Keywords
COVID-19; therapeutic plasma exchange; cytokine storm; treatment
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Coronavirus (COVID-19) collection.
COVID-19; therapeutic plasma exchange; cytokine storm; treatment
Since first reported in December 2019,1 coronavirus disease 2019 (COVID-19) has become an unresolved global pandemic. The challenge of the pandemic management at the present time might be due to the fact that a number of mutations have occurred making the virus more transmissible and causing critical illness.2 The World Health Organization (WHO) has established a living guideline on drugs for the management of COVID-193 and updated it periodically. However, the treatment of critically ill COVID-19 patients remains a serious issue.4 Patients critically ill with COVID-19 have been widely reported to have a poor prognosis, and theory reveals that cytokine storm might underlie this mechanism. In a cytokine storm excessive accumulation of pro-inflammatory cytokines might be responsible for the poor prognosis of COVID-19 patients. No study has found an effective treatment for the management of a cytokine storm in patients critically ill with COVID-19. Therefore, an investigation into the treatment that acts to remove these pro-inflammatory cytokines, for example, using therapeutic plasma exchange (TPE) may be required.
Since first introduced in 1952, TPE has been shown to provide an excellent outcome in patients with multiple myeloma to control hyperviscosity.5 Moreover, the implementation of this therapeutic treatment has also been reported in an Escherichia coli outbreak,6 a Shigella infection,7 infectious toxicosis,8 and septic shock with multiple organ failure9; and reduced risk of mortality was revealed. In the case of COVID-19, the US Food and Drug Administration (FDA) has posited that TPE may have a role as a rescue therapy in critically ill patients with COVID-19.10 However, insufficient evidence has resulted in indecision in applying TPE for the management of critically ill COVID-19 patients. To date, TPE has been studied in Oman,11 Turkey,12 Pakistan,13 and Saudi Arabia.14 However, contradictory findings exist. Therefore, our study aimed to assess the potential of TPE in reducing mortality of critically ill COVID-19 patients using a meta-analysis approach. The findings might add new insight and clarify the true potency of TPE for treating patients critically ill with COVID-19.
From March to August 2021, a meta-analysis following the protocols of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)15 was conducted to evaluate the effectiveness of TPE in reducing the mortality rate of critical COVID-19 patients. The PRISMA checklist in our present study is presented as extended data in Figshare.16 The major databases including PubMed, Scopus, and Web of Science were used to search for potential articles.
Inclusion and exclusion criteria were defined to assess relevant articles. The inclusion criteria of the study were (1) observational or randomized controlled trial studies, (2) having adequate information to calculate the potential association and effect estimates, and (3) applying a well-defined methodological approach to establish a COVID-19 diagnosis. All case reports, case series, letters to the editor, reviews, and commentaries, as well as studies with pre-post test comparison, and poor-quality methodology assessed with the Newcastle-Ottawa scale (NOS) were excluded.
The source databases used in our study were PubMed, Scopus, and Web of Science. We restricted the searching period up to 28 July 2021, and the language was English only. The Medical Subject Headings were: (“COVID-19” or “SARS-CoV-2”) and (“plasma exchange” or “therapeutic plasma exchange” or “TPE”). The reference lists of all potential related articles were also assessed to retrieve additional relevant articles. Data extraction was performed for all included papers, including: (1) name of the first author; (2) year of publication; (3) country of origin; (4) sample size of cases and controls, (5) TPE, and (6) mortality rate between groups.
All included articles were assessed for their quality using NOS for observational studies17 and the Jada-modified scale for RCTs.18 The article quality was interpreted as low, moderate, and high. Low quality articles were excluded from our study. The assessment was performed by two independent authors (MI, HAM), and when a discrepancy was observed an assessment by a senior researcher (JKF) was conducted.
The main outcome of the study was all causes of mortality among critical COVID-19 patients treated with and without TPE. The diagnosis of COVID-19 was established by using RT-PCR of SARS-CoV-2 RNA from nasal or oropharyngeal swab samples, and critical COVID-19 patients were defined by following the guideline (requires life sustaining treatment, acute respiratory distress syndrome, sepsis, or septic shock).3,19
The calculation of potential publication bias, heterogeneity among studies, and the association between the use of TPE and the risk of mortality among patients with COVID-19 were assessed using an Egger test, a Q test, and a Z test; respectively. The Egger test with a p-value more than 0.05 indicated the presence of potential publication bias. Moreover, the heterogeneity among studies was considered when the p-value of a Q test indicated less than 0.10. The pooled association was calculated using a Z test, where the p-value of less than 0.05 indicates a significant association. The estimated effect was presented as an odds ratio with 95% confidence interval (OR 95% CI). The cumulative calculation was presented as a forest plot. An R package software (R Studio version 4.1.1, MA, USA, (RRID:SCR_000432) was used to perform the analyses.
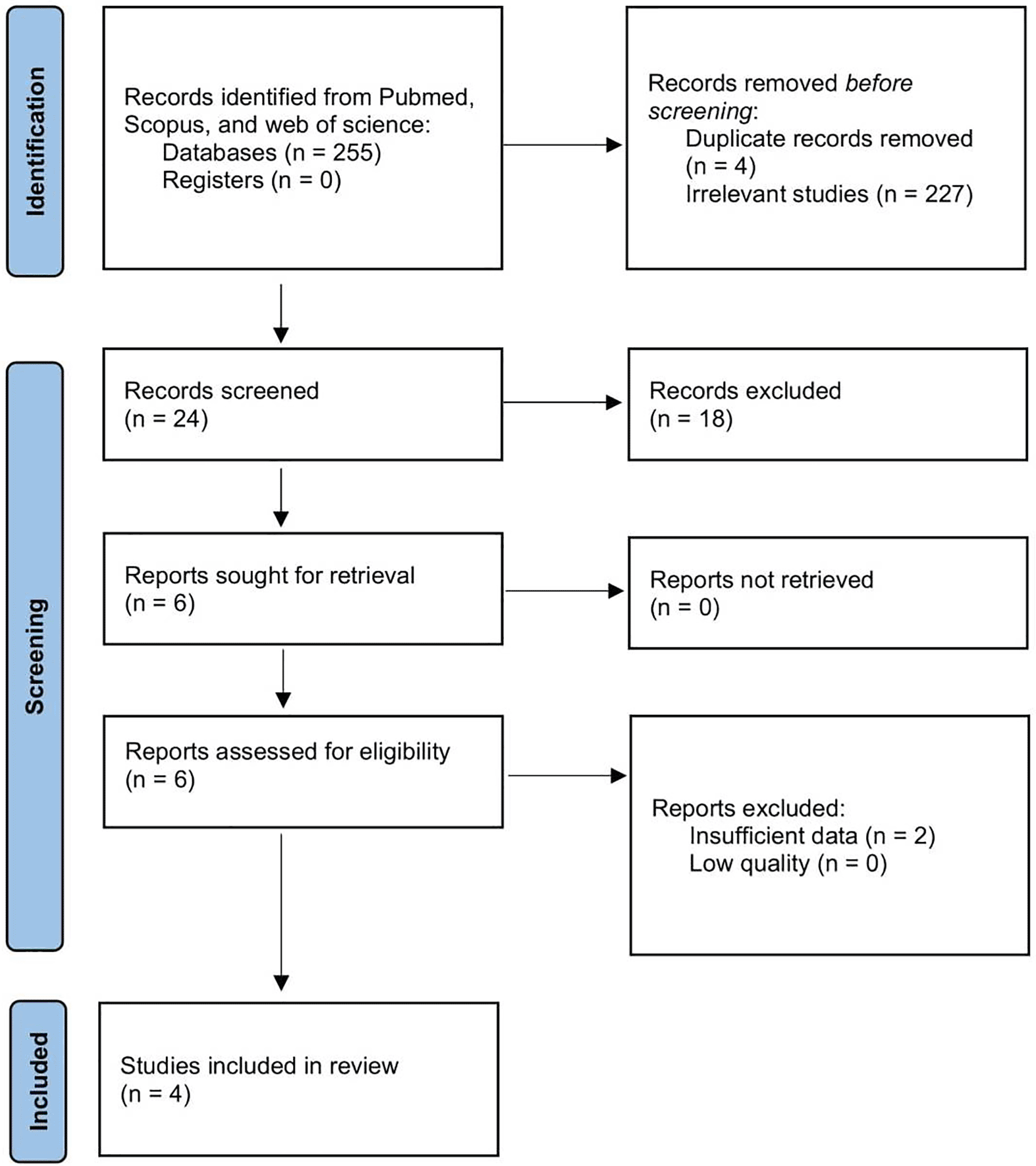
We identified a total of 255 papers. Among them, four papers were excluded due to duplication and additional 227 papers due to irrelevant context. There were 24 papers in total included for full-text assessment. Then, 20 of the 24 papers were further excluded since 18 were reviews and case reports and two papers had insufficient data. Four papers were included in the final analysis.11–14 The article selection PRISMA flowchart is presented in Figure 1 and the baseline characteristics are described in Table 1.
| Study and year | Country | Study design | Quality | TPE | Control | ||
|---|---|---|---|---|---|---|---|
| Total | Mortality | Total | Mortality | ||||
| Khamis et al., 202011 | Oman | Cohort Retrospective | High | 11 | 1 | 20 | 9 |
| Gucyetmez et al., 202012 | Turkey | Cohort Retrospective | Moderate | 12 | 1 | 12 | 7 |
| Kamran et al., 202013 | Pakistan | Cohort Retrospective | High | 45 | 4 | 45 | 18 |
| Faqihi et al., 202014 | Saudi Arabia | RCT | High | 43 | 9 | 44 | 15 |
A total of 111 COVID-19 patients treated with TPE and 121 COVID-19 patients without TPE, retrieved from three retrospective cohort studies and one RCT, were included in our analysis. Our results found that COVID-19 patients treated with TPE had reduced mortality rate compared to COVID-19 patients without TPE treatment (OR: 0.21; 95% CI: 0.05, 0.85) (Figure 2).
Our analysis revealed the absence of the evidence of heterogeneity. Therefore, we applied a fixed-effect model to assess the correlation. For the potency of bias assessment across the studies, our analysis using an Egger test found no publication bias.
Our study identified that TPE treatment in critically ill COVID-19 patients reduced the mortality rate. To date, our study is the first meta-analysis to report on the use of TPE for the management of COVID-19. In our analyses, we included four studies from Oman,11 Turkey,12 Pakistan,13 and Saudi Arabia14; and all reports revealed similar findings in which TPE treatment reduced mortality among patients with COVID-19. TPE has been applied and proved to reduce the risk of mortality in the management of several infectious diseases, such as Escherichia coli O157:H7-associated hemolytic uremic syndrome,6,20 Shigella infection,7 infectious toxicosis,8 HIV infection,21 peripheral HIV neuropathy,22 Kaposi's sarcoma,21 disseminated cryptococcosis,23 and septic shock with multiple organ failure.9 Moreover, in the case of the Escherichia coli O157 outbreak in 1996, TPE proved beneficial in the reduction of mortality.6 Therefore, as suggested in our study, TPE might possess potential benefits in COVID-19 treatment.
The precise mechanism of how TPE benefits COVID-19 patients remains debatable. In critical COVID-19 patients, the excessive accumulation of cytokines may occur, and this can lead to a fatal outcome. Previous studies have revealed that the levels of pro-inflammatory cytokines/chemokines including interleukin-2 (IL-2), interleukin-6 (IL-6), granulocyte colony stimulating factor (GCSF), IFN-γ inducible protein 10, monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1A, tumor necrosis factor-α (TNF-α) were observed to be higher in patients critically ill with COVID-19 compared to those with mild-moderate disease.24,25 TPE is a therapeutic procedure principally acting to remove (through double filtration) molecules of 60–140 nm in size.5 The molecule size of pro-inflammatory cytokines/chemokines is 80–220 nm.26 Therefore, the elimination of pro-inflammatory cytokines/chemokines, proven to affect those critically ill with COVID-19 might provide benefits to improve the prognosis of COVID-19 patients. Moreover, a previous study also reported that TPE played an important role in eliminating toxic substances by suppressing the cytokine release syndrome.27 It was also suggested that TPE plays a crucial role in restoring normal substances that may be deficient in the plasma,5 leading to stabilization and restoration of endothelial membranes.28 Another possibility is when fresh frozen plasma was used in fluid replacement; TPE was associated with the improvement of coagulopathy in COVID-19 patients.29 Previous evidence suggests that TPE might play an important role in maintaining the balance between anti and pro-inflammatory cytokines in the plasma, and might rectify the prognosis in patients with COVID-19, as reported in our study.
To the best of our knowledge, our study is the first meta-analysis reporting the benefit of TPE in reducing the mortality rate of critically ill COVID-19 patients. We found that COVID-19 patients treated with TPE had a lower risk of mortality compared to those without TPE treatment. Since COVID-19 guidelines suggest that the use of TPE for patients with COVID-19 should be carefully implemented as the evidence of TPE efficacy was only limited to a case report,3 our current findings might strengthen the evidence that the use of TPE is effective in reducing the risk of mortality among patients with COVID-19. However, in real-world implementation, special settings such as appropriate condition, target of treatment, potential complications, and particular case or comorbidity should be investigated.
Since this is the initial evidence on the potential efficacy of TPE for the management of COVID-19, several limitations should be highlighted. First, we did not include any potential confounding factors such as comorbidity, the levels of proinflammatory cytokines, and onset of disease course to describe the association between TPE and the risk of mortality rate. Second, a limited number of investigations on the use of TPE in COVID-19 management resulted in our study including only a limited number of articles. Therefore, further investigation involving a larger sample size is required. Third, the clinical setting on the use of TPE might differ among studies, and therefore, this variation might also govern the potency of bias. Fourth, among the included studies, we obtained only one randomized control trial (RCT) and three observational studies. Further meta-analyses involving only RCT studies might provide better levels of evidence.
The data suggests that the use of TPE for the management of critically ill COVID-19 patients could reduce the mortality rate. The application of TPE for the management of COVID-19 should be considered in well-equipped hospitals.
All data underlying the results are available as part of the article and no additional source data are required.
Figshare: PRISMA checklist for ‘the association between therapeutic plasma exchange and the risk of mortality among patients with critically ill COVID-19: a meta-analysis. https://doi.org/10.6084/m9.figshare.16622572.v116
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We thank Lembaga Pengelola Dana Pendidikan (LPDP) Republic of Indonesia for supporting this project.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
No
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Nees more methodological clarification.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: This reviewer previously published an article with the authors in December 2021, one month before their review on this article. The reviewer confirms that this was an international project with multiple authors and their previous collaboration did not bias their review in any way.
Reviewer Expertise: clinical outcomes and evidence synthesis
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: This reviewer previously published an article with the authors in December 2021, one month before their review on this article. The reviewer confirms that their previous collaboration did not bias their review in any way.
Reviewer Expertise: Public health, epidemiology, statistics
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: This reviewer previously published two articles with the authors in August and December 2021, prior to their review on this article. The reviewer confirms that this was a genuine mistake as they had not previously collaborated with the corresponding or first authors of this article. This did not bias their review in any way.
Reviewer Expertise: Immunology of infectious diseases including COVID-19, HIV/AIDS and Opportunistic infections
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 1 14 Dec 21 |
read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)