Keywords
Axin, AMPK, muscle, aging, C. elegans, LKB1, AAK-2, PRY-1
Axin, AMPK, muscle, aging, C. elegans, LKB1, AAK-2, PRY-1
With aging, there is a decline in skeletal muscle mass and function. Aging muscle undergoes a shift in the balance between myogenic potential and fibrogenic activity that leads to reduced capacity of the muscle to repair and regenerate.1 Studies have shown that age-associated decline in muscle function is multifactorial and affected by genetic and environmental factors. While many genes have been identified that contribute to muscle development and function, their mechanisms of action are not well understood.
This review discusses a novel signaling network involving AXIN and AMP-activated protein kinase (AMPK) in maintaining muscle health that offers a new perspective on promoting healthy aging. Both these proteins are conserved in metazoans. AXIN is an established scaffolding protein that acts to integrate inputs from multiple signaling molecules, leading to the regulation of downstream effectors.2 AMPK plays a crucial role in sensing intracellular energy levels and keeping a balance between cellular metabolism and growth.3
Recent findings from our lab and other published studies involving AXIN and its interacting partner AMPK provide a potential clue into the mechanism of muscle health maintenance. Work in the nematode C. elegans has revealed that the AXIN family member PRY-1 is necessary for animals’ normal motility and health, and its activated form promotes longevity by maintaining muscle mitochondrial homeostasis.4 A similar function was previously ascribed to the AMPK catalytic subunit homolog AAK-2.5,6 The genetic and biochemical experiments revealed that PRY-1 and AAK-2 work together, likely through protein-protein interaction, and PRY-1 is required for AAK-2-mediated beneficial effect on muscle health and lifespan (Figure 1). The interaction between PRY-1 and AAK-2 is not a unique phenomenon, as other AXIN family members also interact with AMPK in different biological contexts. For example, another C. elegans AXIN homolog AXL-1 forms a complex with AAK-2 following metformin treatment. Here, AXL-1 is necessary for metformin-mediated lysosomal localization and activation of AAK-2 in a VHA-3-LMTR-3-PAR-4 (v-ATPase-Ragulator-LKB1) complex dependent manner7 (Figure 2).
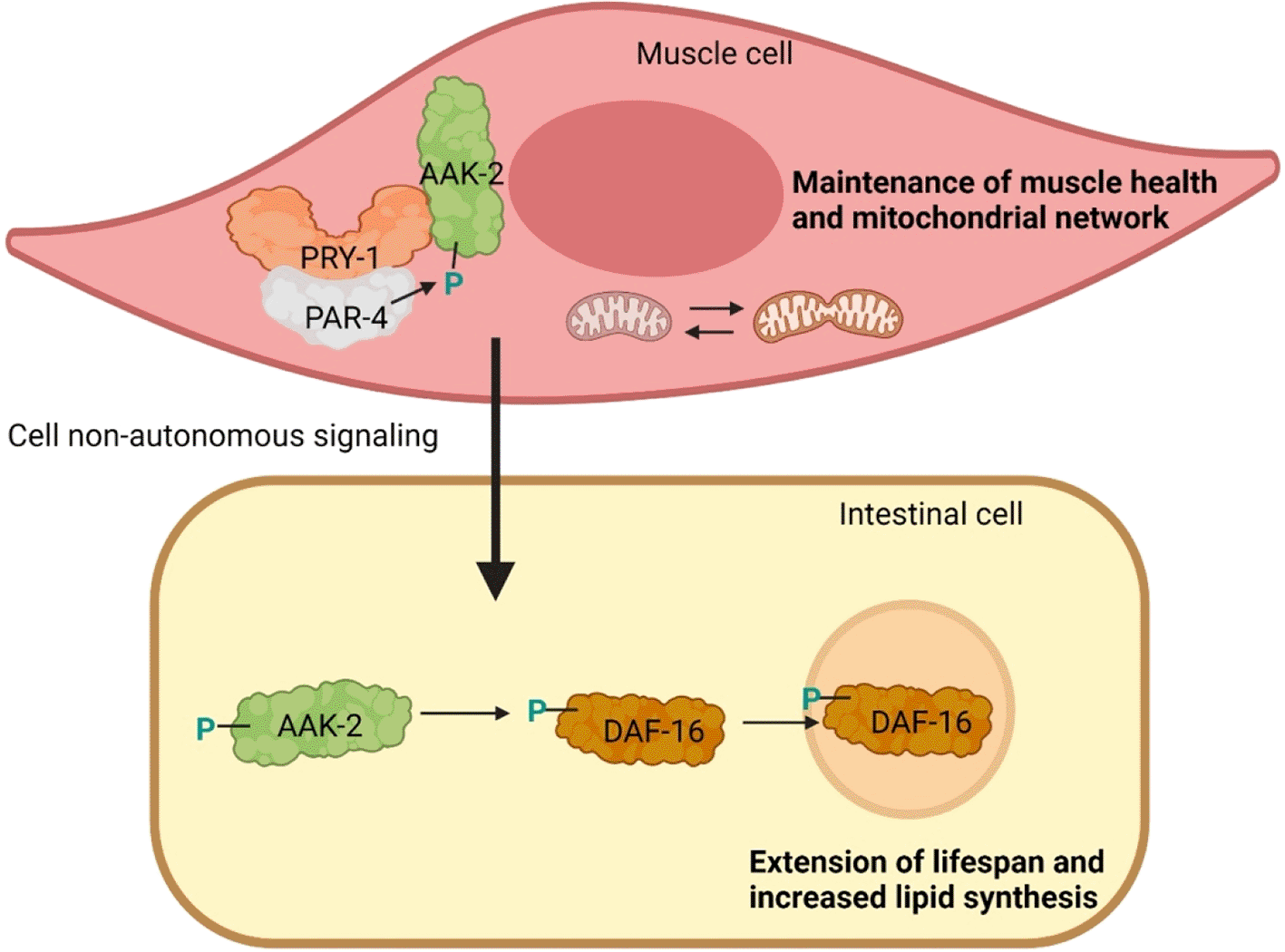
Genetic and biochemical studies have shown that PRY-1/AXIN interacts with PAR-4/LKB1 and AAK-2/AMPK in muscles to promote AAK-2/AMPK phosphorylation. AAK-2 in turn activates DAF-16/FOXO cell non-autonomously in the intestine and promotes DAF-16/FOXO nuclear localization. Green colored P indicates activating phosphorylation.
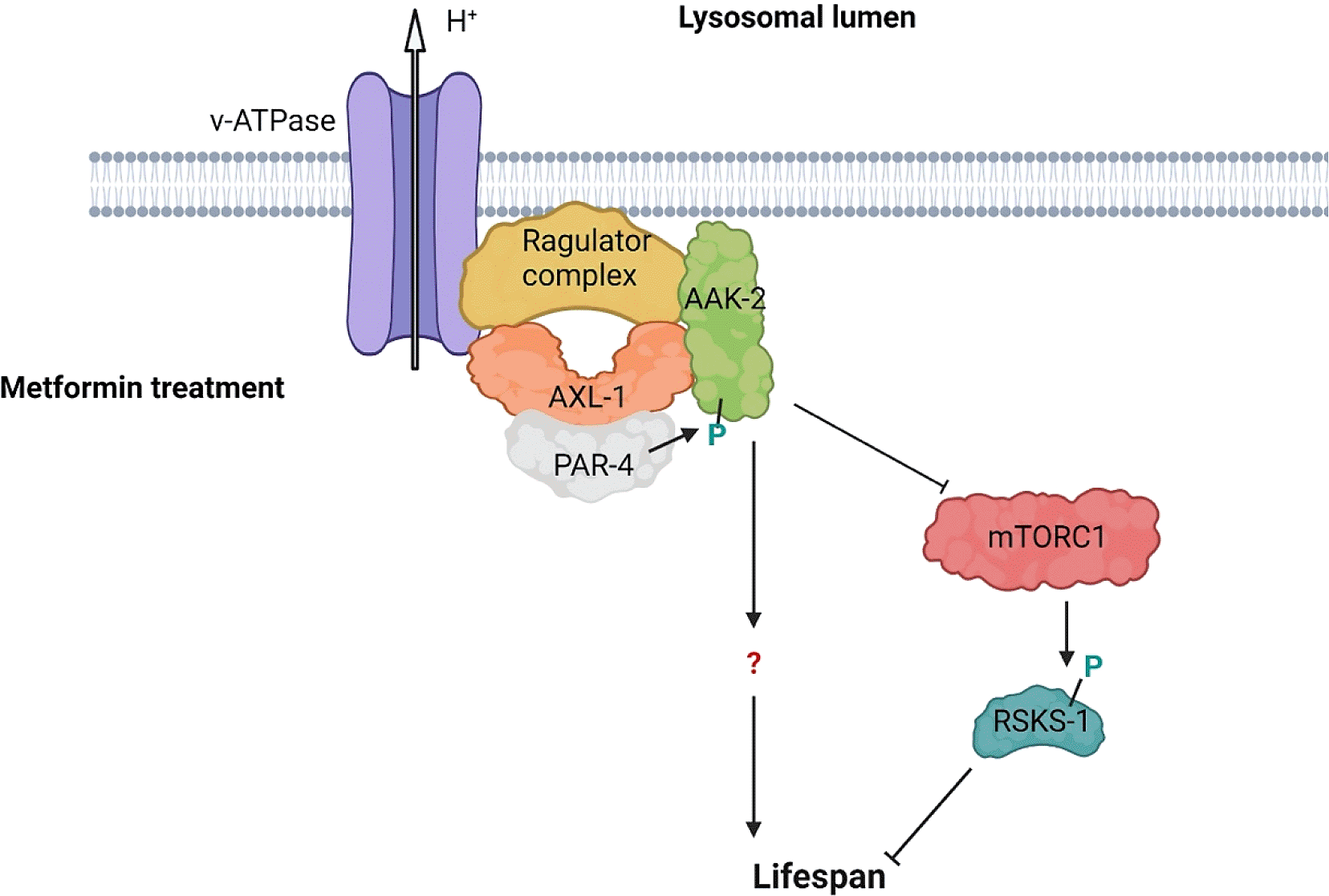
Metformin treatment induces formation of AXL-1/AXIN-based lysosomal VHA-3-LMTR-3-AXL-1/PAR-4-AAK-2 (v-ATPase-Ragulator-AXIN/LKB1-AMPK) complex that is required for AAK-2/AMPK activation. The effect of metformin is partially attained via inhibition of mTORC1, but other targets of the pathway remain unknown. Green colored P shows activating phosphorylation.
The Axin-containing complexes are also reported in mammalian systems. Following metformin treatment and glucose deprivation,8–10 the AXIN-based lysosomal pathway, consisting of v-ATPase-Ragulator complex (v-ATPase-Ragulator-AXIN/LKB1-AMPK), promotes AMPK phosphorylation by LKB1, leading to AMPK activation. In a separate study involving myotubes and mice gastrocnemius muscle tissue, exercise stimulated both AMPK and Rac1 while increasing the cellular levels of AXIN1. Accordingly, reducing the AXIN1 function blocked GTP loading of Rac1, AMPK activation, and glucose uptake in the exercising muscles.11 Additionally, it was shown that muscle-specific knockout (KO) of the AXIN1-binding Ragulator subunit LAMTOR1 completely abolished treadmill exercise-stimulated AMPK activation in gastrocnemius muscle.10 Together, these data demonstrate the crucial role of AXIN tethering in activating AMPK, which promotes muscle metabolism and benefits linked to exercise.
Investigations of cellular mechanisms underlying AXIN and AMPK interaction have revealed a regulatory relationship that depends on AMP levels12 (Figure 3). While low glucose triggered AMP-dependent activation of AMPK through the AXIN-based lysosomal pathway, a modest increase in AMP resulted in AXIN-dependent activation of both lysosomal and cytosolic AMPK. Finally, extreme nutrient starvation or high AMP concentrations caused phosphorylation of AMPK independently of AXIN function.12
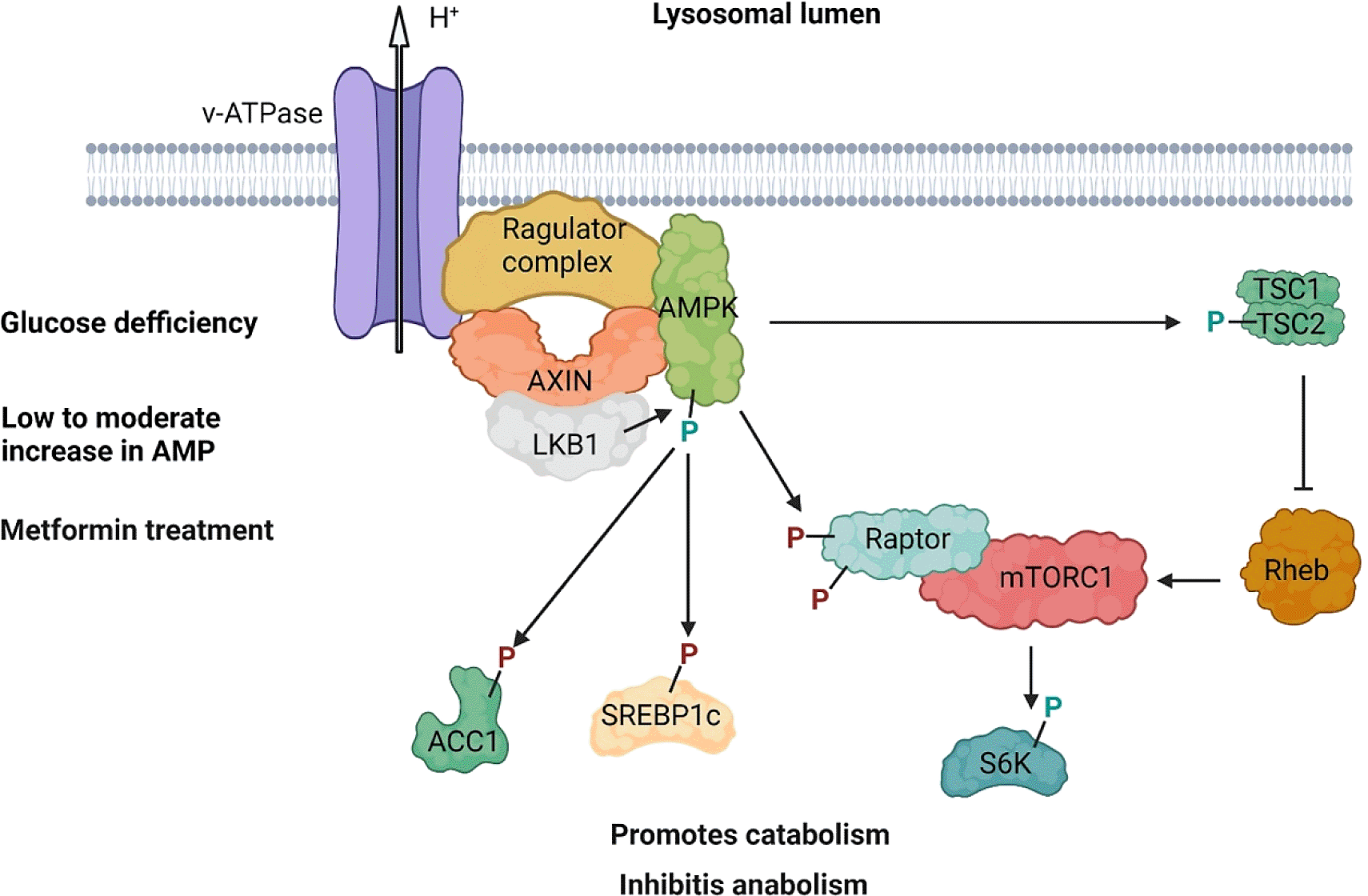
The complex is formed following glucose deprivation, low to moderate increases in AMP levels, and metformin treatment. Once activated, AXIN-AMPK signaling promotes catabolism and inhibits anabolism by phosphorylating downstream targets that include ACC1, SREBP1c, Raptor and TSC2. Green and purple colored P indicate activating and inhibitory phosphorylation, respectively.
Intriguingly, it was shown recently that skeletal muscle-specific AXIN1 knockout (AXIN1 imKO) mice are phenotypically normal and exhibited no impairment of AMPK regulation or glucose uptake.13 Such a phenotype may be explained by redundancies between AXIN1 and its homolog AXIN2. Both proteins are expressed in skeletal muscles, and AXIN2 can functionally replace AXIN1 in regulating AMPK.12,14 Moreover, AXIN2, a negative regulator of WNT signaling, appears to be essential for myogenesis, as increased WNT signaling in aged skeletal muscle promoted fibrogenesis, thereby accelerating aging.15–17
Consistent with the role of AXIN in AMPK activation and myogenesis, AMPK is shown to be crucial for regulating skeletal muscle development, growth, and degradation.18 In skeletal muscle, AMPK signaling has been linked to both acute and chronic exercise adaptations, in addition to a broad range of skeletal muscle disease states and ageing.19,20 Together these data support the growing evidence that both AXIN and AMPK and their signaling cascade are crucial to maintaining healthy muscles and slowing organismal deterioration with aging.
Studies in mammalian models revealed that both metformin and glucose deprivation inhibit the mechanistic target of rapamycin complex 1 (mTORC1) activity, a master regulator of anabolic pathways.8,10 Both these treatments cause the mTORC1 components, RAPTOR and mTOR, to dissociate from the v-ATPase-Ragulator and facilitate the formation of the v-ATPase-Ragulator-AXIN/LKB1-AMPK complex. Similarly, research in C. elegans has demonstrated that the VHA-3-LMTR-3-AXL-1/PAR-4-AAK-2 complex negatively regulates phosphorylation of the mTORC1 target S6 kinase B1 (S6K) homolog RSKS-1.7 As the beneficial effects of AXIN-AMPK signaling in the C. elegans study were not directly attributed to mTORC1 inhibition, the authors suggested that the signaling cascade may utilize additional factors7 (Figure 2).
The downstream effectors of AXIN-AMPK have been reported in several other studies. Specifically, in a low glucose condition, the pathway phosphorylates proteins such as acetyl-CoA carboxylase (ACC1) and endoplasmic reticulum-localized sterol regulatory element-binding protein-1c (SREBP1c), thereby inhibiting fatty acid synthesis12 (Figure 3). Interestingly, in C. elegans, PRY-1 promotes transcription of SREBP1 homolog SBP-1 to regulate fatty acid synthesis4,21; however, the precise mechanism of this regulatory relationship is unknown. Another effector of PRY-1 appears to be the CREB-regulated transcriptional coactivator (CRTC) homolog.22 CRTC-1 is known to function downstream of AAK-2 and affects calcineurin-mediated lifespan and stress regulation in C. elegans.23,24 While AMPK and calcineurin signaling in mammalian systems regulate CRTCs in an antagonistic manner, the involvement of Axin in this regulatory network remains to be determined.25–28
Given that AMPK regulates many targets, it is expected that a subset may be co-regulated by AXIN. We recently reported that both pry-1 and aak-2 mutant transcriptomes significantly overlap with mutually up and downregulated genes. These common differentially expressed genes are associated with muscle structure development, muscle contraction, aging, and lipid metabolism. Moreover, we found that PRY-1-AAK-2 signaling functions in muscles leading to activation of AAK-2 in a cell-non-autonomous manner and phosphorylation and translocation of the FOXO transcription factor homolog DAF-16 into the intestinal cell nuclei4 (Figure 1). These results are supported by previous studies showing that activated DAF-16 is indispensable for muscle mitochondria homeostasis and lifespan extension. It is worth mentioning that FOXO3 is also phosphorylated by AMPK in the mammalian system; however, the involvement of AXIN in this process and the function of activated FOXO3 are unknown.29
Unlike C. elegans, little is known about the role of AXIN and AMPK in regulating muscle health in another leading invertebrate model, namely the fruit fly D. melanogaster. Overexpression of D-axin in wing disc-associated myoblasts in larvae causes partial to complete loss of indirect flight muscles.30 However, the precise role of D-axin and the involvement of AMPK and TORC1 in adult muscles is unknown. In terms of other processes, it has been reported that a hypomorphic allele of D-axin alters the expression of metabolic genes and is hypersensitive to metabolic stress induced by fasting. Such a phenotype depends on TORC1 activity and involves increased ROS production.31
While much has been learned about Axin, AMPK, and their interactions, there are gaps in our understanding of the mechanisms regulating the complex formation, downstream effectors, and their role in maintaining muscle health. Some of the relevant questions are discussed below.
The existing data supports that AXIN function in the muscle is beneficial. AXIN2 is required for myogenesis and linked to muscle aging, whereas AXIN1 mediated signaling is necessary for glucose uptake in the exercising muscles.11,15,17 Both AXIN1 and AXIN2 are expressed in the skeletal muscle. Research in C. elegans hints that muscle-specific overexpression of pry-1 promotes mitochondrial network, muscle development, and muscle physiology.4 Whether such a role of Axin is conserved in higher eukaryotes is unknown.
While AXIN1 and AXIN2 possess similar domains, they show differences in their regulation and expression pattern (subcellular localization and cell type-specific expression).14,32 Additionally, AXIN2 is required for muscle development. Interestingly, exercise-induced glucose uptake requires AXIN1 in skeletal muscles. While it remains to be seen whether AXIN2 plays a redundant role in this process and regulates AMPK, Li et al.13 reported no change in AMPK activation following AXIN1 imKO in the skeletal muscle. Furthermore, Zong et al.12 showed that AXIN2 could substitute AXIN1 in forming a complex between LKB1 and AMPK.
In C. elegans, PRY-1 and AXL-1 possess the characteristic domains for the AXIN family of proteins2 and negatively regulate WNT signaling.33,34 It has been shown that AXL-1 functions redundantly with PRY-1 to regulate the WNT effector protein BAR-1/β-catenin during the formation of the vulva and migration of Q neuroblast. However, both AXINs are functionally not equivalent and play roles independently to control specific processes. For example, PRY-1 is necessary for lipid metabolism, healthspan, lifespan, and seam cell development, whereas AXL-1 regulates excretory cell development.4,21,33–36 Recent experiments from our lab also highlight functional differences between the two Axin proteins. While PRY-1 and AXL-1 are necessary for metformin-induced lifespan extension,7 only PRY-1 is required for glucose deprivation mediated longevity in C. elegans (Mallick et al., unpublished). These same treatments, i.e., metformin and glucose deprivation, are known to extend the lifespan in an AAK-2-dependent manner.37,38 Overall, these studies demonstrate that AXIN homologs in every system have shared as well as unique functions. However, whether these proteins can redundantly activate AMPK remains to be investigated.
Recent reports demonstrate that the lysosomal AXIN-AMPK signaling can be activated by glucose deprivation independently of AMP/ATP ratios. However, the medium-to-high elevation of AMP extends the activation of both cytosolic and lysosomal AMPK, which is also dependent on AXIN1.12 By contrast, very high AMP levels phosphorylate AMPK in a manner that does not involve AXIN1 and probably occurs via a conformational change in AMPK. Whether AXIN-dependent activation of AMPK also requires a similar change in AMPK conformation is unclear. Furthermore, it is unknown how glucose levels facilitate the complex formation and differential activation of AMPK by LKB1.
Several other factors may also limit AXIN and AMPK mediated signaling. One of these is post-translational modification. AXIN activity is known to be regulated by phosphorylation.2,39,40 Another could be subcellular localization. While the AXIN-AMPK complex is localized to lysosomes and cytoplasm, the changes in their activities in response to external stimuli are poorly understood.10,12 Both factors are broadly expressed and in overlapping domains; however, whether their interactions are global or restricted to specific tissues remains to be determined. In this regard, it is worth mentioning that AMPK functions cell non-autonomously in C. elegans,24 and we have reported that the protein is needed in both muscles and intestine to mediate beneficial effects of constitutive expression of AXIN in muscles.4
Given that AMPK is involved in many different processes and regulates many downstream targets, one might expect that AXIN-AMPK interaction co-regulates a subset of the targets. In support of this, a recent paper suggests that AXIN-AMPK signaling phosphorylates targets that are different from ATP/AMP-dependent AMPK signaling.12 As mentioned above, our analysis of C. elegans pry-1 and aak-2 transcriptomes has revealed many overlapping genes that are differentially expressed. However, more work is needed to identify and validate common targets of AXIN-AMPK signaling that are involved in maintaining muscle health in different systems. Identification of such target genes could lead to a better understanding of molecular mechanisms underlying the signaling network and the development of diagnostic markers and therapeutic interventions to promote muscle health.
We envisage several exciting research avenues involving AXIN-AMPK signaling. While substantial knowledge has been gained in terms of processes that each one participates in and mechanisms underlying their function, little is known how the interactions between the two proteins are regulated, leading to changes in the expression of target genes that carry out various roles. Below are some of the potential research directions to address the questions in the previous section.
While it has been shown that the AXIN homologs in both C. elegans (PRY-1 and AXL-1) and mammalian systems (AXIN1 and AXIN2) can activate AMPK,4,7,9,12 the redundancies between the homologs and their tissue-specific interactions with AMPK are unknown. Moreover, the differences in lifespan and lipid metabolism phenotypes between the two AXIN mutants in C. elegans raise the question of functional equivalency regarding AMPK activation in physiological conditions. Future research along these lines should refine our understanding of AXIN-AMPK signaling and its conservation in eukaryotes.
Depending on the context, signaling pathways may utilize different mechanisms to regulate their responses. In this regard, research in the following areas should improve our understanding of the regulatory mechanism of AXIN-AMPK signaling. First, whether a conformational change in AMPK following AXIN binding occurs similar to the AMP-dependent mechanism. Second, the role of post-translational modification of AXIN in activating AMPK. Third, identifying a specific region of the multidomain AXIN protein required for AMPK interaction that, in turn, may uncover potential competitors to modulate the signaling. And, finally, the discovery of factors affecting subcellular localizations of both AXIN and AMPK and, in turn, their interactions.
Other modes of regulation of AXIN-AMPK signaling may include spatial and temporal changes in AXIN expression. AXIN is not only a negative regulator but also a downstream target of the WNT signaling.21,41 Consistent with this, PRY-1/AXIN is required for MOM-2/WNT mediated lifespan regulation,4 and MOM-2 is expressed in the body wall muscles of C. elegans. It remains to be explored whether AXIN function in muscles is regulated in a WNT-dependent manner in eukaryotes.
Research from our group has shown that overexpression of PRY-1/AXIN in C. elegans extends the lifespan and improves muscle health in older adults. Whether forced expression of mammalian AXIN in muscles may also promote the healthspan of animals by activating AXIN-AMPK signaling requires investigation. In line with this, expression analysis of AXIN1 and AXIN2 in old adults and patients with a muscle disease should prove valuable.
As mentioned above, AXIN and AMPK are crucial for muscle development and physiology. Furthermore, exercise promotes the activation of AMPK in an AXIN-dependent manner. Given that exercise promotes muscle health and delays aging,42–44 it is conceivable that AXIN and AMPK are involved in this process. More work is needed to understand the role of AXIN-AMPK signaling in exercise-mediated benefits.
AXIN family of scaffolding proteins control a wide array of cellular processes by recruiting multiple factors and forming protein complexes. One of the interactors of AXIN is the well-known energy sensor AMPK. AMPK functions as a nexus between energy conservation and aging, and perturbations of its function lead to various age-related pathologies. AXIN-AMPK signaling promotes muscle health and delays age-associated deterioration. Future studies on the pathway, its interacting proteins, and tissue-specific effectors hold promise to uncover key candidates that may be targeted in the future to delay age-associated muscle degeneration and improve muscle health during aging.
Authors thank Gupta lab members for discussions on the topic and feedback on the manuscript. Figures were created using BioRender.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the opinion article discussed accurately in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Are arguments sufficiently supported by evidence from the published literature?
Yes
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: C. elegans, transcription, metabolism, stress response, longevity,
Is the topic of the opinion article discussed accurately in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Are arguments sufficiently supported by evidence from the published literature?
Yes
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Genetics, C elegans, neural circuit development, neuronal regeneration, aging
Is the topic of the opinion article discussed accurately in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
Partly
Are arguments sufficiently supported by evidence from the published literature?
Partly
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Metabolic signaling pathways; structural biology; cell biology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 08 Dec 21 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)