Keywords
Functional Food, Siamese Pumpkin, Tuna, HbA1c, HDL-C, Chol/HDL Ratio, Prediabetes, Dyslipidemia
This article is included in the Agriculture, Food and Nutrition gateway.
This article is included in the The Role of Nutrition in Healing and Improving Health collection.
Functional Food, Siamese Pumpkin, Tuna, HbA1c, HDL-C, Chol/HDL Ratio, Prediabetes, Dyslipidemia
We have replaced and simplified the sentence "Before and after the intervention, pre-test and post-test examinations were performed, factors examined including hemoglobin A1c (HbA1c), lipid profile, blood pressure, food intake with the SQ-FFQ interview, BMI, and physical activity using IPAQ-SF. During the intervention, blood pressure and BMI data were taken before and after the study. Statistical analysis included descriptive tests, independent and paired T-tests, and analysis of variance (ANOVA)." with "Pre-test and post-test measurements were performed for body mass index (BMI), physical activity (using IPAQ-SF), hemoglobin A1c (HbA1c), lipid profile, blood pressure, and food intake (using SQ-FFQ) . Statistical analyzes performed included descriptive tests, independent and paired T-tests, and analysis of variance (ANOVA)."
Prediabetes is a condition that initiates the incidence of diabetes mellitus (DM), and its prevalence continues to increase.1 This condition increases the risk of type 2 DM by about 3-10 times, and other chronic diseases such as cardiovascular disease by 1.5 times, compared to healthy people.2 In addition, cardiovascular disease due to atherosclerosis of the walls of blood vessels and thrombosis is one of the leading causes of death in Indonesia.3 One of the main risk factors is a degenerative disease, such as hypertension, DM or dyslipidemia. Cardiovascular diseases, especially dyslipidemia and type 2 DM, are diseases whose risk factors increase with prediabetes.2 Nevertheless, prediabetes is a condition that can be managed by changing lifestyle, losing weight, regulating diet, and doing regular exercise.2
Treatment of prediabetes and diabetes using plant extracts (herbal medicine) and functional foods has been widely used since ancient times in various countries, one such plant is the siamese pumpkin (Sechium edule (Jacq.)).4 Experimental animal studies have been conducted extensively and the results support the positive effects of the siamese pumpkin extract on cardiovascular and glucose metabolism.5 The siamese pumpkin is a natural ingredient that has antioxidant, antimicrobial, diuretic, antihypertensive and hypocholepertensive effects, as well as hypoglycemic effects.6 Additionally, siamese pumpkin has cardioprotective effects that can help in the prevention and treatment of dyslipidemia, and can potentially be processed into several food products that have more economic value.6
In addition to siamese pumpkin, foods high in omega 3 fatty acids or eicosapentaenoic acid (EPA) and decosahexaenoic acid (DHA), such as tuna (Thunnus sp.) can also prevent atherosclerosis and even reduce the risk of obesity and other non-communicable diseases.7 Tuna is a good source of protein and omega 3 fatty acids that are good for heart health and help improve the lipid profile.8 In addition, omega 3 fatty acids affect glucose metabolism by changing cell membrane function, enzyme activity, insulin signals, and gene expression.8
It can be concluded that the combination of siamese pumpkin and tuna has potential nutritional content with protective against cardiovascular disease and type 2 DM. Therefore, this study tried to innovate both ingredients into functional foods in the form of powder as a diet for people with prediabetes and dyslipidemia. The study aimed to examine the effect of administering CHAGURO (a product name of a combination between siamese pumpkin and tuna-based powder) on serum levels of hemoglobin A1c (HbA1c), high-density lipoprotein cholesterol (HDL-C), and cholesterol/high-density lipoprotein (chol/HDL) ratio in prediabetes individuals with dyslipidemia.
The formulation used is the formulation selected (best) based on the analysis of macronutrients, micronutrients, and organoleptic substances that have been published in the journal of the Ministry of Health of the Republic of Indonesia.9-11 Here are the formulations:
1. Processing details
CHAGURO products are made from tuna and siamese pumpkins on the market, thus the basic ingredients are easy to find. Tuna aged 2-3 years with an average weight of 2.5 kg was used. The young siamese pumpkin was chosen because they tend to have a sweeter taste than the old one. The process of making CHAGURO products started with tuna that was cleaned of blood, thorns, head, gills, other body parts, and dirt. The tuna meat was steamed until cooked for about 25 minutes. Next, the tuna meat was mashed until it formed flakes.
The finely shredded siamese pumpkin is placed in the oven at 55 °C for 4 hours. Tuna and pumpkin that have dried and formed flakes are mixed in one container with a ratio of 3:1.9 The selection of tuna and pumpkin ratio was based on research done in mice. The study showed that a 3:1 ratio of tuna and pumpkin could have the best effect on HbA1c, triglycerides, HDL, low-density lipoprotein (LDL), and cholesterol levels in diabetic and dyslipidemia mice compared to other formulas.12 The 3:1 formula was the most preferred or likely formula chosen by 57 semi-trained panelists based on organoleptic tests.
2. Results of micronutrient, and organoleptic siamese pumpkin and tuna-based powder products (CHAGURO)
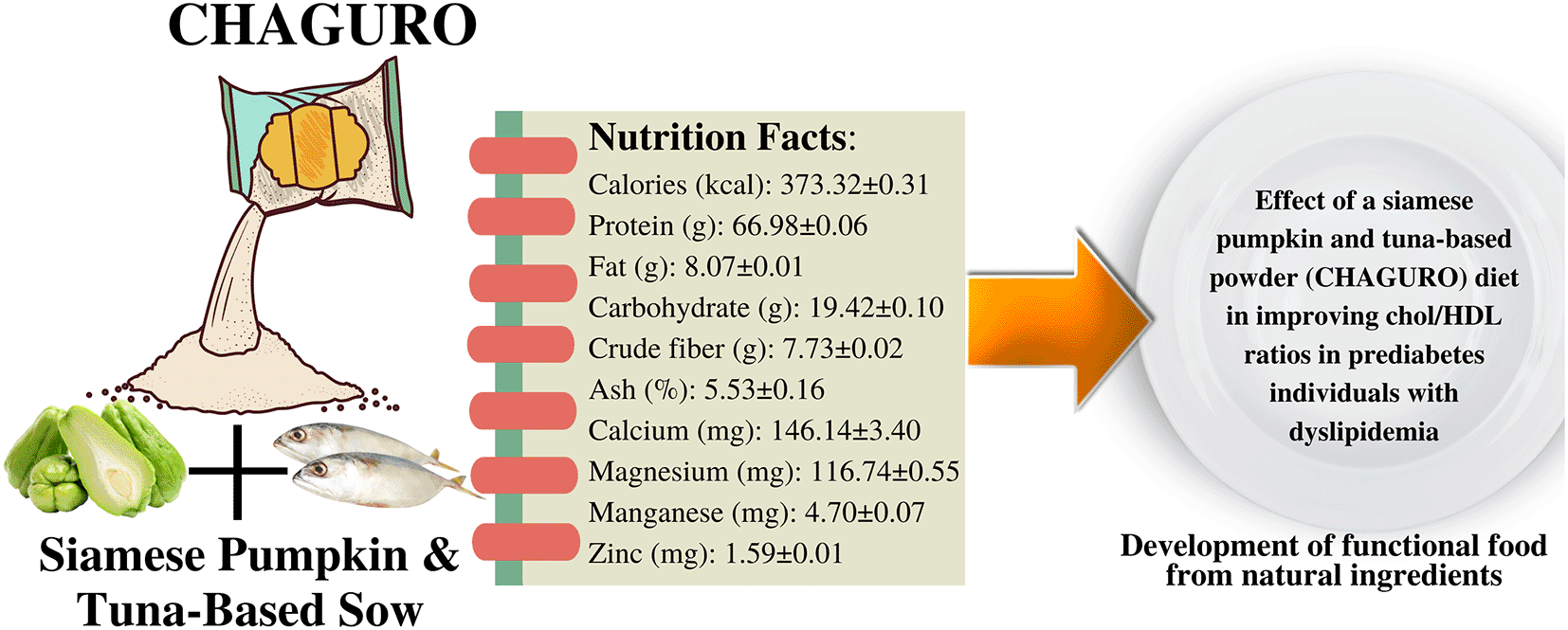
The results of the macronutrient tests in 100g of products showed that the selected CHAGURO contained Calories (kcal): 373.32 ± 0.31; Protein (g): 66.98 ± 0.06; Fat (g): 8.07 ± 0.01; Carbohydrate (g): 19.42 ± 0.10; Crude fiber (g): 7.73 ± 0.02; Ash (%): 5.53 ± 0.16; Calcium (mg): 146.14 ± 3.40; Magnesium (mg): 116.74 ± 0.55; Manganese (mg): 4.70 ± 0.07 and Zinc (mg): 1.59 ± 0.01. The levels of macronutrients and micronutrients in the CHAGURO products are in accordance with the standard SNI Abon (shredded fish) 01-3707-1995.11
The formulation of selected CHAGURO products were tested for effects through experimental intervention tests, involving respondents who passed the screening and have received ethical approval from The Medical and Health Research Ethics Committee (MHREC), Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada - Dr. Sardjito General Hospital, under trial protocol register No.KE/FK/0951/EC/2020 (August 27, 2020).12 Based on the calculation of samples using the formula designed by Lwanga & Lameshow,13 which was corrected with an attrition rate of 20%, samples for each group of 29 and 32 respondents were determined.
The study was used a clustered quasi-experimental design. This experimental test was conducted with two intervention groups (see Figure 1), where each group received a different dose of 7.5 g/70 kg body weight (BW)/day (low dose or LD) and 15 g/70 kg BW/day (high dose or HD) for 60 days. Respondents were divided into intervention groups using simple randomization. Before and after the intervention, a pre-test and post-test was performed that included a HbA1c examination, lipid profile, blood pressure, food intake using the SQ-FFQ (Semi Quantitative-Food Frequency Questionnaire) interview, body mass index (BMI, referring to the Asia Pacific), and physical activity based on the duration of time respondents used to move every 7 days. Physical activity data were measured using the IPAQ-SF (International Physical Activity Questionnaires-Short Form). Furthermore, the intervention period of the administration of the CHAGURO product runs, according to the group dose, for 60 days. During the intervention, blood pressure and BMI data were before and after the study, starting from August to October 2021. Statistical analysis includes descriptive tests, independent and paired T tests, as well as ANOVA.
All respondents were made aware of the research activities and signed an informed consent form. The inclusion criteria in this study (Figure 1) included the following: the respondents were over 30 years of age and less than 60 years of age, had a fasting blood sugar of 100-125 mg/dL and cholesterol of 200 mg/dL based on rapid tests, and domiciled in the Sleman Regency, Yogyakarta.
Exclusion criteria (Figure 1) included the following: respondents who were diagnosed with chronic diseases or took medications that affect HbA1c levels and blood lipids, women who were pregnant and/or breastfeeding or menopause. The dropout criteria were less than 80% compliance with CHAGURO products, experiencing dangerous conditions such as diarrhea or allergies, and the respondents died.
In the two public health centers (puskesmas) that we chose, 111 patients with DM2 were matched with the criteria and participated in 60 days of intervention at Sleman Regency, Yogyakarta. At the end of the recruitment, out of a total of 111 individuals who were included and randomized, 84 respondents were selected for this subgroup analysis. A total of 23 respondents experienced dropout during follow-up, including loss of follow-up for personal reasons (n = 16), dislike of fish smell (n = 6), and hospitalization (n = 1).
Blood samples were taken from veins and stored in a sample tube that already contained ethylene diamine tetra-acetic acid (EDTA) fluid so that blood does not freeze. Blood sampling was carried out by a registered analyst and samples were analyzed at the Prima Diagnostika Clinical Laboratory, KH. Wakhid Hashim, No. 27 Yogyakarta.
HbA1c was analysed using the immunoassay nephelometry method; triglycerides were analysed using Trinder, and HDL and LDL using the Direk (precipitation) method that compresses HDL and LDL using polyvinyl sulfate or heparin at low pH, HDL and LDL levels. These were calculated as a difference from the total cholesterol value and levels contained in the supernatants. Cholesterol examination used the CHOD-PAP method (Cholesterol Oxidase–Peroxidase Aminoantypirin) method with blanks and standards, the sample was then mixed and incubated at a temperature of 37 °C for 10 minutes, the sample absorbance reading and standard against blank was recorded in 60 minutes with a wavelength of 500 nm.
Statistical analysis includes independent and paired T-tests to analyze the effects of CHAGURO products on HbA1c, HDL-C, low-density lipoprotein cholesterol (LDL-C), triglycerides, and total cholesterol before and after the intervention. Qualitative data were analyzed using chi-squares and Mann-Whitney tests. Data were analyzed using Stata version 12.1 for Windows 8. All statistical tests were two-sided, with the significance level at 0.05.
This experimental study was conducted to determine the clinical effects of CHAGURO products as functional foods for individuals with prediabetes with dyslipidemia. This study is also follow-up from a previous study in experimental mice.10 In particular, the 60-day intervention of functional food CHAGURO could lower the chol/HDL ratio in our subjects. However, an in-depth exploration of the variables related to obesity is required, in order to understand the complex schemes or mechanisms of siamese pumpkin and tuna-based powder (CHAGURO) as a functional food which is both antiobesity and antidiabetic.
Dietary compliance was monitored through the number of CHAGURO packs consumed by respondents per week (Figure 2). The result showed that there were no significant differences in self-reported dietary compliance per week between both groups (p > 0.05).
Follow-up was conducted once every two weeks in the first and second months of the intervention. The duration of each follow-up session was about 5 minutes. Patients who did not comply with CHAGURO consumption, with a rate of less than 80% compliance, were excluded from the study.
The characteristics of the respondents in this study can be seen in Table 1 below.
| Variables | CHAGURO 7.5 g (n = 29) | CHAGURO 15 g (n = 32) | t/X2 | p |
|---|---|---|---|---|
| Mean ± SD/n (%) | Mean ± SD/n (%) | |||
| Demographic data | ||||
| Age (years) | ||||
| 25-45 | 12(41.38) | 16(50.00) | 0.455a | 0.500 |
| 46-60 | 17(58.62) | 16(50.00) | ||
| Gender | ||||
| Male | 27(93.10) | 22(68.75) | 5.709a | 0.017* |
| Female | 2(6.90) | 10(31.25) | ||
| Marital status | ||||
| Single | 1(3.45) | 1(3.13) | 0.005a | 0.944 |
| Married | 28(96.55) | 31(96.88) | ||
| Job employment | ||||
| Not employed | 10(34.48) | 19(59.38) | 3.779a | 0.052 |
| Employed | 19(65.52) | 13(40.63) | ||
| Education level | ||||
| College or more | 7(24.14) | 4(12.50) | 2.677a | 0.262 |
| Middle, high school | 16(55.17) | 24(75.00) | ||
| Less than primary school | 6(20.69) | 4(12.50) | ||
| Physical activity level | ||||
| Low | 11(37.93) | 11(34.38) | 0.854a | 0.652 |
| Moderate | 15(51.72) | 15(46.68) | ||
| High | 3(10.34) | 6(14.75) | ||
| Clinical data | ||||
| Height (cm) | 156.60 ± 4.58 | 154.20 ± 7.24 | 1.522b | 0.066 |
| Weight (kg) | 62.91 ± 11.66 | 66.13 ± 12.59 | −1.030b | 0.846 |
| BMI (kg/m2) | 25.64 ± 4.66 | 27.92 ± 4.95 | −1.843b | 0.964 |
| SBP (mmHg) | 132.31 ± 24.80 | 128.15 ± 19.89 | 0.267c | 0.789 |
| DBP (mmHg) | 81.03 ± 13.06 | 82.20 ± 12.76 | −0.731c | 0.435 |
| Waist circumference (cm) | 85.67 ± 10.97 | 85.98 ± 9.23 | −0.119b | 0.547 |
| Hip circumference (cm) | 101.40 ± 11.16 | 101.59 ± 10.80 | −0.065b | 0.525 |
| Smoking | ||||
| No | 28(96.55) | 26(81.25) | 3.506a | 0.061 |
| Yes | 1(3.45) | 6(18.75) | ||
| Hereditary diabetes mellitus | ||||
| No | 22(75.86) | 26(81.25) | 0.263a | 0.608 |
| Yes | 7(24.14) | 6(18.75) | ||
| Accompanying diseases | ||||
| No | 25(86.21) | 25(78.13) | 0.672a | 0.412 |
| Yes | 4(13.79) | 7(21.88) | ||
The general characteristics of the participants enrolled in each group is shown in Table 1. The two study groups were matched in terms of mean age, marital status, job employment, education level, and physical activity level. However, gender differ significantly between the two groups. Among the two groups, there were no significant differences in the baseline characteristic of anthropometric variables, blood pressure, hereditary diabetes mellitus, and the accompanying disease.
The changes in macronutrient and micronutrient intake are presented in Table 2. There was a significant difference in carbohydrate intake in the LD group before and after 60 days of intervention. This may result in bias because carbohydrate intake is not controlled. There was an insignificant decrease in fat intake in the HD group, and fiber intake in both groups. Other nutrients such as energy, carbohydrate, protein, cholesterol, sugar, and sodium intake increased at the end of the intervention.
| Variables | Pre-test | Post-test | pa |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Nutrition intake | |||
| Energy (kcal/day) | |||
| CHAGURO 7.5 g | 1059.15 ± 360.20 | 1263.46 ± 521.52 | 0.098b |
| CHAGURO 15 g | 1309.64 ± 448.00 | 1343.86 ± 478.89 | 0.477b |
| pd | 0.030* | 0.435 | |
| Carbohydrate (g/day) | |||
| CHAGURO 7.5 g | 153.70 ± 50.87 | 188.25 ± 78.20 | 0.023b,‡ |
| CHAGURO 15 g | 176.78 ± 67.91 | 188.95 ± 78.88 | 0.217b |
| pd | 0.157 | 0.828 | |
| Protein (g/day) | |||
| CHAGURO 7.5 g | 36.37 ± 17.92 | 41.58 ± 20.06 | 0.442b |
| CHAGURO 15 g | 42.81 ± 14.82 | 43.97 ± 15.97 | 0.708b |
| pd | 0.935c | 0.288 | |
| Fat (g/day) | |||
| CHAGURO 7.5 g | 36.03 ± 20.32 | 39.66 ± 23.42 | 0.738 |
| CHAGURO 15 g | 48.48 ± 23.45 | 42.47 ± 21.65 | 0.355b |
| pd | 0.038* | 0.544 | |
| Cholesterol (g/day) | |||
| CHAGURO 7.5 g | 140.41 ± 158.85 | 197.66 ± 175.09 | 0.173b |
| CHAGURO 15 g | 119.59 ± 104.12 | 147.12 ± 143.7 | 0.687b |
| pd | 0.965 | 0.382 | |
| Fiber (g/day) | |||
| CHAGURO 7.5 g | 13.66 ± 18.45 | 11.74 ± 16.45 | 0.393b |
| CHAGURO 15 g | 9.22 ± 3.9 | 9.17 ± 4.02 | 0.476 |
| pd | 0.544 | 0.822 | |
| Sugar (g/day) | |||
| CHAGURO 7.5 g | 22.47 ± 15.85 | 28.44 ± 25.85 | 0.721b |
| CHAGURO 15 g | 30.37 ± 29.42 | 32.78 ± 29.55 | 0.574b |
| pd | 0.389 | 0.506 | |
| Sodium (mg/day) | |||
| CHAGURO 7.5 g | 521.63 ± 399.41 | 623.14 ± 582.58 | 0.325b |
| CHAGURO 15 g | 413.91 ± 392.8 | 427.25 ± 346.01 | 0.859b |
| pd | 0.184 | 0.230 |
Changes in variables and mean differences in changes between the two groups after 60 days of intervention are presented in Table 3. Compared to baseline, there were no significant changes in the level of HbA1C, total cholesterol, LDL-C, and triglycerides after the intervention; although there was an insignificant decrease in total cholesterol and LDL-C. A significant improvement in the chol/HDL ratio was observed in the LD group (p < 0.05). However, HDL-C has decreased significantly in the HD group.
| Variables | Period | Men (Mean ± SD) | pa | Women (Mean ± SD) | pa | ||
|---|---|---|---|---|---|---|---|
| CHAGURO 7.5 g | CHAGURO 15 g | CHAGURO 7.5 g | CHAGURO 15 g | ||||
| HbA1c (mg/dL) | Initial | 8.25 ± 3.74 | 5.31 ± 0.38 | 0.103b | 6.22 ± 1.85 | 5.35 ± 0.77 | 0.029b,‡ |
| End | 8.6 ± 4.66 | 5.37 ± 0.56 | 0.448b | 6.35 ± 2.02 | 5.32 ± 0.73 | 0.026b,‡ | |
| Mean difference (95% CI) | −0.35(−8.60-7.90) | −0.06(−0.44-0.32) | 0.666b | −0.13(−0.48-0.21) | 0.03(−0.28-0.34) | 0.607b | |
| pc | 0.657 | 0.634 | 0.539d | 0.423 | |||
| Total cholesterol (mg/dL) | Initial | 197.5 ± 21.92 | 175.6 ± 53.45 | 0.914b | 189.14 ± 35.60 | 202.72 ± 30.93 | 0.917 |
| End | 192.5 ± 30.40 | 177.5 ± 53.27 | 0.357 | 183.48 ± 37.87 | 197.22 ± 45.16 | 0.873 | |
| Mean difference (95% CI) | 5(−71.23-81.23) | −1.9(−18.94-15.14) | 0.648 | −4.56(−4.56-15.89) | 5.5(−7.79-18.79) | 0.508 | |
| pc | 0.278 | 0.596 | 0.132 | 0.199 | 0.029b,‡ | ||
| LDL-C (mg/dL) | Initial | 133.7 ± 25.31 | 105.09 ± 41.26 | 0.188 | 117.60 ± 29.75 | 131.15 ± 30.47 | 0.938 |
| End | 121.3 ± 35.49 | 106.31 ± 45.57 | 0.337 | 113.97 ± 32.89 | 125.4 ± 36.84 | 0.871 | |
| Mean difference (95% CI) | 12.4(−79.08-103.88) | −1.22(−13.35-10.91) | 0.845 | 3.63(−6.38-13.66) | 4.81(−4.27-15.77) | 0.381 | |
| pc | 0.16 | 0.587 | 0.231 | 0.123 | |||
| HDL-C (mg/dL) | Initial | 44.5 ± 0.70 | 44.2 ± 11.38 | 0.486 | 49.48 ± 10.62 | 49.77 ± 9.02 | 0.911b |
| End | 48 ± 4.24 | 37.8 ± 11.28 | 0.125 | 51.22 ± 10.00 | 47.40 ± 8.52 | 0.081 | |
| Mean difference (95% CI) | −3.5(−35.26-28.26) | 6.4(−2.60-15.40) | 0.155 | −1.74(−4.66-1.18) | 2.36(−2.08-6.81) | 0.053 | |
| pc | 0.802 | 0.071 | 0.883 | 0.140 | |||
| Triglycerides (mg/dL) | Initial | 96.5 ± 20.50 | 138.6 ± 90.16 | 0.729 | 89.37 ± 45.36 | 121.36 ± 57.22 | 0.024b,‡ |
| End | 116 ± 46.66 | 191.1 ± 148.27 | 0.519b | 93.81 ± 40.48 | 112.09 ± 47.26 | 0.099b | |
| Mean difference (95% CI) | −19.5(−254.56-215.56) | −52.5(−118.60-13.60) | 0.746b | −4.44(−17.25-8.37) | 9.27(−13.56-32.10) | 0.355b | |
| pc | 0.758 | 0.947 | 0.400d | 0.204 | |||
| Chol/HDL ratio (mg/dL) | Initial | 4.42 ± 0.41 | 4.13 ± 1.49 | 0.398 | 3.91 ± 0.82 | 4.16 ± 0.84 | 0.853 |
| End | 3.99 ± 0.27 | 5.01 ± 1.90 | 0.757 | 3.72 ± 1.00 | 4.18 ± 0.83 | 0.956 | |
| Mean difference (95% CI) | 0.43(−0.84-1.70) | −0.88(−1.74-(−0.01)) | 0.915 | 0.19(−0.01-0.39) | −0.02(−0.32-0.28) | 0.888 | |
| pc | 0.07 | 0.976 | 0.034† | 0.562 | |||
Functional food has been widely studied and, most commonly, chayote (Sechium edule) and tuna have shown beneficial effects in improving glycemic status and inflammation.11,14,15 Tuna fish contain docosahexaenoic acid (DHA), polyunsaturated fatty acids (PUFA) and eicosapentaenoic acid (EPA), which are known to have benefits in dyslipidemia and insulin resistance, and also physiological effects.16 This study showed that 7.5 g and 15 g CHAGURO did not result in a reduction in HbA1c, which is less consistent with previous research results.14,17 Comparison of the HbA1C level before and after the 60-day intervention did not differ significantly between groups (Figure 3). This means that CHAGURO consumption cannot lower the HbA1C level. This may be caused by a failure to control the level of carbohydrate intake during the intervention, which can be seen from the increase in carbohydrate intake before and after the intervention in both groups (Table 2). This study has not produced comprehensive results on the effect of CHAGURO on HbA1C levels. The HbA1C level can be used to evaluate long-term glycemic control of patients and can reflect mean blood glucose over the last 8 to 12 weeks. One of the reasons for this study result is the short-term duration of the intervention.18 Sakung et al. also reported no differences in blood glucose with chayote consumption in subjects with prediabetes.19
Total cholesterol, LDL-C, HDL-C, and tryglyceride levels were not significantly altered with 7.5 g and 15 g of CHAGURO in this study, except HDL-C levels, which decreased significantly in the 15 g group of CHAGURO. However, the cholesterol-lowering effects of CHAGURO are shown to be greatest in the group with lower baseline lipids.
The chol/HDL-C ratio has a high correlation with the levels of LDL particles. Previous studies have shown that it is a strong cardiovascular risk marker.20–22 Omega-3 fatty acids in tuna can be responsible for lowering the lipid profile as a cardiovascular protective nutrient. Through activation of peroxisome proliferator-activated receptor (PPAR), omega-3 fatty acids have the ability to lower the fasting concentration of plasma trygliserides, reduce inflammation, and increase insulin sensitivity.23
Limitations of the study design should be noted. Potential dietary confounders to evaluate the effects of CHAGURO were homogenized between groups by providing a general diet. The limitation of this study lies in the short-term intervention. Considering that the duration of a 60-day intervention is too short for some variables such as HbA1C to show significant changes. Another limitation was that the subjects were not fully blinded. However, this study shows that CHAGURO has an effect in lowering the chol/HDL ratio.
In prediabetes individuals with dyslipidemia, experimental research intervention or administration of 7.5 g of siamese pumpkin and tuna seed (CHAGURO) can significantly improve the chol/HDL ratio. Thus, CHAGURO can be a functional food alternative for individuals with prediabetes and dyslipidemia in improving chol/HDL ratio, but further studies are needed to support this data.
Figshare: The Effect of Siamese Pumpkin and Tuna-Based Sow (CHAGURO) Data. https://doi.org/10.6084/m9.figshare.16908859.v211
The project contains the following underlying data:
Figshare: Copy of Trial Registry Approval for The effect of a siamese pumpkin and tuna-based powder (CHAGURO) diet in improving HbA1c, HDL-C, and chol/HDL ratios in prediabetes individuals with dyslipidemia: a randomised trial. https://doi.org/10.6084/m9.figshare.17142368.v112
This project contains the following extended data:
- KE-FK-0951-EC-2020 -Dr. Toto Sudargo, SKM., M. Kes - KE-0745-07-2020.pdf (Ethical approval from The Medical and Health Research Ethics Committee (MHREC), Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada - Dr. Sardjito General Hospital, under trial protocol register No.KE/FK/0951/EC/2020 (August 27, 2020).
Figshare: CONSORT checklist for ‘The effect of a siamese pumpkin and tuna-based powder (CHAGURO) diet in improving HbA1c, HDL-C, and chol/HDL ratios in prediabetes individuals with dyslipidemia: a randomised trial’. https://doi.org/10.6084/m9.figshare.17125214.v124
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The authors thank everyone who has contributed and cannot be mentioned one by one. Especially thanks to the funding provider in the implementation of this research, The Directorate of Research and Community Service – Ministry of Research, Technology and Higher Education (DRPM – KEMENRISTEKDIKTI) (DECENTRALIZATION PROGRAM RESEARCH, NATIONAL COMPETITIVE, AND ASSIGNMENT IN 2019) with No. 2522/UN1.DITLIT/DIT-LIT/LT/2019.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: clinical nutrition, obesity, metabolic diseases, anti aging medicine.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical Nutrition, Food Sciences, Obesity and Non-communicable diseases.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical Nutrition, Food Sciences, Obesity and Non-communicable diseases.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical Nutrition, Food Sciences, Obesity and Non-communicable diseases.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 24 Aug 22 |
read | read |
|
Version 2 (revision) 23 Feb 22 |
read | |
|
Version 1 20 Dec 21 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)