Keywords
Zuotai, 70 Wei-Zhen-Zhu-Wan (Rannasangpei, Qishiwei), HgCl2, MeHg, Cytochrome P450, Nuclear receptors.
Zuotai, 70 Wei-Zhen-Zhu-Wan (Rannasangpei, Qishiwei), HgCl2, MeHg, Cytochrome P450, Nuclear receptors.
Figure 1 legend: "total" was added to "total RNA".
Introduction: "Pamda-28" is removed.
All other comments are minor and already shown in the prior work with references cited.
See the authors' detailed response to the review by Xingguo Cheng
Tibetan Medicine is one of the important medical heritages of the world1. Zuotai, a Tibetan medicine mixture containing β-HgS, has been included in many famous Tibetan medicines for the treatment of diseases2–4. A systematic review of available studies of Tibetan medicine, however, indicates that the literature in Western industrialized countries is scarce5. Traditional Tibetan medicines use polyherbo-metallic mixture recipes as opposed to a single ingredient in the treatment of diseases. For example, in a review of 193 herbo-metallic Tibetan medicine recipes for liver diseases, herbs/plants (181 kinds), animal products (7 kinds), and minerals (5 kinds) were frequently used6. Well-designed pharmacology and clinical studies are encouraged to elucidate the pharmacology, safety, and clinical efficacy of Tibetan medicines5,6.
We have recently indicated that chemical compositions of minerals (metals) are a major determinant of their therapeutic effects and toxicity in Tibetan medicines7. 70W Zhen-Zhu Wan (70W, also called Rannasangpei, Qishiwei) is such an example 8. 70W was developed in the middle of fifteenth century and is composed of herbo-metallic mixtures, mainly from pearl, Hong-sik, Albergia odorifera, Nine stone, Saffron, Bezoar, Musk and Zuotai (a mineral mixture) in the treatment of cardiovascular, gastrointestinal, and neurodegenerative diseases8, and is listed in the 2015 edition of Pharmacopoeia of China9. 70W is effective experimentally against vascular dementia in rats10, and protects cerebral ischemia-reperfusion injury via blood-brain barrier and metabonomics with 18 identified active ingredients11. We have recently demonstrated that 70W is effective in protecting against LPS plus MPTP-induced chronic neuroinflammation and dopaminergic neuron loss8 and could modulate gut microbiota as a means of protection8,12. 70W dose-dependently protected against CCl4-induced liver injury, probably by activation of the Nrf2 antioxidant pathway13.
CCl4 is metabolized via cytochrome P450 (CYP450), particularly CYP2E1, to produce reactive metabolites14. Whether the protective effects of 70W against CCl4 hepatotoxicity is related to CYP450 inhibition is not known. In addition, 70W might be used in combination with other medications since it has many beneficial effects because it contains many ingredients. It has the potential to cause herb-drug interactions, especially on the liver CYP450 gene, similar to other Chinese medicine formulae15. CYPs are the mixed function oxidase system mainly existing in the liver, and play roles in the metabolism of over 80% drugs16. Induction or inhibition of CYP450 is implicated in traditional medicine-induced hepatoprotection and/or hepatotoxicity15,17,18. CYP450 genes are regulated by corresponding nuclear receptors, their coordinated regulation affects hepatic phase I and phase II metabolisms19.
This study was therefore designed using 1–5 times clinical doses of 70W (0.15, 0.5 and 1.5 g/kg, po) for oral administration to mice for 7 days and comparing its effects with equivalent Hg contents of Zuotai, HgCl2, and 1/10 Hg contents of MeHg, in an attempt to obtain information for the safe use of Zuotai-containing 70W in the clinic.
70W and Zuotai was provided by Tibetan Medicine Manufacture Factory as described previously8, based on the 2015 edition of Pharmacopoeia of China for QA/QC control (Lot number Z20110561). 70W was prepared by grinding the pill into powder, adding distilled water to prepare the suspension for oral administration. Mercury chloride (HgCl2 Cat# M1136) and methylmercury (MeHgCl Cat# 442534) were from Sigma (St. Louis, MO, USA). All other chemicals were commercially available reagents.
Male Kunming mice (20 ± 2 g) were purchased from Animal Experimental Center of theThird Military Medical University (Chongqing, China). Animals were maintained in the SPF-grade facilities at Zunyi Medical University, with a controlled environment (22 ± 1°C, 50 ± 2% humidity and a 12 h: 12 h light: dark cycle) and free access to purified water and standard laboratory feed. Efforts were made to ameliorate distress and harm to animals by daily monitoring and humane treatment of the animals. To reduce the use of animals, the minimal number of mice (n=5)/group according to the experiment requirement was used which are sufficient for statistical analysis. All animal care and experimental protocols are complied with the Animal Management Guidelines of the Chinese Ministry of Health and approved by Animal Use and Care Committee of Zunyi Medical University (2015-07).
Mice were randomly divided into seven groups of five mice each (Total number n=35), respectively as the control, 70W (0.15, 0.5, 1.5g/kg), Zuotai (30 mg/kg, the amount contained in 70W), HgCl2 (33.6 mg/kg, equivalent Hg as HgS) and MeHgCl (MeHg, 3.1 mg/kg, 1/10 of Hg). Mice were given oral administration for seven consecutive days. The dose regimen selection was based on our prior publications for 70W (at clinical dose)8 or for zuotai and mercury compounds20. Twenty-four hours after the last dose, the animals were euthanized and the livers were collected and stored at 80°C prior to analysis.
The activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined by commercial kits (Jaingcheng, Nanjing, China)21. Liver samples were fixed in 10% formalin prior to routine processing and paraffin embedding. Liver sections (4 µm) were dewaxed in xylene, rehydrated in different concentrations of alcohol (100%, 95%, 80%, 75%) and stained with hematoxylin, followed by counterstaining with eosin. After rinsing, the slides were rehydrated with series of alcohol (75%, 95%. 100%) and mounted with cover glass slip. The slides were examined in nine random fields under a light microscope (Leica Microsystems Ltd., Wetzlar, Germany)21,22.
Approximately 50–100 mg of tissue was homogenized in 1 ml TRIzol (TakaRa Biotechnology, Dalian, China) and the total RNA was extracted according to manufacturer’s instructions. The quality and quantity of RNA were determined by the Nanodrop (Thermo Scientific, ND-2000, USA), with 260/280 ratio >1.8. Total RNA was reverse transcribed with a High Capacity Reverse Transcriptase Kit (Applied Biosystems, Foster City, CA, USA). The primers were designed with Primer3 software and listed in Table 1.
The 15 µL PCR reaction mix contained 3 µL of cDNA (10 ng/µL), 7.5 µL of iQTM SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA), 0.5 µL of primer mix (10 µM each), and 4 µL of ddH2O. After 5 min denature at 95°C, 40 cycles were performed: annealing and extension at 60°C for 45 seconds and denature at 95°C for 10 seconds. Dissociation curve was performed after finishing 40 cycles to verify the quality of primers and amplification. Relative expression of genes was calculated by the 2-ΔΔCt method and normalized to the house keeping gene β-actin or expressed as a percentage of controls8,21.
Approximately 80 mg of liver tissue was homogenized with RIPA lysis buffer containing 1 mM PMSF and freshly prepared proteinase inhibitors. The homogenates were centrifuged at 12,000 g at 4⍛C for 10 min, and the protein concentration in the supernatants was determined by the BCA assay, and denatures at 90⍛C for 10 min with Nupage loading buffer. Approximately 30 µg proteins were separated in the 10% Nupage gel and transferred to the PVDF membrane. The membranes were blocked in 5% of the skim milk for 1 hour at room temperature, followed by incubation with primary antibodies (CYP1A2 (1:500), CYP2B1 (1:500), CYP2E1 (1:500), CYP3A4 (1:500), CYP4 (1:500), CYP7A1 (1:500), and GAPDH (1:2000)) at 4°C overnight. After washing the membranes with TBST four times, the secondary horseradish peroxidase (HRP) labelled anti-rabbit, or anti-mouse antibodies were added (1:5000) (Beyotime, Shanghai, China), and incubated at room temperature for 1 hour. The enhanced chemiluminescent reagents (ECL) were used to detect the intensity of protein-antibody complexes, and intensity was semi-quantified with Quantity One software (Bio-Rad, USA)18.
At the doses of 70W and Zuotai used in the present study, animals were healthy, without body weight loss and no mortality occurred. No significant elevations of serum ALT and AST were evident, and histology did not reveal overt lesions12,20–22. HgCl2 and MeHg groups showed body weight loss and mild histology lesions, consistent with prior publications12,21,22.
Figure 1 illustrates mRNA expression of nuclear receptors (left side) and cytochrome P450 isozyme genes (right side). The aryl hydrocarbon receptor (AhR) mainly mediates the expression of CYP1A enzymes such Cyp1a2; Constitutive androstane receptor (CAR) mediates CYP2 enzymes such as Cyp2b10; Pregnane X receptor (PXR) plays an important role in the regulation of CYP3A enzymes such as Cyp3a11; Peroxisome proliferator-activated receptor α (PPARα) regulates induction of CYP4A enzymes such as Cyp4a1019. The Farnesoid X receptor (FXR) regulates cholesterol 7α hydroxylase (Cyp7a1) induction23. The results show that compared with the controls, 70W at 0.15, 0.5, and 1.5 g/kg doses and Zuotai (β-HgS, 30 mg/kg) had no effects on these nuclear receptor and CYP gene expressions. In contrast, HgCl2 (33.6 mg/kg) and MeHg (3.1 mg/kg) significantly increased AhR, Cyp1a2, CAR, Cyp2b10, PPARα, Cyp4a10, FXR, and Cyp7a1, while having no significant effects on PXR, Cyp3a11 (Figure 1).
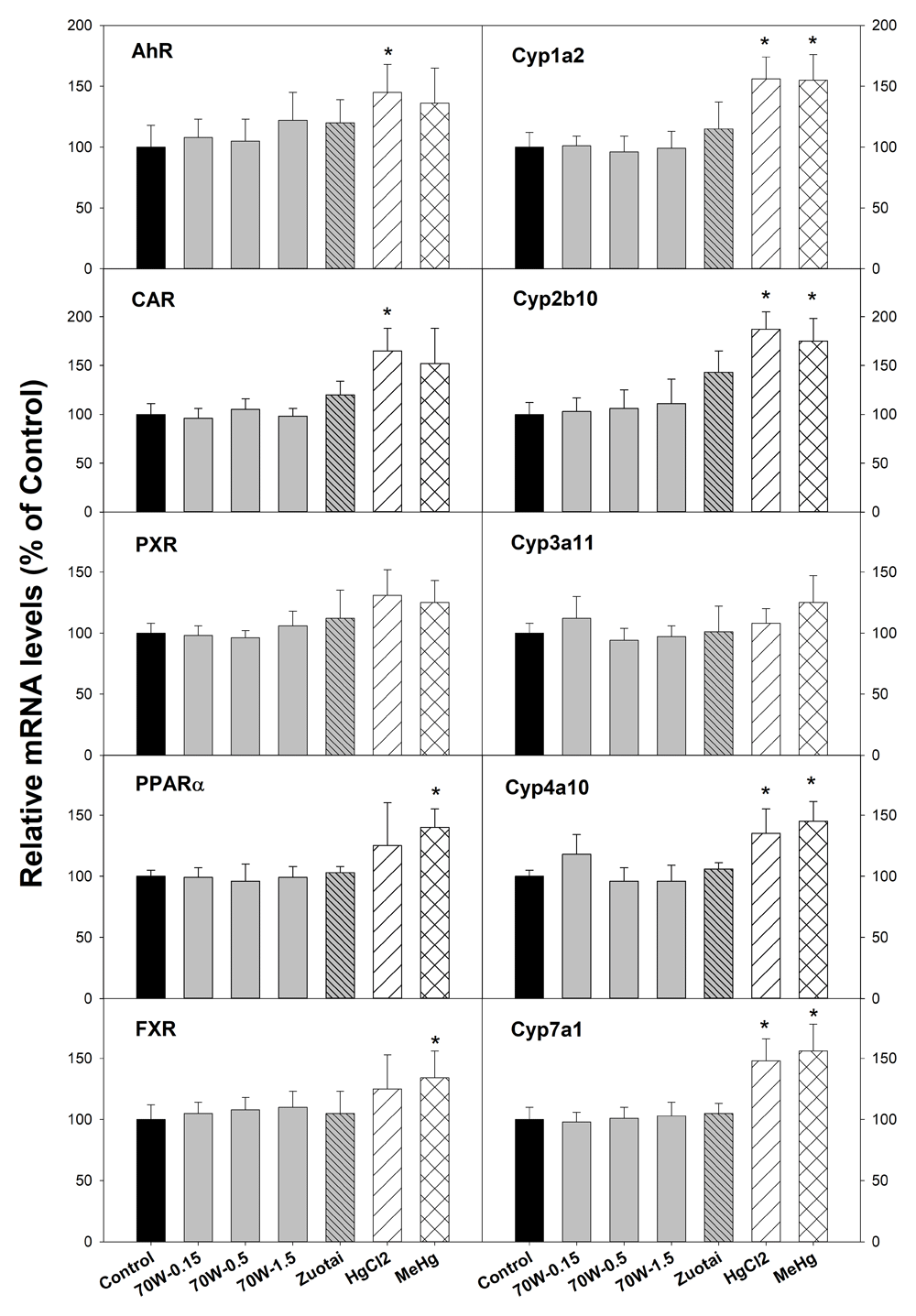
Mice were given 70W 0.15, 0.5, and 1.5 g/kg, po. Zuotai (30 mg/kg, po), HgCl2 (33.6 mg/kg, po), and MeHg (3.1 mg/kg, po) daily for seven days, and hepatic total RNA and protein were extracted for RT-PCR analysis. Data are mean ± SE, n = 5. *Significantly different from control, p< 0.05.
Figure 2 illustrates protein expression of P450 isozymes. Figure 2A shows representative western-blots for CYP1A2, CYP2B1, CYP2E1, CYP3A, CYP4A, and CYP7A1; Figure 2B shows the statistical analysis of 3-5 replicates. Consistent with mRNA expression, 70W at 0.15, 0.5, and 1.5 g/kg doses and Zuotai (β-HgS, 30 mg/kg) had no apparent effects on cytochrome P450 isozyme protein expressions. In contrast, HgCl2 (33.6 mg/kg) and MeHg (3.1 mg/kg) significantly increased CYP1A2, CYP2B1, CYP4A, and CYP7A1, while having no effects on CYP3A (Figure 2).
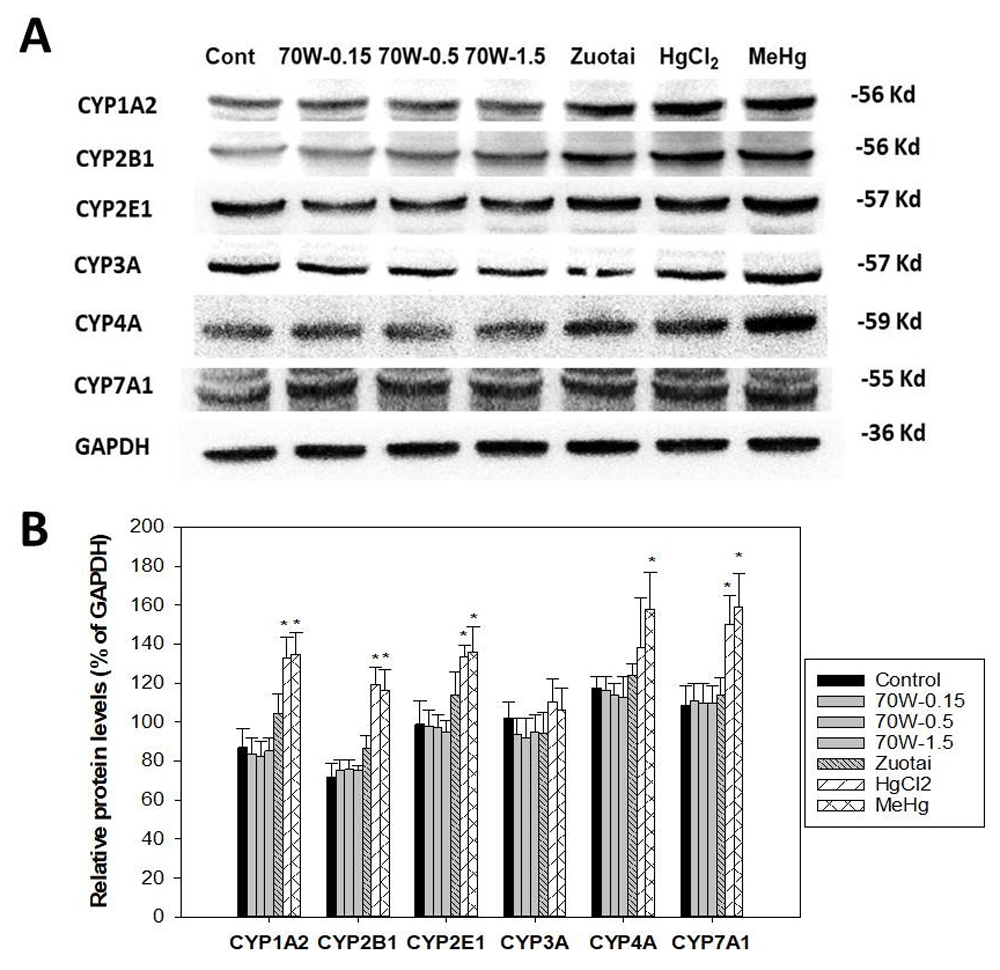
Mice were given 70W 0.15, 0.5, and 1.5 g/kg, po. Zuotai (30 mg/kg, po), HgCl2 (33.6 mg/kg, po), and MeHg (3.1 mg/kg, po) daily for seven days, and hepatic proteins were extracted and pooled for Western-blot analysis. A, the representative western-blot; B, statistical analysis of P450 proteins. Data are mean ± SE of 3-5 replicates. *Significantly different from control, p< 0.05.
The potential efficacy and toxicity of minerals (metals) in traditional medicines is currently a matter of debate7,24. In the present research, we examined the effects of β-HgS-containing Zuotai and Zuotai-containing 70W on hepatic CYP 1-4 and CYP-7 families, and their corresponding nuclear receptors, compared to HgCl2 and MeHg at both mRNA and protein levels. Briefly, 70W at 1 to 5-times clinical doses and Zuotai (β-HgS, 30 mg/kg, po) administered for seven days did not produce significant effects on the liver CYP450 gene and protein expressions in mice. HgCl2 and MeHg at 1/10 Hg dosing increased the expression of CYP1A, CYP2B, CYP2E1, and CYP7A at the mRNA and/or protein levels. These results further demonstrate that chemical forms of metals are a major determinant of their biological effects and that the use of HgCl2 or MeHg for risk assessment on minerals in traditional medicines is inappropriate7.
Tibetan medicine has thousands of years of history and is still used in the world today to treat a variety of diseases, including liver diseases1–4,6. Herbal-metallic preparations are believed to assist the delivery of drugs to the target, contribute to therapeutic effects, and reduce toxicity7. 70W is a famous Tibetan medicine listed in the 2015 Edition of Chinese Pharmacopoeia for the treatment of various diseases3,10. The major ingredients in 70W and the mode of the protection against cerebral ischemia-reperfusion injury has recently been demonstrated11. We have shown that 70W is effective against CCl4-induced liver injury, protected LPS plus MPTP-induced neurotoxicity8, and modulated gut microbiota8,12. The present study further demonstrated that the hepatoprotective effects of 70W is not due to the inhibition of CYP450 to reduce CCl4 bioactivation, rather the activation of the Nrf2 antioxidant pathway13.
Zuotai is a mineral mixture, with 54% of β-HgS25, and is included in a small amount to many valuable Tibetan medicines1–3. Mercury (Hg) is a toxic metal; the safety of Hg-containing traditional medicines is of concern24. The chemical speciation, spatial distribution of mercury from Zuotai are different from that of HgCl27,25, resulting in differential toxicity. A recent human study revealed that Zuotai-containing Tibetan medicines are safe at clinical doses26–28, including 70W29. Indeed, Zuotai differs from HgCl2 and MeHg in producing hepatotoxicity21, nephrotoxicity30, and intestinal toxicity with gut microbiome disruptions20. The present study demonstrated that Zuotai-containing 70W at clinical doses had minimal effects on hepatic CYP450, supporting the notion that Zuotai and 70W at clinical doses are safe26–29.
Cytochrome P450 1A1 (CYP1A1) is a hepatic and extrahepatic enzyme that is regulated by the AhR signaling pathway and is regarded as carcinogen activation CYP450 family31. CYP-1 family includes CYP1A1, CYP1A2, and CYP1B1,and CYP1A1/CYP1A2 has become a therapeutic tool for the bioactivation of prodrugs, particularly cytotoxic agents. Little is known about effects of 70W on CYP1A family. We have shown previously that oral Zuotai (β-HgS) and cinnabar (α-HgS) had minimal effects of hepatic P4501A family gene expression32. However, in rats, Zuotai at higher doses could decrease CYP1A2 activity33. In comparison, the effects of HgCl2 on CYP1A expression were more dramatic. In Zebra fish, a low dose (0.1 LC50) of HgCl2 increased CYP1A1, but at higher doses (0.4 and 0.8 LC50), the expression of CYP1A1 was suppressed34. In the mouse heart, kidney and lung, HgCl2 (2.5 mg/kg, ip) increased CYP1A1, along with other CYP450 isoforms35. In the present study, HgCl2 at 33.6 mg/kg increased CYP1A2 at mRNA and protein levels, largely in agreement with the above literature32–35 In another study, mice that chronically (6 weeks) received HgCl2 (32 mg/kg) and MeHg (2.6 mg/kg), had increased expressions of hepatic Cyp1a1 and Cyp1b1, while cinnabar (HgS, 300 mg/kg) and cinnabar-containing An-Gong-Niu-Huang Wan were ineffective36. Thus, the effects of mercury compounds on CYP1 family are dependent on the mercury forms, the dose, route, and duration of administration.
The CYP-2 family is easily induced by many xenobiotics such as phenobarbital. CAR is shown to play a crucial role in the activation of CYP2B genes by xenobiotics19. The CYP-2 family mainly includes the CYP2B subfamily and CYP2E1. CYP2E1 metabolizes an extensive array of pollutants, drugs, and other small molecules, often resulting in bioactivation to reactive metabolites, which in turn damage mitochondria37. HgCl2-induced hepatotoxicity and oxidative stress is partially mediated through its effects on CYP2E138. HgCl2 (2.5 mg/kg, ip) increased the expression of Cyp2b9 and Cyp2b10 in mice hearts39 and HgCl2 (33.6 mg/kg, po) increased Cyp2b10 expression in the livers of mice32. Under the present experimental conditions, Cyp2b10 mRNA and CYP2B protein expression were increased by HgCl2 and MeHg only.
CYP3A is the most abundant subfamily of CYP450, with the highest content in the liver and intestines, and is involved in the metabolism of clinical drugs17,18. CYP3A can be induced or inhibited by a variety of substances. In the present study conditions, 70W and mercury compounds had minimal effects on Cyp3a11 mRNA and CYP3A protein expression. The length of Hg compound administration could make a difference as compared to the present study.
CYP4A is involved in lipid metabolism and is regulated by PPARα, their dysregulations are implicated in xenobiotics induced adverse effects leading to various human diseases19. Researchers found that HgCl2 exposure is associated with increased risk of cardiovascular disease and profound cardiotoxicity, and their results show that mercury treatment caused a significant induction of the cardiac hypertrophy markers, along with CYP4A genes (Cyp4a10, Cyp4a12, Cyp4a14)35. In the present study, 70W and Zuotai at 1–5 times clinical doses do not have appreciable effects on PPARα and Cyp4a10 mRNA expression and CYP4A protein expression, while HgCl2 and MeHg increased PPARα and Cyp4a10 mRNA, as well as CYP4A protein, consistent with our prior observation that HgCl2 increased PPARα and Cyp4a10 in livers of mice after seven days of administration32. In mice chronically (6 weeks) dosed with HgCl2 (32 mg/kg) and MeHg (2.6 mg/kg), the expression of Cyp4a10 was increased, but cinnabar (HgS, 300 mg/kg) and cinnabar-containing An-Gong-Niu-Huang Wan was ineffective36. Increased expression of the CYP-4A family genes under the dose of HgCl2 and MeHg used in the present study could impact lipid metabolism.
CYP7A1 is a rate-limiting enzyme for bile acid synthesis and is regulated by FXR23. Little is known on the effects of mercury compounds on FXR and CYP7A1 expression. The present study showed that 70W and Zuotai did not affect CYP7A1, while HgCl2 and MeHg increased Cyp7a1 mRNA and CYP7A1 protein. The biological effects of CYP7A1 induction by HgCl2 and MeHg warrant further investigation.
The present study showed β-HgS and β-HgS containing 70W (1–5-times of clinical dose) did not produce appreciable effects on hepatic CYP450 enzyme gene/protein expression compared to equal Hg content as HgCl2 or 1/10 of Hg content as MeHg, suggesting that (1) the protection of 70W against CCl4 hepatotoxicity is not due to inhibition of CYP450 (CYP2E1); (2) 70W appeared to be safe under recommended clinical doses; and (3) HgCl2 and MeHg had significant effects on CYP450 expression, correlated with their potential toxic effects to the liver.
70 Wei-Zhen-Zhu-Wan (70W, also called Rannasangpei; Qishiwei); Cytochrome P450 (CYP450); Aryl hydrocarbon receptor (AhR); Constitutive androstane receptor (CAR); Pregnane X receptor (PXR); Peroxisome proliferator-activated receptors (PPARs); farnesoid X receptor (FXR).
Zenodo: Zuotai (β-HgS)-containing 70 Wei Zhen-Zhu-Wan differs from mercury chloride and methylmercury on hepatic cytochrome P450, http://doi.org/10.5281/zenodo.440371740
This project contains the following underlying data:
- PCR and WB figure data (PCR-WB)
- Raw western-blot data (CYP-WB). Please note that full blot images are not available
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
A previous version of this article is available on Research Square: https://doi.org/10.21203/rs.3.rs-32118/v1
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Toxicology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology and toxicology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Toxicology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 24 Jun 21 |
read | |
|
Version 1 11 Mar 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
However, some suggestions were put forward in the manuscript. For example, in describing the effects of Zuotai and 70 Wei Zhen-Zhu-Wan on animal weight, ALT and AST, and liver histopathology, the manuscript only quoted the links of published articles. However, we suggest that the relevant data should be written appropriately, which is beneficial for the author to read, because this part of data is actually the most important and basic.
However, some suggestions were put forward in the manuscript. For example, in describing the effects of Zuotai and 70 Wei Zhen-Zhu-Wan on animal weight, ALT and AST, and liver histopathology, the manuscript only quoted the links of published articles. However, we suggest that the relevant data should be written appropriately, which is beneficial for the author to read, because this part of data is actually the most important and basic.