between immune system components in isolated structures.
24 Male C57BL/6 healthy mice (11–12 weeks old, 30 gr.) were obtained from Harlan Laboratories (Jerusalem, Israel) and maintained in the Animal Core of the Hadassah-Hebrew University Medical School. Mice were administered standard laboratory chow and water ad libitum and kept in a 12-hour light/dark cycle. Mice were administered standard laboratory chow and water ad libitum and kept in a 12-hour light/dark cycle. Animal experiments were carried out according to the guidelines of the Institutional Committee for Care and Use of Laboratory Animals and with the committee's approval. All efforts were made to ameliorate any suffering of animals by using mild anesthesia.
Six groups of mice (Table 1) were studied, with four mice per group in each of the four experiments. The number of animals was determined based on statistical analysis. Data on all mice in each of the studies is included in the Underlying data.21 Groups were selected based on the different parameters tested with the appropriate controls. Table 2 provides a representation of the research protocol.
| Group | Mouse in cage | Splenectomy | Fasting | Autogeneic cells on top of the cage | Syngeneic cells on top of the cage |
|---|---|---|---|---|---|
| A | + | + | + | + | - |
| B | + | + | + | - | + |
| C | + | + | - | + | - |
| D | + | + | - | - | + |
| E | + | - | + | - | + |
| F | + | - | - | - | + |
| G/H | - | - | - | - | - |
The mice in groups A and B underwent splenectomy followed by fasting. Each mouse was kept in a cage with a tube containing its own autogeneic (group A) or syngeneic (group B) lymphocytes on top. The mice in groups C and D underwent splenectomy but did not fast, and they were kept in cages with tubes containing autogeneic (group C) or syngeneic (group D) lymphocytes on top. The mice in groups E and F did not undergo splenectomy and were kept in cages with a tube containing syngeneic lymphocytes on top. The mice in group E fasted, while those in group F did not. Tubes marked G and H contained lymphocytes harvested from syngeneic donors and were kept on top of empty cages. The study design determined the number of animals to enable significance. Controls were used for each of the experiments based on the parameters tested. Studies were repeated in an outside laboratory for verification of the effect. Studies were conducted under the Arrive guidelines.
The described system enabled us to study two types of triggers for the associations between two immune system constituents. Mice underwent splenectomy using standard procedure6 or fasting (21-24 hours). Each mouse was kept in a separate cage for the duration of the experiment. Autogeneic or syngeneic splenocytes were prepared from splenectomized mice and kept for 24 hours in a tube on top of the cage at a distance of 10-20 cm from the mouse itself. Control cells were kept in a tube placed on top of the cages containing either non-splenectomized or non-fasting naïve mice or were kept on top of empty cages without a mouse. In splenectomized mice, we tested the effect of splenectomy alone or combined with fasting on either autogeneic or syngeneic cells. In naïve, non-splenectomized mice, we tested the effect of fasting on syngeneic cells. The effects of these triggers on the associations between immune components were examined by determining the expression of surface markers and cytokine secretion by the out-of-body lymphocytes. The study was replicated three times, and for each study, four mice per group were analyzed (marked 11A, 11B, and 11C). For epitope parameters, the experiment was repeated for the fourth time by an independent control laboratory (marked OL) for several parameters. Cytokine study was performed once in the controlled laboratory.
Splenocytes were isolated, as described previously.7 Flow cytometry was performed on splenocytes resuspended in 1 mL of Flow Cytometry Staining (FACS) buffer. Cells were stained with diluted antibodies (50 μL/sample). Cells were examined using FACS at times 0 and 24 hours for the expressions of epitopes, CD3, CD4, CD8, CD25, FoxP3, and NK1.1, on the lymphocytes (antibodies by BD Biosciences). Flow cytometry was performed using an LSR-II flow cytometer (BD Biosciences, DeNovo software). The following subsets were analyzed: CD4, CD25, CD4 CD25, Foxp3, CD4 Foxp3, CD4 CD25 Foxp3, CD4 CD25 Foxp3, CD8, CD8 Foxp3, CD8 CD25, CD8 CD25 Foxp3, CD8 CD25 Foxp3, CD3, NK1.1, and CD3 NK1.1. Full data of the study can be accessed from the Underlying Data21 section.
One set of mice marked OL (n = 4) was analyzed in the control laboratory for IFNγ, TNFα, IL10, and TGFβ serum levels, IL10, and TGFβ. Determination of IFNγ, TNFα, and IL10 in supernatants was performed concurrently, using Flow Cytomix (Thermo Fisher Scientific (eBioscience)). TGFβ levels were determined separately using ELISA Quantikine (MB100B) (R&D Systems). Cytokine levels were determined according to the manufacturer's instructions.
The Mann-Whitney SPSS test (software version 1.0) was performed for each experiment. Data of all individual mice in all studies is provided in the Underlying data.21 Only parameters that showed significant changes in two experiments or more, including changes in opposing directions, were used for the final analysis.
The effect of fasting and/or splenectomy on promoting correlations between immune systems was studied by determining the alterations in expressions of cell membrane epitopes and in cytokine secretion by out-of-body autogeneic and syngeneic lymphocytes.
The effect of fasting as a trigger for altering the association that can modify epitope expression on out-of-body lymphocytes kept in tubes on top of cages was investigated in splenectomized mice. The effect on autogeneic cells was determined by comparing groups A (fasting) and C (non-fasting).
Figure 1A shows the effect of fasting on autogeneic lymphocytes; decreased expression of CD8 and CD25 was found in the mice in group A compared with mice in group C in experiments 11B (p = 0.02) and 11C (p = 0.019).
Fasting significantly affected cytokine secretion by the out-of-body syngeneic lymphocytes in non-splenectomized mice.
Figure 1B shows a significant increase in the TNFα levels (p = 0.047) when comparing group E (fasting) to group F (non-fasting, the TNFα levels were undetectable in-group F).
The data suggested that correlations between immune system components can be induced after fasting in splenectomized and non-splenectomized mice targeting out-of-body autogeneic and syngeneic lymphocytes. Fasting did not affect the syngeneic lymphocytes kept in a tube on top of the cages of splenectomized mice, as indicated by comparing groups B (fasting) and D (non-fasting).
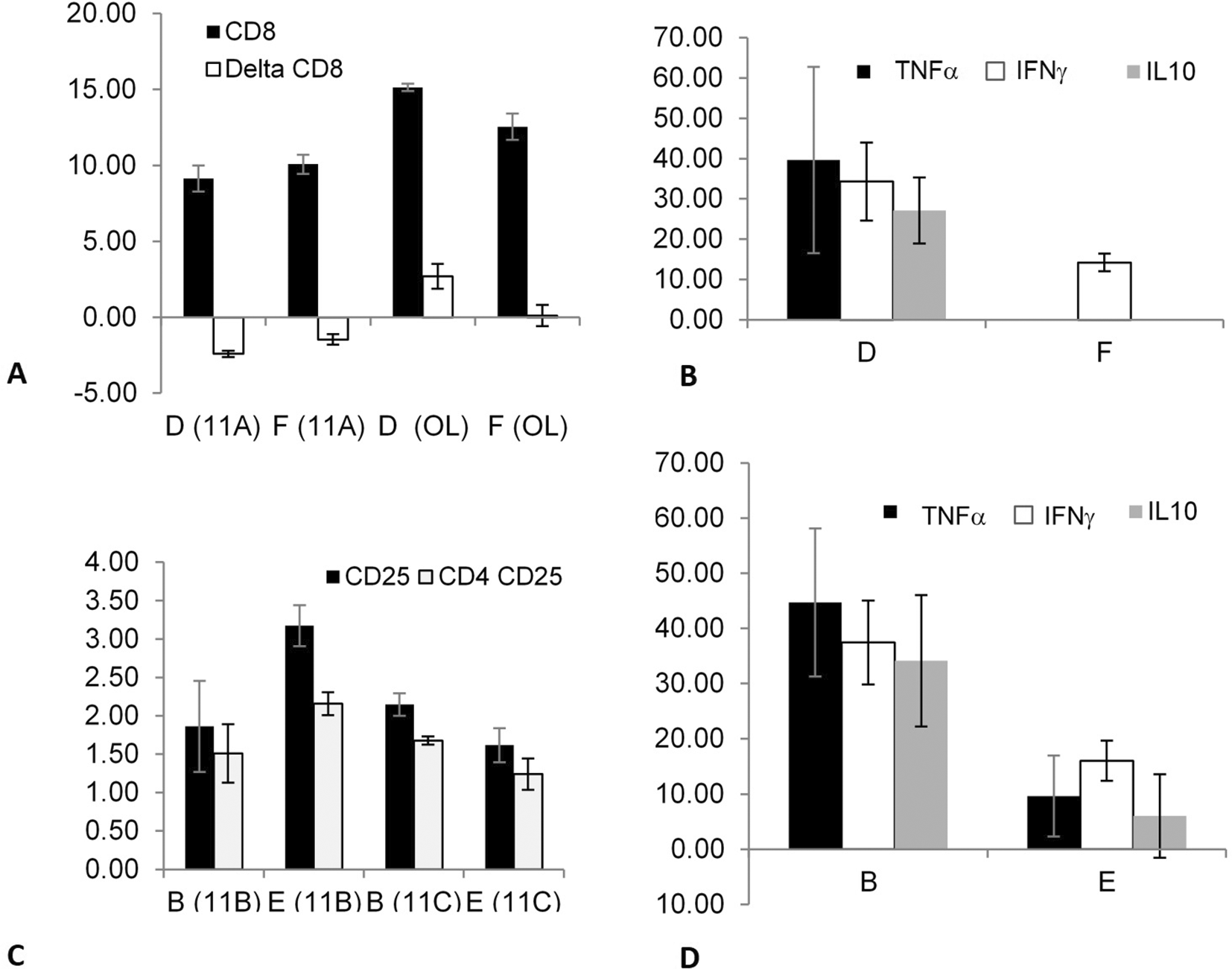
The effect of fasting as a trigger on splenectomized mice was determined by comparing the syngeneic lymphocytes kept in tubes on top of the cages of the mice in groups B (fasting) vs. D (non-fasting). The effect of fasting as a trigger for an association that may alter the epitope expression and/or cytokine secretion of out-of-body lymphocytes kept in tubes on top of cages was determined by comparing the syngeneic lymphocytes of the mice in groups E (fasting) vs. F (non-fasting); there was a significant effect on the TNFα secretion.
The effect of splenectomy as a trigger for altering the association that can modify epitope expression and/or cytokine secretion from out-of-body syngeneic lymphocytes kept in tubes on top of cages was investigated in non-fasting mice by comparing groups D (splenectomized) and F (non-splenectomized).
Figure 2A shows a significant increase in the CD8 expression (between time points 0 and 24 h) in experiments 11A (p = 0.021) and OL (p = 0.021) in group D compared with that in group F; however, the changes in CD8 expression in experiment 11A were opposite to those in experiment OL. The secretion of three cytokines, TNFα (p = 0.014), IFNγ (p = 0.021), and IL10 (p = 0.013), significantly increased in group D compared with group F, as shown in Figure 2B.
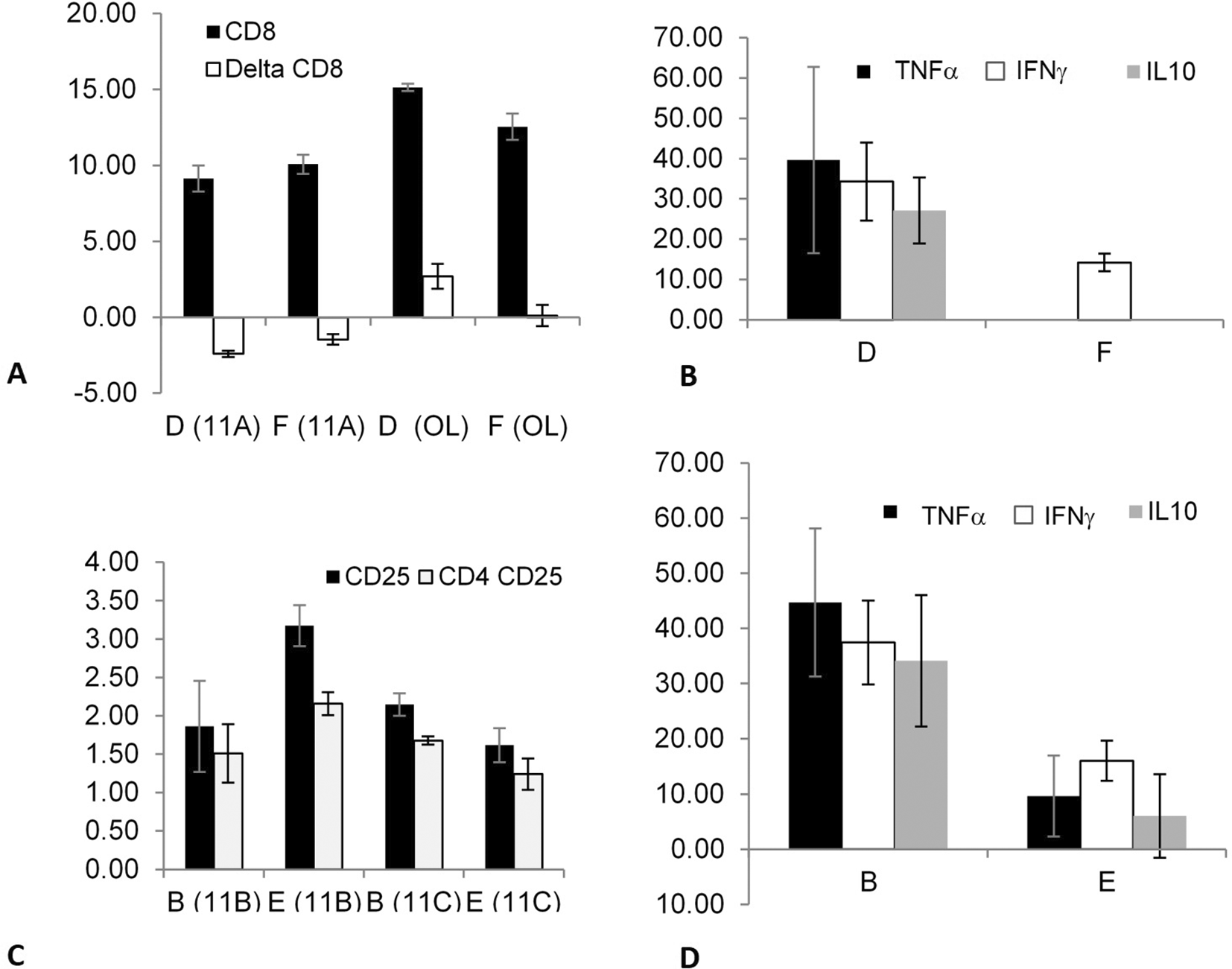
The effect was significant for CD8 expression and for TNFα, IFNγ, and IL10 secretion. The effect was also determined by comparing the syngeneic lymphocytes that were kept in tubes on top of the cages containing fasting mice from groups B (splenectomized) vs. E (non-splenectomized). It was significant for CD25 and CD4 CD25 expressions and TNFα, IFNγ, and IL10 secretion.
The effect of splenectomy as a trigger for modifying the epitope expression and/or cytokine secretion of out-of-body syngeneic lymphocytes was investigated in fasting mice by comparing groups B (splenectomized) and E (non-splenectomized).
Figure 2C shows that splenectomy significantly affected several parameters, as indicated by comparing the two groups in experiments 11B and 11C; however, the effects in group B were opposite to those in group E. The expression of CD25 (p = 0.02 and p = 0.03 for 11B and 11C, respectively) and CD4 CD25 (p = 0.021 and p = 0.034 for 11B and 11C, respectively) differed significantly between groups B and E.
The secretion of TNFα (p = 0.021), IFNγ (p = 0.02), and IL10 (p = 0.02) were significantly higher in group B compared with that in group E, as shown in Figure 2D. The results suggested that a link can be induced between immune system components after splenectomy in fasting and non-fasting mice in syngeneic lymphocytes.
No significant differences were observed when assessing differences between the effects on out-of-body autogeneic vs. syngeneic cells; these differences were determined by performing a group A (autogeneic) vs. B (syngeneic) comparison of the lymphocytes harvested from the fasting-splenectomized mice and by performing a group C (autogeneic) vs. D (syngeneic) comparison of the non-fasting splenectomized mice.
The results suggested that the associations made for autogeneic cells did not differ significantly from that made for syngeneic cells.
To determine the effect of keeping the cells in an out-of-body location on the expression of lymphocyte epitopes, tubes kept on top of the cages of the fasting mice from groups A, B, and E were compared with tubes kept on top of empty cages (G), as shown in Figure 3. A significant change in the CD8 expression was noted by comparing groups B and G in 11B (p = 0.034) and OL (p = 0.043), as shown in Figure 3A; however, the changes in group B were opposite to those in group G. No significant effect was observed when comparing groups A and G.
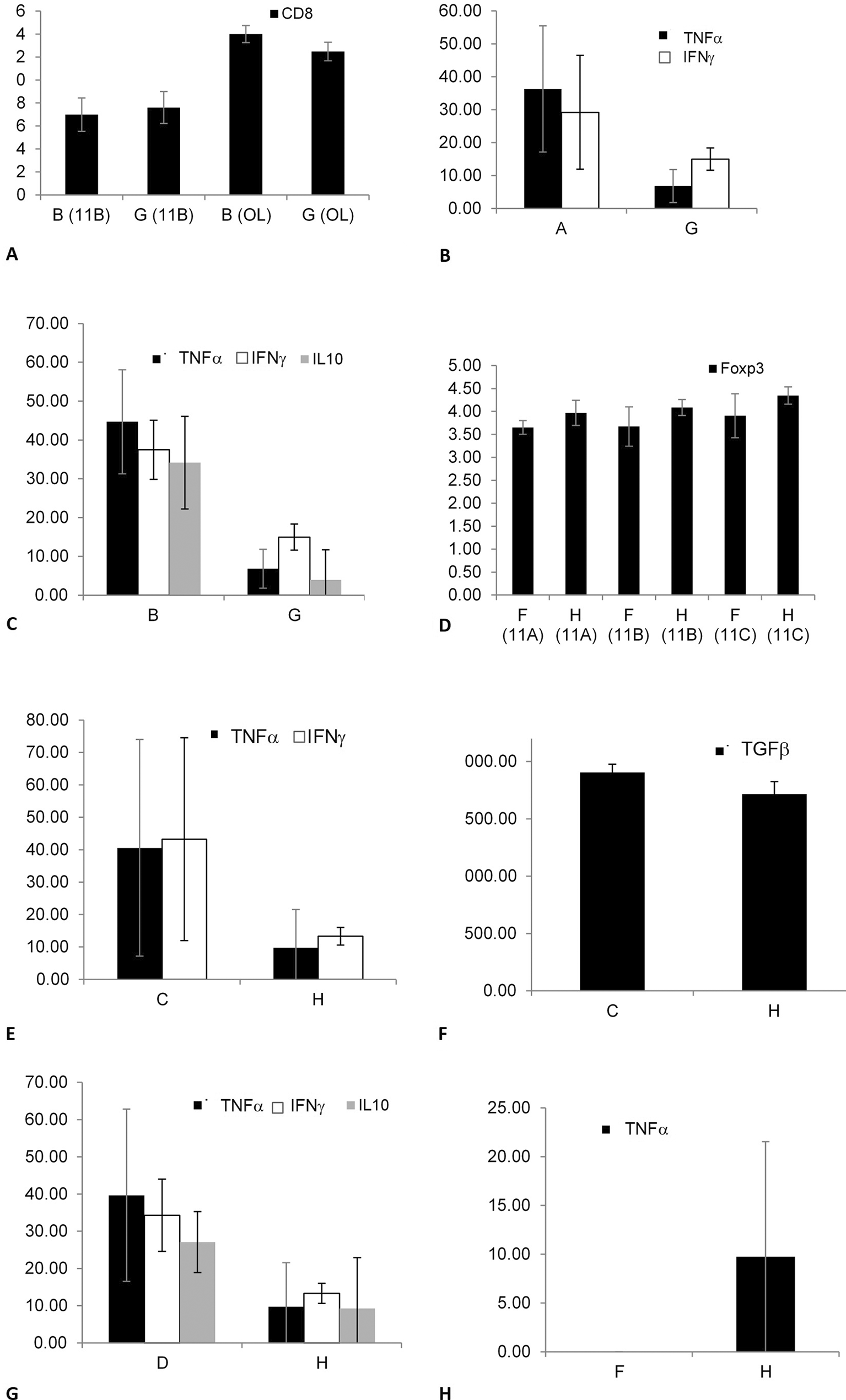
The effect was significant for CD8 expression when comparing groups B and G (3A); for the secretion of TNFα and IFNγ when comparing groups A and G (3B); for the secretion of TNFα, IFNγ, and IL10 when comparing groups B and G (3C); for the expression of Foxp3 when comparing groups F and H (3D); for TNFα, IFNγ, and TGFβ secretion when comparing groups C and H (3E, 3F); for IFNγ, TNFα, and IL10 secretion when comparing groups D and H (3G); and for TNFα secretion when comparing groups F and H (3H).
To determine the effect of keeping cells in an out-of-body location on cytokine secretion, tubes kept on cages were tested for cytokine levels. A significant decrease was noted for the secretion of TNFα (p = 0.021) and IFNγ (p = 0.028) between groups A and G, as shown in Figure 3B. Similarly, a significant decrease was noted between groups B and G for the secretion of TNFα (p = 0.021), IFNγ (p = 0.021), and IL10 (p = 0.018), as shown in Figure 3C. No significant difference was observed between groups E and G for the epitope expression or cytokine secretion.
To determine the effect of keeping cells in an out-of-body location on the expression of lymphocyte epitopes, tubes kept on top of cages containing the non-fasting mice from groups C, D, and F were compared with tubes kept on top of empty cages (H). The Foxp3 expression significantly increased between groups F and H in all three experiments, as shown in Figure 3D (for 11A, p = 0.083; for 11B, p = 0.081; and 11C, p = 0.083; p = 0.006 for all three; this parameter was not tested in OL). No significant difference was observed when comparing groups C vs. H and D vs. H.
To determine the effect of keeping cells in an out-of-body location for cytokine secretion, tubes kept on top of cages were assessed for cytokine levels. A significant decrease in IFNγ, TNFα, and TGFβ secretion was observed when comparing groups C and H, as shown in Figure 3E (p = 0.020) and Figure 3F (p = 0.43). A significant decrease in IFNγ, TNFα, and IL10 secretion was observed between groups D and H, as shown in Figure 3G (p = 0.021). A significant increase in the TNFα secretion was observed between groups F and H, as shown in Figure 3H (p = 0.047). The data suggested that a fasting or non-fasting mouse in a cage affects its connections with syngeneic cells.
The present study presents measurements of parameters over a set of mutually unbiased states, which demonstrated an ability to induce a correlation between components of the immune system through an indirect effect in an isolated system. The described experiment showed that a mouse could affect cells at a distance and alter the expression of surface markers and cytokine secretion following two triggers: fasting and/or splenectomy. In the described laboratory setting, only the measured observables resulting from this effect can be detected. The effects were compared for cells kept on top of cages containing mice that did not undergo a splenectomy and did not fast with cells kept in empty cages (Figures 1 and 2 vs. Figure 3).
In biological systems, different levels of associated states may be present. These levels might depend on the degree of comparison and/or correlation between donors and recipients that act simultaneously on various immune system components.3–5 In the present schematic model, a correlation between two systems, a cell or other immune system component, allows for the recovery of a new state of a lymphocyte or cytokine-secreting cell.
The lymphocyte expression of cell surface markers and cytokine secretion involved different pathways.7–10 The classical transportation of immune signals is based on messages delivered by molecules (e.g., chemokines) secreted by different subsets of cells that transmit a direct signal to a target cell or a subcellular organelle (e.g., receptor) through physical contact or a messenger molecule. The present data demonstrated that a correlation can be induced independent of a direct interaction in an isolated system. The observed effects cannot be explained by classical immunology suggesting a correlation-dependent phenomenon in the described system (Figures 1 and 2).
The effect that underlines the outcome observed in the present study on autogeneic cells can be explained by an inherent association between two immune system components, suggesting an undefined historical correlation between the two parts of the system. At the same time, the effects observed on syngeneic cells may imply a wave-dependent phenomenon between foreign components of the immune system.
The fidelity obtained in the current schematic model shows excellent adherence correlations properties when targeting the expression of cell membrane epitopes and cytokine secretion of lymphocytes. The results from each mouse in the described system suggest an inherent pattern that repeats itself. Each attempt for separate mice is viewed as independent of all others. Using a standard trigger, fasting or splenectomy, enables our protocol to succeed without filtering results, and the effects are relatively reliable. The experiments were repeated in an independent laboratory, further supporting the feasibility of setting up isolated conditions under which these effects can be measured (Figuers 1-3).
The lack of effects for all parameters in all experiments (see Underlying data21) and changes in opposing directions for some of the measured observables may result from the multiple confounding factors that act simultaneously, which are hard to control, or from the disordered nature of the immune pathways and networks. These are inherent to any biological system.11–14 The out-of-body correlation was significant for several investigated parameters in repeated experiments. For some of the observables, the changes were small and could be claimed to result from intra-test variability. However, each mouse was tested in a separate cage, which can be considered independent of the others. Only parameters that significantly changed in two separate experiments were used for the final data analysis.
The results described in the present study may support built-in memory in an isolated system, which is mandatory for such correlations to occur. Although memory in immune systems is based on the direct delivery of mediators or messenger molecules secreted by immune cells,15,16 a “wave type of memory” is required at both the transmitting and receiving sites for such a correlation to occur. This type of immune memory may explain some of the observations in the present study.
The effects observed in the present study may occur at a cellular or subcellular level and may act per theories that apply some of the principles of quantum physics to biological systems.11,17–19
A model using a non-natural computer-brain interface to induce an out-of-body effect, which showed non-invasive information transfer between the brains of different species, has been reported.20 A translation of the intention of a human volunteer to stimulate the rat brain motor area responsible for tail movement has also been demonstrated. The data suggested the feasibility of a computer-mediated brain–brain interface that can link the neural functions between two biological entities.
The system described here provides a robust model for biological applications of correlations in immunology and biology. The model demonstrated here could also be used as an elementary constituent of a wave-type repeater in other biological systems. The state implemented in this protocol is based on complex systems, which enables modifying the surface markers and cytokine secretion of lymphocytes. Even with a relatively low success probability, this system can be scaled to more complex biological systems.
The optimal function of the immune system may require an alliance between classical and correlative immunology. The present data shed light on several ununderstood biological phenomena, including inter-immune cell interactions, brain–immune component connections, environmental impacts, genotype-phenotype interfaces, and organism-host interactions. By enabling diagnostic and therapeutic procedures to be performed on out-of-body autogeneic or syngeneic tissues or organisms, these concepts may apply to developing improved methods for diagnosing and treating immune-associated disorders.
Study limitations include using the defined model and extrapolations to other biological systems that may require further studies.
This study measured parameters on a group of mutually unbiased states, demonstrating the ability to induce a correlation between immune system components through an indirect effect in an isolated system. The experiment revealed that a mouse could affect cells at a distance and alter the expression of surface markers and cytokine secretion following two triggers: fasting and/or splenectomy. The findings of this research characterized a system for establishing a correlation between two components of the immune system without the need for mediators. The data suggests that two immune system components could induce an out-of-body correlation.
Dryad: Correlations between components of the immune system, https://doi.org/10.5061/dryad.prr4xgxn3. 21
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
PLoS Biol: ARRIVE checklist for ‘Correlations between components of the immune system’, https://doi.org/10.1371/journal.pbio.3000411. 22
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Ethnopharmacology and Natural Product Chemistry
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Ethnopharmacology and Natural Product Chemistry
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biomedical genetics and diganostics
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology of Inflammatory and Infectious diseases
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 12 Mar 24 |
read | ||
|
Version 2 (revision) 28 Sep 23 |
read | read | |
|
Version 1 22 Nov 21 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)