Keywords
Stroke, Autonomic Modulation, Transcranial Direct Current Stimulation, Physical Exercise, Hemiplegia
Stroke, Autonomic Modulation, Transcranial Direct Current Stimulation, Physical Exercise, Hemiplegia
tDCS: transcranial direct current stimulation
F3: Left dorsolateral prefrontal cortex
HRV: heart rate variability
DLPFC: dorsolateral prefrontal cortex
DT: linear time-domain
FD: frequency-domain
IP: Pulse interval
rMSSD: square root of the mean of the squares of the differences between adjacent normal RR intervals
HF: Absolute high frequency
LF: Absolute low frequency
LF / HF: low frequency and high-frequency ratio
ACE: Addenbrooke Cognitive Exam questionnaire
SSQOL: stroke specific quality of life questionnaire
BDI: beck depression inventory
MMSE: mini mental state examination quiz
The contribution of complications to the mortality of patients with stroke is variable between 12.5% to 22.7%1,2.
Individuals with stroke have depressed parasympathetic activity mainly in the acute phase, exacerbated cardiovascular responses with increased sympathetic activity, unbalanced heart rate and blood pressure, decreased Heart Rate Variability (HRV), arrhythmias, and a higher risk of sudden death3–6.
Dorrance & Fink (2015)7 observed that post-stroke Autonomic Nervous System (ANS) dysfunction increases the circulation of catecholamine levels in the heart, and enhances post-stroke patients' morbidity and mortality. However, it is not yet clear whether this disfunction is an effect of increased sympathetic nervous system (SNS) activity, reduced parasympathetic nervous system (PNS) activity, or a change in the balance of the two.
Thus, a great concern about the impact of physical fitness on stroke survivors to prevent cardiovascular risks has been observed in recent times, which shows the importance of physical exercise. It is notorious that physical exercise can prevent and mitigate heart problems. However, it requires prudence, since physical activity itself can increase cardiac output and increase blood pressure in these patients, even with the use of medications8–12.
Therefore, supportive therapies that can improve the rehabilitation process are relevant, such as Transcranial Direct Current Stimulation (tDCS). Transcranial direct current stimulation associated with aerobic exercise has shown significant effects on autonomic modulation in athletes and healthy individuals, as noted by some researchers13–15. Heinz et al. (2019)16 observed that the application of tDCS in individuals with stroke sequelae tends to modulate parasympathetic action. However, the effectiveness of tDCS was not noticed by Nguyen et al. (2015)17 in this same population.
Rossi et al. (2016)18 showed in a systematic review that tDCS performance is a therapeutic option in autonomic modulation, allowing instantaneous ('online') and lasting ('offline') modulation in cortical excitability. However, the tDCS protocol to obtain the better performance of the autonomic balance of individuals affected by stroke remains unclear.
To investigate the effect of adding tDCS to aerobic training in the autonomic modulation of chronic stroke survivors immediately after the first therapy, after the 12th, 24th, and 36th interventions, and 30 days after the end of interventions.
To evaluate the additional effect of tDCS on aerobic training in the distance covered (meters), quality of life, and cognition after the 12th, 24th, and 36th interventions and 30 days after the end of the interventions.
This study assumes that the application of tDCS over the left dorsal prefrontal cortex (DPFC) will stimulate this area and, therefore, increase the effects of aerobic training on the autonomic modulation of individuals with stroke sequelae. This effect may occur because the left DPFC, when activated, has the function of inhibiting the sympathetic excitatory circuit of the amygdala, helping with autonomic regulation19.
The hypothesis is that after a single stimulation session, it will be possible to verify some effect on the ANS, as observed by Heinz et al. (2019)16, Okano et al. (2015)14, Petrocchi et al. (2017)20. However, these may be even more significant after a 3-month aerobic training, a time described in the literature to obtain effects on cardiovascular conditioning21. Another hypothesis is that these results will remain one month after the end of the training.
It is possible to observe effects on cognition since exercise can improve it and because the area that will be stimulated with tDCS is also responsible for behavior modulation, planning, temporal sequencing, language, and memory22–24.
Through the possible beneficial effects of exercise for the body, the improvement in the quality of life can be a consequence25,26.
This is a protocol for a double-blind study (evaluator and participants), controlled by sham and randomized that will follow the recommendations of the Consolidated Standards of Reporting Trials (CONSORT) (Figure 1) and the recommendations of the standard protocol items for clinical trials (SPIRIT) (Reporting guidelines). The study was approved by the ethics committee of Universidade Nove de Julho, São Paulo, Brazil (CAAE: 97475718.5.0000.5511) - and registered in the Brazilian Registry of Clinical Trials (ReBEC) (U1111 -1222-4588) Participants will be informed about the research, procedures, risks, and benefits. If they agree, they will sign an informed consent form (Extended data: Appendix 127).
There will be enrolled 34 participants of both sexes at the physiotherapy clinics at Nove de Julho University, in São Paulo.
The inclusion criteria are as follows: individuals of both sexes, aged between 21 and 74 years old, minimum of six months of stroke injury, medical authorization to participate in the study, with the functional capacity of lower limbs that allow them to pedal the exercise bike, even if with the help of the therapist. The participants who usually ingest beta-blockers will not be excluded, but after the end of the research, an analysis will be carried out to compare the HRV of the individuals who use the medication with those who do not use them. Exclusion criteria include individuals with cognitive impairment (≤17) assessed by the mini-mental status exam (MMSE)28, severe heart problems, use of a pacemaker, and/or contraindications to the use of tDCS29.
Participants can freely withdraw from assessment and therapy at any time. Criteria for the termination in the study include participants who were absent more than a week away from the start date; who became ill or acquire any injury making it impossible to perform physical activity.
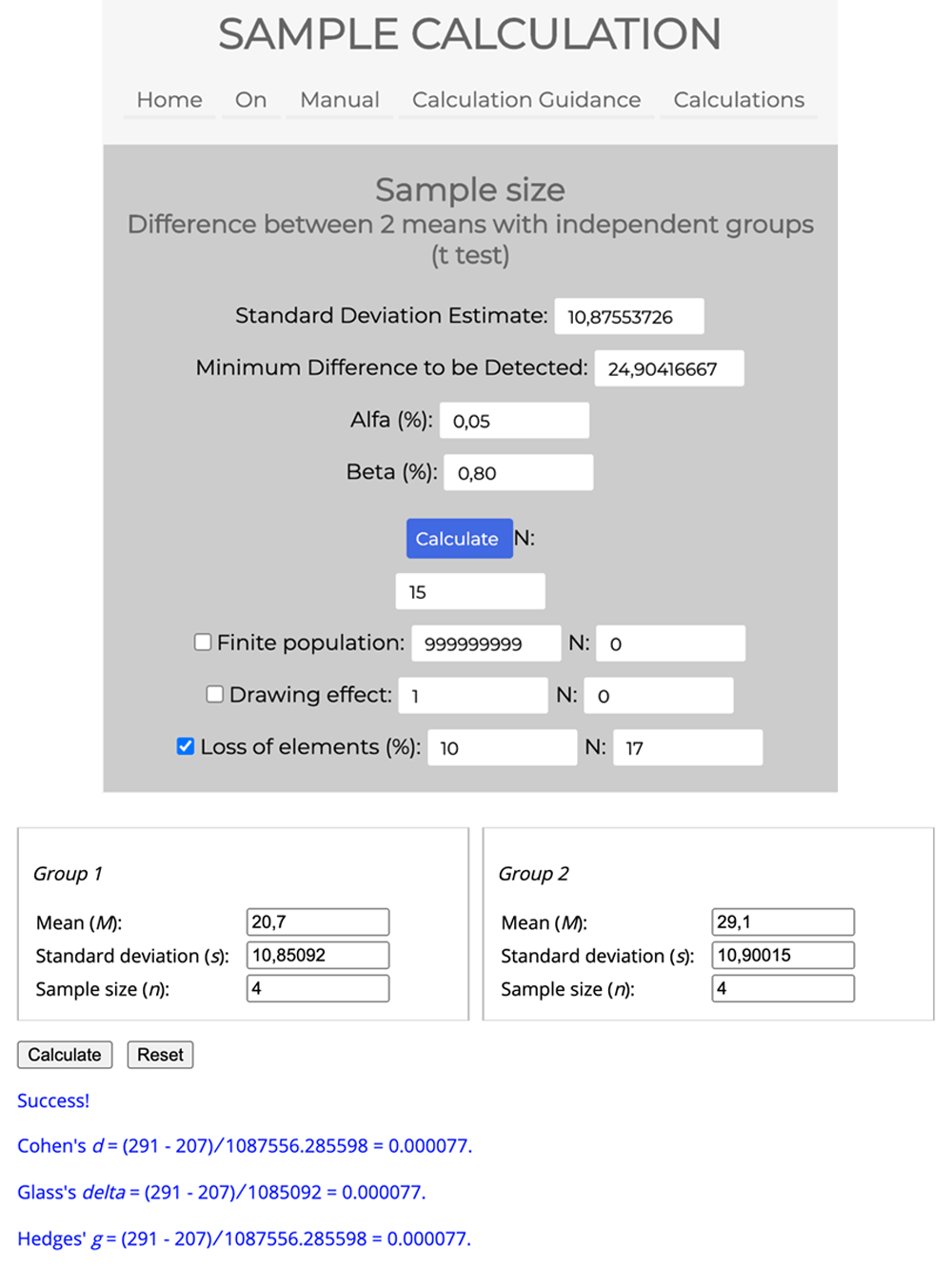
The sample size was calculated utilizing a pilot study with 8 individuals (4 for the active tDCS group and 4 for the tDCS sham group) and, with the sample power considering the rMSSD outcome variable by linear time-domain (DT) methods, assuming α of 0.05 and β of 0.80. Using the sample calculation tool on the website: calculoamostral.bauru.usp.br, the difference between two means with independent groups (t-test) was calculated. A total N of 15 individuals, considering possible losses, 10% was added totaling N of 17 individuals for each group and, therefore, 34 individuals were recruited, with an effect size of d = 0.000077 by Cohen (Figure 2).
The allocation of individuals to Group 1 (active tDCS combined with aerobic exercise on the stationary bike) and Group 2 (tDCS sham combined with aerobic exercise on the stationary bike) will take place using the website www.randomized.com by a researcher not involved in the evaluation and intervention.
A study evaluation schedule with standard protocol items is provided in Table 1.
Note: Heart Rate Variability (HRV); -T1: one week before intervention; T0: one day before intervention; T1: 20 minutes before intervention; T2: 1st-day session; T3: 12th-day session; T4: 24th-day session; T5: 36th-day session; T6: immediately after the session; T7: 30th-day follow-up after the last training session.
The assessments and interventions will be carried out in the morning, always at the same time, to minimize the effects of the circadian cycle. The recommendations will be to continue to use the medications in their regular schedule, to have a light diet on the test days, to abstain from caffeine or alcoholic beverages, and smoking, and to avoid moderate or excessive efforts on the day before the test day.
Transcranial direct current stimulation. The therapy of tDCS DC-Stimulator Plus (NeuroConn) (active or sham) will be combined with aerobic exercise on the stationary bike. The anode electrode will be placed over the left dorsolateral prefrontal cortex (F3), and the return electrode (cathode) will be placed over the contralateral supra-orbital region, defined by the 10/20 electroencephalogram system. The intensity of current will be 2mA, applied for 20 minutes, 10-second linear ramp up / down.
The electrodes used will be of conductive rubber, anode 5x5 cm, and cathode 5x7 cm, wrapped in cellulose sponge moistened in 0.9% saline solution.
For sham stimulation, all electrode placement procedures will be performed equally with the active tDCS. Still, the stimulator will only be on for 30 seconds, considered a valid method for a control in tDCS studies30.
The NeuroConn DC-STIMULATOR PLUS device has settings that allow the selection of the active or sham stimulation mode by inserting codes. A researcher not involved in the procedures will allocate the participant. The external functioning of the device will not perceive the stimulus mode. Therefore, neither the researcher who will apply the intervention nor the individual will know what treatment will be used (double-blind).
After using the tDCS is carried out, participants and researchers will be asked to complete questionnaires about blinding (Extended data: Appendix 227), adverse effects (Extended data: Appendix 327), and satisfaction of therapy (Extended data: Appendix 427).
The participant will performer the aerobic activity on a Reebok® RT 445 model N° RBEX49021 exercise bike, 30 minutes a day, with the initial 5 minutes of warm-up, 20 minutes of aerobic activity associated with active tDCS or sham, and the final 5 minutes of cooling.
The treatment will be carried out three times a week, for 12 weeks, totaling 36 sessions31. The initial intensity will be 50% of the reserve heart rate, as tolerated, and will be rising until 5% of the reserve heart rate each week32. The target aerobic intensity will be 50% to 70% of the reserve heart rate.
The following formula {% reserve HR = [(maximum HR - resting HR) x%] + resting HR} will be used to obtain the reserve heart rate (HR). If one of the individuals uses β-blockers, the maximum corrected HR should be calculated using the following formula [(the dosage taken with the drug + 95.58) / 9.74 =% that should be removed from the maximum HR]. The maximum heart rate (HRmax) will be estimated using the Karvonen formula33.
The HR and oxygen saturation (SpO₂) will be monitored as a protective measure by a portable pulse oximeter UT-100 Polar V800 frequency meter every two minutes of exercise; as well as blood pressure (BP) and the perception of dyspnea and fatigue of the lower limbs by the modified Borg questionnaire34.
The evaluations will be carried out before, after the 12th, 24th, and 36th interventions and 30 days after the end of the interventions. Personal data about the individual and the disease will be collected (Extended data: Appendix 527). The other data will be:
Evaluation of heart rate variability (HRV). The CardioSeries software (http://www.danielpenteado.com/cardioseries) will be used to identify correct premature ectopic beats, and undesirable transients will be removed using linear interpolation that alters the signal stationarity. The variances of the pulse interval (IP) will be evaluated in the domain of time and frequency by the linear method.
HRV will be measured using the Polar® V800 heart rate monitor device. The evaluation of cardiac modulation will be performed by recording the RR interval, processed using the Flow software (https://flow.polar.com), calculating the transducer indices of cardiac cycle fluctuation, high-frequency waves (0.15 and 0.4 Hz), low frequency (0.04 to 0.15 Hz), and the interrelation between low frequency and high frequency (0.15 and 0.4 Hz). The raw, unfiltered data will be exported, converted, and stored in an Excel file, used later for the domain of time and frequency.
The indexes obtained by analyzing the RR intervals in the time domain will be the average of the RR pulse interval utilizing absolute variance and the square root of the squared mean of the difference between the normal adjacent RR intervals (rMSSD), expressed in ms². As for the frequency domain, the data will be analyzed through the analysis of absolute high frequency (AF), low absolute frequency (BF), and the vagal sympathetic balance between low frequency and high frequency (BF / AF).
Traveled distance. The distance covered will be measured at the end of the 30 minutes of aerobic exercise performed by the participant on the exercise bike.
The results of the distance covered will be compared intragroup (active and sham tDCS), for each moment pre, post 12th, 24th, 36th interventions, and 30 days after the end of the interventions to verify the evolution in both.
Cognitive Performance. The cognitive performance of chronic stroke patients will be assessed using the Addenbrooke Cognitive Exam (ACE)35 questionnaire. The evaluator will apply the questionnaire in three moments:
The first moment will be before randomization for the intervention groups, the second will be after the 36th intervention, and the third moment will be 30 days after the end of the training.
Quality of life assessment. Quality of life will be measured by the Stroke Specific Quality of Life questionnaire (SSQOL)36.
Depressive symptoms. The Beck Depression Inventory (BDI)37 will be used to assess the depressive symptoms. The results will be correlated with the performance of the physical activity38.
Statistical analysis. The program SPSS Statistic version 17.0 will be used for statistical analysis.
For measures of central tendency and dispersion will be used descriptive statistical analysis. To measure the parametric variables will be used the mean and standard deviation, to measure the non-parametric variables will be used the median and the interquartile range, and to measure the categorical variables will be used the frequency and percentage.
HRV data (linear methods) will be analyzed in the time domain with the variable rMSSD and in the frequency domain with absolute and normalized high frequency. The data will be submitted to the Shapiro-Wilk normality test, using the unpaired t-test for parametric data and Mann-Whitney for non-parametric data, considering the significance level p≤0.05 for all conditions.
This article provides a detailed description of a prospective, randomized, controlled, double-blind clinical trial designed to demonstrate the effects of combining tDCS and aerobic training with an exercise bike on the autonomic modulation of individuals with hemiparesis due to chronic stroke.
We will publish the results, and the evidence found can contribute to the cardiovascular rehabilitation process of this population. In this sense, if positive, it allows a better prognosis in the cardiovascular rehabilitation of these individuals and reduces the likelihood of a stroke with more severe recurrence.
So far, participants have been enrolled, and the allocation is being made from the perspective of completion of collections in June 2021.
The results will be communicated to the public through publication as a data set and original research in the relevant scientific journals.
We consider some topics as possible limitations of our study:
The possible difficulty in recruiting patients due to their limited mobility;
A possible analysis difficulty due to a decrease in vagal withdrawal and use of ß-blockers;
Absence of control over modifiable risk factors.
Difficulty getting complementary examinations for an accurate diagnosis regarding the location and extent of the lesion.
Harvard Dataverse: Effect of Transcranial Direct Current Stimulation associated with aerobic exercise on the autonomic modulation of hemiparetic individuals due to stroke: a study protocol for a double-blind randomized controlled trial, https://doi.org/10.7910/DVN/MUNWDB27
This project contains the following extended data:
Appendix 1: Clarified Free Consent Term
Appendix 2: Blinding Questionnaire tDCS Researcher
Appendix 3: Adverse effect tDCS
Appendix 4: Evaluation of treatment satisfaction
Appendix 5: Personal data about the individual
Register approved by the ethics committee
Registered in the Brazilian Registry of Clinical Trial (ReBEC)
Harvard Dataverse: SPIRIT checklist and CONSORT flow diagram for ‘Effect of Transcranial Direct Current Stimulation associated with aerobic exercise on the autonomic modulation of hemiparetic individuals due to stroke: a study protocol for a double-blind randomized controlled trial’, https://doi.org/10.7910/DVN/MUNWDB27
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
The authors thank the Brazilian fostering agency Coordination for the Improvement of Higher Education Personnel (CAPES) for granting a scholarship to the first author and the University Nove de Julho. Funding provided by CAPES.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for, and objectives of, the study clearly described?
No
Is the study design appropriate for the research question?
Partly
Are sufficient details of the methods provided to allow replication by others?
Partly
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Stroke recovery, neuroplasticity, brain stim
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Assessment of cardiovascular autonomic function, heart rate variability, Blood pressure variability, baroreflex sensitivity, exercise physiology, yoga, electroencephalography
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 11 Oct 21 |
read | read | |
|
Version 1 08 Mar 21 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)