Keywords
5-alpha-reductase, Testosterone, Dihydrotestosterone, Finasteride, Dutasteride, Phytochemicals, Phytosterols, Polyphenols, Androgens, Prostate cancer
5-alpha-reductase, Testosterone, Dihydrotestosterone, Finasteride, Dutasteride, Phytochemicals, Phytosterols, Polyphenols, Androgens, Prostate cancer
Reply to the comments made by the Reviewer:
Thank you for reviewing and suggesting some constructive amendments to the review article.
Following are the actions taken in response to the reviewer’s comments. The reviewer could be able to see all the changes made to the article in response to the comments by downloading/reviewing the latest version of the review article.
See the authors' detailed response to the review by Binesh Shrestha
See the authors' detailed response to the review by Martin L. Read
Prostate cancer (CaP) is the second most deadly malignancy in men after lung cancer and the fifth leading cause of death worldwide, accounting for 7.1% (1,276,106) of the new cases and 3.8% (358,989) of total death in males in 2018 (Rawla, 2019). According to the United Kingdom Cancer Research Centre, over 47,500 men are diagnosed with CaP each year, where one man dies from it every 45 minutes. CaP is also estimated to be the most common cancer by 2030, as one in eight men destined to be diagnosed with CaP in their lifetime. CaP is a malignant tumour that is caused by unregulated prostate cell division resulting in an abnormal cellular growth that leads to a potential spread of cancer to other body parts (Ochwang’i et al., 2014; Packer & Maitland, 2016). The current primary treatments for CaP are surgery, radiation therapy, proton beam therapy, chemotherapy, cryosurgery, high intensity focused ultra-sound and hormonal therapy, depending on the clinical conditions, outcomes and disease progression among others (Chen & Zhao, 2013). The latter strategy was largely anticipated, considering CaP as being hormones-driven disease especially during the initial stage (Taplin et al., 1995). Therefore, targeting the hormones involved in the CaP’s pathway mechanisms seems to be a potentially useful approach in developing CaP prevention and treatment strategies.
The physiologic functions and pathologic conditions of the prostate are regulated by numerous hormones and growth factors. For instance, androgens are essential for prostatic development and function as well as for cells’ proliferation and survival (Banerjee et al., 2018). Testosterone (T), synthesised by the Leydig cells of the testes under the control of hypothalamus and anterior pituitary gland, is the most abundant circulating androgen in males, where from it, more potent form dihydrotestosterone (DHT) is synthesised (Figure 1). The microsomal enzyme 5αR mediates a rapid and irreversible T conversion to DHT within the prostate where it then binds to androgen receptor (AR) to exert its biological function (Azzouni et al., 2012). 5αR exists in two isoforms, namely type 1 (5αR1) and type 2 (5αR2), which differ in their molecular genetics, structural and biochemical properties, and tissue localisation (Nahata, 2017). 5αR1 occurs predominantly in non-genital skin, the scalp, the sebaceous gland, the liver, the kidney and the brain, whereas 5αR2 is found extensively in the prostate, genital skin, seminal vesicles and in the dermal papilla (Nahata, 2017; Othonos & Tomlinson, 2019; Strauss & FitzGerald, 2019). Both isozymes are expressed at a much lower level in other peripheral tissues. Due to the tissue specific expression of 5αRs, DHT concentration is much higher than T concentration in the prostate. Androgens, although necessary for the development of prostate, could also allow CaP cells to grow. They promote the growth of cancerous prostate cells by binding to and activating the AR, resulting in the expression of specific genes responsible for the proliferation of CaP cells. Augmented levels of androgens, particularly DHT, are detrimental towards CaP later in life.
Progression of cancer in prostate is typically dependent on the levels of androgens present during the initial stages of cancer growth (Taplin et al., 1995). Therefore, reducing the production of androgen provides a useful approach to androgen deprivation where it restricts the availability of T, allowing minimal conversion to DHT by 5αRs and androgen-receptor binding activity. The inhibition of 5αRs will subsequently limit the production of DHT and therefore represents a valid target for CaP risk prevention and reduction as well as treatment strategies as a whole.
Synthetic 5αR inhibitors (5αRis) can be broadly classified into two categories, namely steroidal and nonsteroidal, where their development was aimed to bind to 5αR with little or no affinity for the androgen or other steroid receptors. The most promising and well-studied 5αRis by far are finasteride and dutasteride. Clinical treatment with finasteride and dutasteride have shown to decrease both mean serum and intraprostatic level of DHT in CaP patients (Andriole et al., 2004; Clark et al., 2004; McConnell et al., 1992; Span et al., 1999). Finasteride is the first synthetic steroidal 5αRi approved for the treatment of benign prostatic hyperplasia (BPH) and male pattern baldness (Aggarwal et al., 2010; Brough & Torgerson, 2017). Finasteride, a synthetic 4-azasteroid compound, is a potent competitive inhibitor of 5αR2 that also inhibits 5αR1 but less effectively (Figure 2a). Finasteride has been reported to decrease LNCaP cell growth rate in vitro in a dose dependent manner (Bologna et al., 1995). Meanwhile, dutasteride, also a synthetic 4-azasteroid compound and an approved drug for BPH treatment, is known as a dual 5αRi with a 45-fold more effective in inhibiting 5αR1 and 2-fold more effective in inhibiting 5αR2 than finasteride (Figure 2b). Dutasteride has been reported to inhibit T and DHT-induced LNCaP cell proliferation by targeting the 5aRs activity and displaying a more potent DHT inhibition than finasteride (Lazier et al., 2004). Dual inhibition of 5aRs is more beneficial than selective type 2 inhibition as it suppresses the DHT level to a great extent by also preventing the type 1 mediated synthesis of DHT production.
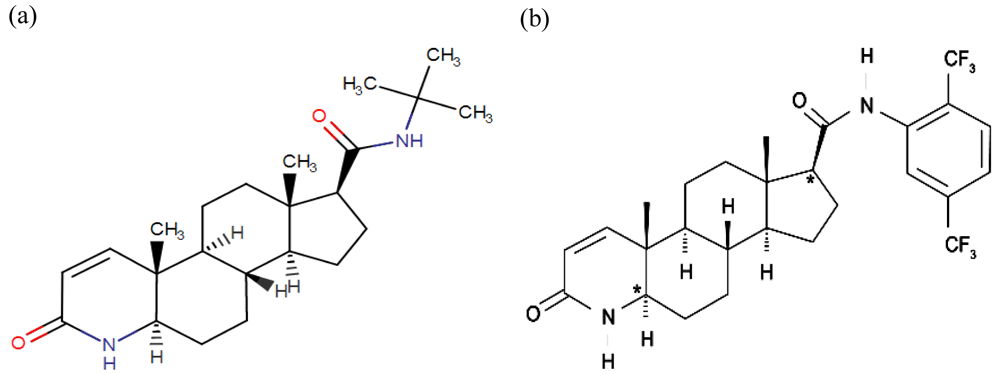
The chemical structure of 5αRis; (a) Finasteride, and (b) Dustasteride. The figure is adapted and modified from National Center for Biotechnology Information (2020).
These observations, among others, provide a strong rationale for CaP risk reduction and prevention using 5αRis finasteride and dutasteride, although their use as a targeting therapeutic drug continues to be widely discussed. One of the main issues that halt the progression of 5αRis, considered as an effective CaP therapeutic agent, is the numerous undesirable side effects including erectile dysfunction, ejaculatory dysfunction and loss of libido (Erdemir et al., 2008). 5αRis, which are also commonly prescribed for women with hair loss, demonstrate headache, gastrointestinal discomfort and decreased libido as the most common reported side effects (Hirshburg et al., 2016). Other factors include the controversy that 5αRis appear to only preferentially prevent low-grade cancers and now concern lingers that 5αRis may induce or selectively promote growth of high-grade disease (Hamilton & Freedland, 2011).
Synthetic drugs are known to have various adverse effects, hence, safer alternative drugs have been sought, focusing on herbal sources. Older people often use traditional plants as complementary and/or alternative remedies to sustain healthy life or cure diseases. Traditional plants are known to be in medicinal practices for treatment of various diseases since ancient times (Falodun, 2010; Leroi-Gourhan, 1975; Pan et al., 2014) and the use of medicinal plants in the search of new drugs from nature has increased since then (Savithramma et al., 2011). Plants contain numerous bioactive compounds for treatment of many conditions, including cancer (Mohan et al., 2011). The plant kingdom is comprised of approximately 250,000 plant species and only around 10% have been studied for the treatment of different diseases (Iqbal et al., 2017). Approximately 25% of the modern drugs in clinical use are derived from plants, where the majority of these drugs were discovered as a direct result of studies focusing on the isolation of active compounds from traditional plants (Calixto, 2019).
Herbal drugs, which have been increasingly used in cancer treatment, represent a rich pool of new, and interesting bioactive entities for the development of CaP therapeutic agents. This is because herbal plants exhibit favourable side effects and toxicity profiles compared to conventional chemotherapeutic agents. Therefore, the aim of this review is to discuss the use of phytochemicals found in various plants that have been proven to exhibit anti-CaP as alternative herbal CaP medicines and to focus on the types of phytochemical present in plants that exhibit inhibitory activities on 5αRs isozymes.
Phytochemicals are the bioactive non-nutrient plant compounds that are found present in fruits, vegetables, grains and other plant foods, where its consumption has been linked to reduction on risk of many major chronic diseases (Sathishkumar & Baskar, 2014). Six major phytochemical categories that have been identified are phenolics, alkaloids, nitrogen-containing compounds, organosulfur compounds, phytosterols and carotenoids (Liu, 2013). The surge of interest in finding new natural bioactive entities as a template for new drug discovery and/or studying existing bioactive compounds for other biological and medicinal properties has kept scientists constantly conducting more chemical studies, particularly focusing on fractionating, isolating and identifying the active compounds. Phytochemicals offer a promising array of entities that can be further formulated into complementary or alternatives to conventional medicines that are less costly and have no/less harmful side effects. Many in vivo and in vitro studies have shown anti-CaP properties of various phytochemicals via numerous pathways as well as their ability to inhibit 5αR activity, particularly the phytosterols and phenolics, probably due to their structural similarity with the current inhibitors of 5αRs. Fatty acids, which differ in structure to any 5αRis, are also found to exhibit anti-5αR activity. Table 1 summarises the inhibitory action of various phytochemicals on 5αRs.
| Phytochemical | Structures | Effect on 5αRs | Model of Study | Source of Plant | References |
|---|---|---|---|---|---|
| Phytosterols; | |||||
| β-sitosterol | 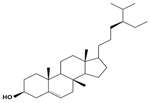 | Inhibition on type I and II | In vitro | Sepenoa repen, Hypoxis rooperi, Secale cereale (Rye Grass Pollen), Urtica dioica, Prunus africana | (Madersbacher et al., 2007), (Pais, 2010), (Dawid-Pać et al., 2014), (Komakech et al., 2017) |
| Stigmasterol | 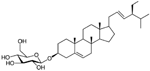 | Inhibition on type I and II | In vitro | Sepenoa repen, Phyllanthus urinaria, Croton sublyratus, Ficus hirta, Eclipta alba (L.) Hassk, Eclipta prostrate, Parkia speciosa, Gypsophila oldhamiana, Eucalyptus globules, Aralia cordata, Emilia sonchifolia, Akebia quinata, Desmodium styracifolium, Heracleum rapula | (Pais, 2010), (Dawid-Pać et al., 2014), (Kamei et al., 2018) |
| Lupeol | 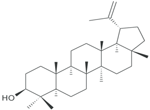 | Inhibition on type I and II | In vitro | Sepenoa repen, American ginseng, Shea butter plant, Tamarindus indica, Allanblackia monticola, Himatanthus sucuuba, Celastrus paniculatus, Zanthoxylum riedelianum, Leptadenia hastata, Crataeva nurvala, Bombax ceiba, Sebastiania adenophora | (Siddique et al., 2011) (Rainer et al., 2007) |
| Phenolics (Polyphenols); | |||||
| Quercetin | 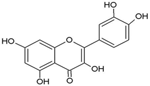 | Inhibition on type I | In vitro | Morus alba L, Camellia chinensis, Allium fistulosum, Calamus scipionum, Moringa oleifera, Centella asiatica, Hypericum hircinum, Hypericum perforatum | (Hiipakka et al., 2002), (Salvamani et al., 2014), (Yang et al., 2015), (Kashyap et al., 2019) |
| Myricetin | 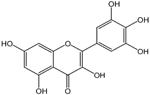 | Inhibition on type I | In vitro | Ampelopsis cantoniensis, Myrica cerifera L, Calamus scipionum, Chrysobalanus icaco L, Moringa oleifera, Aloe vera | (Hiipakka et al., 2002), (Salvamani et al., 2014) |
| Fisetin | 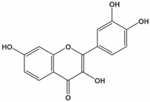 | Inhibition on type I | In vitro | Butea frondosa, Gleditsia triacanthos, Quebracho colorado, Curcuma longa, Rhus verniciflua, Acacia greggii, Acacia berlandieri | (Hiipakka et al., 2002), (Salvamani et al., 2014) (Kashyap et al., 2019) |
| Kaempferol | 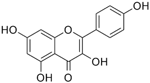 | Inhibition on type II | In vitro | Moringa oleifera, Centella asiatica, Euonymus alatus, Kaempferia galanga L, Ginkgo biloba, Equisetum spp., Tilia spp., Sophora japonica, propolis | (Hiipakka et al., 2002), (Park et al., 2006) |
| Biochanin A | 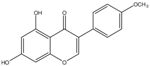 | Inhibition on type II | In vitro | Trifolium pratense L, Glycine max, Lupinus | (Jlan, 2009), (Spagnuolo et al., 2015), (Zhang et al., 2016) |
| Genistein | 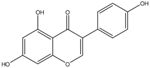 | Inhibition on type II | In vitro | Glycine max, Lupinus | (Jlan, 2009), (Spagnuolo et al., 2015), (Zhang et al., 2016) |
| Daidzein | 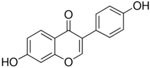 | Inhibition on type II | In vitro | Glycine max, Lupinus | (Jlan, 2009), (Spagnuolo et al., 2015), (Zhang et al., 2016) |
| Phenolics (Catechins); | |||||
| Epicatechin-gallate | 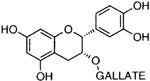 | Inhibition on type I | In vitro | Camella sinensis, Betula pubescens, Betula pendula, Cocos nucifera, fruit pulp of Argania spinosa, Cassia fistula | (Hiipakka et al., 2002) |
| Epigallocatechin- gallate | 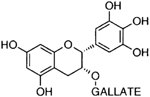 | Inhibition on type I | In vitro | Camella sinensis, Betula pubescens, Betula pendula, Cocos nucifera, fruit pulp of Argania spinosa, Cassia fistula | (Hiipakka et al., 2002) |
| Fatty Acids; | |||||
| Oleic Acid | 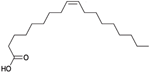 | Inhibition on type I | In vitro | Sepenoa repens, Helianthus annuus | (Raynaud et al., 2002), (Sheeba et al., 2015) |
| Linoleic Acid | 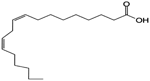 | Inhibition on type I | In vitro | Sepenoa repens, Prunus africana, Cocos nucifera, Helianthus annuus | (Raynaud et al., 2002), (de Lourdes Arruzazabala et al., 2007), (Nyamai et al., 2015), (Sheeba et al., 2015) |
| Myristic Acid | 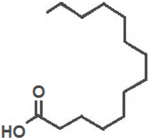 | Inhibition on type II | In vitro | Sepenoa repens, Prunus africana | (Raynaud et al., 2002) |
| Lauric Acid | 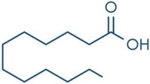 | Inhibition on type I and II | In vitro | Sepenoa repens, Prunus africana, Cocos nucifera | (Raynaud et al., 2002), (de Lourdes Arruzazabala et al., 2007), (Nyamai et al., 2015) (Komakech et al., 2017) |
1. Phytosterols
Plant sterols or phytosterols (PS) are bioactive components in plants with 28- or 29-carbon alcohols and double bonds at the C-5 position of the ring that resemble cholesterol in vertebrates in terms of both of their structure and function (Zaloga, 2015). More than 200 different types of phytosterols have been reported, with β-sitosterol, campesterol and stigmasterol being the most abundant type of PS (Miras-Moreno et al., 2016). The toxicity profiles of PS have shown that there are no obvious side effects after long-term feeding of PS in both animals and humans (Ling & Jones, 1995). PS play essential roles in the reduction of cholesterol in blood that eventually decrease cardiovascular morbidity, therefore are well known for their beneficial effect on cardiovascular disease risk. Katan et al. (2003) reported that the intake of 1–2 g of PS daily can effectively lower low-density lipoprotein cholesterol levels by 8%-12%. However, little attention was received with regard to PS on their potential in cancer aetiology, although increasing evidence of biochemical and molecular effects of PS may make them strong candidates for cancer therapeutic agents.
Being structurally similar with four rings to synthetic 5αRis finasteride and dutasteride, PS could stand as the strongest promising candidate for plant-derived 5αRis. A study by Awad et al. (2001) showed that β-sitosterol inhibits the growth and migration of PC-3 human CaP and slows down the growth of prostate tumour in SCID mice, which suggests an involvement of androgenic mechanism of action as CaP is dependent on androgen. An in vitro metabolic study in hamster prostate by Marisa Cabeza and colleagues revealed that β-sitosterol inhibits the enzymatic activity of 5αRs in dose-dependent manner, which therefore confirms the ability of β-sitosterol as a 5αRi (Cabezal et al., 2003).
Another PS, stigmasterol, was reported to be associated with a reduction in common cancer risks including colon cancer, breast cancer and CaP (Bradford & Awad, 2007). Kamei et al. (2018) studied Phyllanthus urinaria where the extract was shown to suppress androgen activity of DHT in LNCaP cell lines and has inhibitory activity against 5αRs, of which the active bioactive compound responsible for the activity was identified as stigmasterol isolated from an activity-guided fractionation. An in vitro study of Serenoa repens extract (SPE) using baculovirus-directed insect cell expression system demonstrated the inhibition of both 5αR1 and 5αR2 in a non-competitive and uncompetitive manner, respectively (Iehlé et al., 1995). The major active compounds from PS of SPE includes β-sitosterol and stigmasterol (Suzuki et al., 2009). SPE, a well-known phytotherapeutic agent, most frequently used to treat lower urinary tract symptoms and as a BPH medicine, not only targets the regulation of 5αRs activity but also hampers the binding of DHT to androgenic receptors (Dawid-Pać et al., 2014). Pais (2010) reported in his study that in a cell-free test system, ethanolic extract of Serenoa repens was a potent inhibitor of 5αR2 with 61% inhibition. From these observations, β-sitosterol and stigmasterol are found to exhibit inhibitory activity on both isozymes of 5αRs. Various plants reported to have β-sitosterol as their major active compound include Hypoxis rooperi extract (Harzol®), Secale cereal (Rye Grass Pollen), Urtica dioica and Prunus Africana (Komakech et al., 2017; Madersbacher et al., 2007). A study by Nahata & Dixit (2014) analysing the inhibitory effects of different types of Urtica dioica extracts on the activity of 5αR2, demonstrated that ethanolic extracts were the best 5αRis, followed by petroleum ether and aqueous extracts. Stigmasterol, with known 5αR2 inhibitory activity, is also reported to be present in various medicinal plants including Croton sublyratus, Ficus hirta, Eclipta alba (L.) Hassk, Eclipta prostrate, Parkia speciosa, Gypsophila oldhamiana, Eucalyptus globules, Aralia cordata, Emilia sonchifolia, Akebia quinata, Desmodium styracifolium, Heracleum rapula (Chaudhary et al., 2011).
Lupeol, another PS, has also been shown to exhibit various pharmacological properties including anti-CaP activity (Siddique & Saleem, 2011). Siddique et al. (2011) demonstrated in their study that lupeol inhibited the growth of various CaP cells i.e LAPC4, LNCaP and CRPC cells, in vitro. Another in vivo study using implanted CaP cells as xenograft tumours in mice also revealed that lupeol treatment effectively halts tumour growth, which further suggests the ability of lupeol as an effective agent that can potentially inhibit the tumourigenenicity of CaP cells. Lupeol has also been observed to have a striking ability to preferentially kill CaP cells while sparing normal prostate epithelial cells (Saleem et al., 2005). SPE, which contains lupeol as its bioactive compound, has been shown to possess a dual 5αRs inhibition activity (Iehlé et al., 1995; Rainer et al., 2007), therefore confirming the ability of lupeol to inhibit both 5αR1 and 5αR2. Lupeol can also be found in other numerous medicinal plants such as American ginseng, Shea butter plant, Tamarindus indica, Allanblackia monticola, Himatanthus sucuuba, Celastrus paniculatus, Zanthoxylum riedelianum, Leptadenia hastata, Crataeva nurvala, Bombax ceiba and Sebastiania adenophora (Siddique & Saleem, 2011). PS, being able to exhibit dual inhibition on both isoforms of 5αRs, further strengthens its potential as the most promising candidate as plant-derived 5αRis.
2. Phenolics
a) Polyphenols
Polyphenols (PP) are generally subdivided into two large groups: flavonoids and non-flavonoids. For centuries, preparation containing PP-flavonoids were applied as major active components in different remedies which were used to treat different human diseases (Salvamani et al., 2014). PP exert various pharmacological effects such as anti-oxidant, anti-hypertensive, anti-inflammatory and anti-thrombotic activity that can further help in promoting human health (Hollman et al., 1997; Kleemann et al., 2011; Manach et al., 2005; Vinson et al., 1995). The toxicity profiles have shown that PP exert their therapeutic effect in a dosage-dependent manner in animal studies, whereas moderate dosages of PP do not seem to elicit any adverse effects, hence indicating its beneficial effects and safe use. Conversely, at high dosages, PP might show parallel adverse effects and/or toxicity, particularly due to accumulation of high levels of PP (Silva & Pogačnik, 2020).
PP, although lacking one ‘ring’, exhibit a chemical structure similar to the synthetic 5αRis, hence representing a potential plant-derived 5αRis candidate. Quercetin, one of the PP-flavonoids, has a 3-OH group on its pyrone ring and is abundant in many fruits and vegetables. It has been shown to be non-toxic and possesses an anti-cancer property in various human cancer cell lines both in vitro and in vivo including CaP (Piao et al., 2014). In vitro, quercetin exhibits significant arrest of cell cycle, decreases cell viability, inhibits proliferation, and induces cell apoptosis especially in PC-3, LNCaP and DU-145 cell lines, whereas when used in vivo, growth of a CaP cell xenograft tumour was effectively halted at a selective dosage (Yang et al., 2015). Another PP, myricetin, possesses an aglycone structure that has been thought to attribute strongest inhibitory effects on enzymes such as DNA polymerases and DNA topoisomerase II and hence interferes with cellular proliferation activities (Shiomi et al., 2013). Myricetin has been reported to exhibit anti-tumour activity in in vitro (DU-145 and PC-3 cell lines) and in vivo (thymic nude mice) models, by promoting cell apoptosis and inhibition of cell migration and invasion (Ye et al., 2018).
Another PP, fisetin, which has two aromatic rings linked via a 3-C oxygenated heterocyclic ring with four hydroxyl groups and one oxo group, has also shown remarkable anti-cancer effects in multiple in vitro and in vivo systems. Fisetin-promoted apoptotic activation was seen in DU-145, LNCaP, and PC-3 human CaP cells (Szliszka et al., 2011). Khan & colleagues (2008) conducted a study to determine whether fisetin inhibits cell growth and induce apoptosis in human CaP cells, where the study revealed fisetin treatment decrease the viability of LNCaP, CWR22Rupsilon1 and PC-3 cells while exerting only minimal effects on normal prostate epithelial cells. Fisetin arrested the G1-phase cell cycle activity in LnCAP cells and induced cell apoptosis (Khan et al., 2008). A study by Szliszka et al. (2011) has also demonstrated fisetin’s ability to enhance cytotoxicity and apoptosis in LNCaP, DU-145 and PC-3 cells. From all of the outcomes, the PP quercetin, myricetin, and fisetin present a significant role and impact towards CaP treatment strategies via numerous pathways and this includes targeting the inhibition of 5αRs activity. An extensive study conducted by Hiipakka et al. (2002) to determine inhibition of 5αRs using varieties of polyphenols in cell-free assay and whole-cell assay, showed that PP quercetin, myricetin and fisetin were more potent against 5αR1 than 5αR2 isozyme (IC50 < 100 µM) in cell-free assay but showed little or no activity in whole-cell assay. Structure-activity relationships were also examined where it appeared that the number and position of B-ring hydroxyl groups were important for inhibitory activity against 5αR1. Many plants are reported to contain PP like quercetin, myricetin and fisetin. For example, Camellia chinensis, Allium fistulosum, Calamus scipionum, Moringa oleifera, Centella asiatica, Hypericum hircinum and Hypericum perforatum have been reported to have high contents of quercetin (Salvamani et al., 2014). High contents of myricetin has also been reported in Myrica cerifera L, Calamus scipionum, Chrysobalanus icaco L, Moringa oleifera and Aloe vera (Salvamani et al., 2014). While plants like Butea frondosa, Gleditsia triacanthos, Quebracho colorado, Curcuma longa, Rhus verniciflua, Acacia greggii and Acacia berlandieri are rich sources of fisetin (Salvamani et al., 2014).
Several other PP have also exhibited anti-CaP effects. The effect of the PP, genistein, daidzein, and biochanin A on the growth of LNCaP and DU-145 human CaP cell lines was studied where all except daidzein inhibited the cells growth (Peterson & Barnes, 1993). Wang & colleagues (2003) studied the PP reduction effect on CaP cell proliferation and apoptotic resistance in vitro using a AT6.3 rat CaP cell line and revealed that the PP kaempferol, biochanin A, and genistein were responsible for inhibited cell proliferation in a dose-dependent manner and induced apoptotic effects, except for daidzein, which counteracted the effect (Wang et al., 2003). Szliszka et al. (2013) in their study demonstrated that biochanin A remarkably augmented selective-cancer cell cytotoxicity and apoptosis in both LNCaP and DU-145 cell lines. Many in vivo and in vitro studies have demonstrated PP’s ability as 5αRis in combating CaP (Evans et al., 1995; Hiipakka et al., 2002; Park et al., 2003). Kaempferol, biochanin A and genistein were found to be more effective as inhibitors of 5αR2 than 5αR1 in a cell-free assay as well as significantly inhibit 5αR2 in a whole-cell assay (Hiipakka et al., 2002). A previous study has also demonstrated genistein and biochanin A as potent inhibitors of 5αRs, more specifically on type 2 in human genital skin fibroblasts and BPH tissue homogenates and on type 1 in prostate tissue homogenates (Evans et al., 1995). A study that used isolated kaempferol from Camellia sinensis showed good inhibition on 5αR2 in HEK-293 cells lines that expressed both 5αRs type 1 and 2 (Park et al., 2006). Park et al. (2003) revealed that Thujae occidentalis semen (TOS) extract showed high inhibition activity on 5αR2 that were expressed in HEK-293 cell lines. Previous studies have shown that TOS extracts contain PP flavonoids, which suggests a promising potential of PP as strong inhibitors of 5αRs (Hidehiko et al., 1996). Kaempferol has been identified in many other plants including Centella asiatica, Euonymus alatus, Kaempferia galanga L, Ginkgo biloba, Equisetum spp., Tilia spp., Sophora japonica and propolis (Salvamani et al., 2014). Genistein, daidzein and biochanin A which are the isoflavones that are mostly found in soybean (Glycine max), lupin (Lupinus) and red clover (Trifolium pratense L).
b) Catechin
Catechin is a type of PP that is found abundant especially in green tea. Two out of four major types of catechin are discussed herein, namely epigallocatechin-gallate (EGCG) and epicatechin-gallate (ECG). An in vivo study where PC-3 and LNCaP cell lines from tumour-induced mice was injected with EGCG revealed that within seven days the EGCG rapidly inhibited the growth and reduced the size of the CaP tumours (Liao et al., 1995). Kao et al. (2000) found that EGCG reduces blood levels of T as well as prostate growth. Stadlbauer et al. (2018) studied the anti-tumour effect of ECG in vitro and demonstrated that the treatment of LNCaP and PC-3 cell lines using ECG inhibited cell viability in a dose-dependent manner. Both EGCG and ECG were also reported to have significant inhibitory effects on cell proliferation and induced apoptosis in DU-145 cells (Agarwal, 2000; Chung et al., 2001). In regard to catechin as a 5αRi, a previous study using rat liver microsomes that expressed different types of 5αRs via retroviral expression vector pMV7 system has shown that ECG and EGCG are potent inhibitors of 5αR1 but not of 5αR2 (Liao & Hiipakka, 1995). A further extensive 5αRis study by Hiipakka et al. (2002) using a similar method as previous has demonstrated that ECG and EGCG were better inhibitors against 5αR1 than 5αR2. An in vitro study by Koseki et al. (2015) showed the reduction in DHT conversion from T in 5αRs enzymatic activity in rat liver microsomes using Quercus acutissima extract where both EGCG and ECG were identified as being amongst the major components in the extract. Catechins are found in other plants such as Betula pubescens, Betula pendula, Cocos nucifera, fruit pulp of Argania spinosa and Cassia fistula (Hiipakka et al., 2002).
3. Fatty acids
Fatty acids (FA) are monocarboxylic acids containing long hydrocarbon chains found naturally in various plants and in general can either be saturated or unsaturated (Jóźwiak et al., 2020). Saturated FA includes myristic acid (MA) and lauric acid (LA), which are a long-chain fatty acid with a 14-carbon backbone and medium-chain fatty acid with a 12-cabon backbone, respectively. Oleic acid (OA) and linoleic acid (LNA) are mono-unsaturated omega-9 FA and poly-unsaturated omega-6 FA, respectively. Toxicity profiles of FA demonstrate positive impacts on various tissues as they generally pose no significant safety concern but have only low systemic toxicity potential (Burnett et al., 2017; Karacor & Cam, 2015).
There are various studies that showed a decreased incidence of CaP with consumption of a FA-rich diet, especially from marine-derived FA, although knowledge on the effect of plant-derived FA on CaP remains limited. A clinical study that aimed to investigate the association of FA with risk of CaP in a case-control study of 209 CaP patients and 224 cancer-free men revealed that FA reduced the risk of CaP (Jackson et al., 2012). In an in vivo study by de Lourdes Arruzazabala et al. (2007) that determined the effect of coconut oil (CO), which is rich in MA and LA, on uncontrolled growth of prostate gland using Sprague-Dawley rats, it was found that CO significantly reduced the prostate growth, suggesting that CO MA/LA-rich content could be attributed to the outcomes. This is further supported by a 14-day study by Babu et al. (2010) that showed MA/LA treatment in rats significantly inhibited prostate enlargement, and a four-week study by Patil & Yadav (2016) where treatment with MA and LA in rats led to significant reduction in prostate weight and DHT level in prostate.
An in vitro study showed that LA, OA and LNA showed proliferation inhibitory effect on LNCaP cell lines (Liu et al., 2009). Another study also demonstrated LNA effects on CaP cell proliferation where it inhibited cell viability in PC-3 and LNCaP cell lines (Eser et al., 2013). Prunus africana bark extracts, where amongst the major compounds identified are MA, LA and LNA, exhibit a very strong anti-androgenic activity and can prevent proliferation and kill CaP tumour cells (Nyamai et al., 2015). Oils of Cocos nucifera and Helianthus annuus contains unsaturated FA, OA, and LNA as their major components (de Lourdes Arruzazabala et al., 2007; Sheeba et al., 2015). FA therefore represent a noteworthy contribution in both prevention and treatment of CaP through animal model and cell culture studies by mediating its effect in various pathways including via the inhibition of 5αRs enzymatic activity. Raynaud et al. (2002) conducted an extensive study on Serenoa repens lipido-sterolic extracts, which are mainly constituted of FA MA, LA, OA and LNA, for its inhibitory effects on 5αR enzymatic activity. The study determined the specificity of each FA inhibitory effect on both isozymes of 5αRs that have been cloned and expressed in the baculovirus-directed insect cell expression system Spodoptera frugiperda (Sf9). The results showed OA and LNA to be more potent against 5αR1 than 5αR2, while LA was found to be potent against both 5αR1 and 5αR2, whereas, the inhibitory effect of MA was found only active against type 2 and therefore, is a potent inhibitor of 5αR2.
CaP is one of the leading causes of death in men worldwide (Daniyal et al., 2014). Until today, various preventive and treatment strategies have been carried out to tackle the disease (Tindall & Rittmaster, 2008). The androgens, which are the modulator of prostate growth, are also thought to contribute to the pathogenesis of CaP. This in turn, has led to a surge of interest in studies that aim to block the activity of 5aRs using available synthetic inhibitors of 5aRs resulting in androgens deprivation as part of the strategies. The idea therefore represents a valid strategy for CaP prevention and treatment. However, the use of synthetic 5αRis such as finasteride and dutasteride as 5aR activity-targeting CaP medicines continues to be widely discussed. 5aRis have been reported to have numerous adverse side effects (Erdemir et al., 2008; Hirshburg et al., 2016). Due to this, study interests have switched to finding a safer remedy with no/less harmful side effects by means of natural-derived entities found in plants as an alternative to synthetic 5αRis. Plants are constituted of numerous bioactive compounds and are proven to have various powerful medicinal properties that could contribute significantly towards a healthier life (Mohan et al., 2011; Sathishkumar & Baskar, 2014). The phytochemical PS, PP and FA are discussed in this review for their potential as CaP medicines and 5αRis. Numerous in vitro studies using different type of CaP cell lines and in vivo studies using xenograft/tumour-induced animal models have revealed the ability of PS, PP and FA as potential CaP medicines targeting various mechanisms including inhibiting cell proliferation, migration and invasion, as well as promoting selective tumour cell apoptosis. In addition, the ability of PS, PP and FA as potential naturally-derived 5αRis is also demonstrated in many studies, which further validates their exhibition of anti-5αR enzymatic activity that can produce beneficial interference in androgen-dependent CaP progression. In terms of structural similarities to current synthetic 5αRis, PS that are characterised with four ‘rings’ stand as the most promising candidate for naturally-derived 5αRis and they are found to be potent against both 5αR1 and 5αR2. PP have also demonstrated anti-5αR activity on both 5αR1 and 5αR2 despite lacking one ‘ring’. FAs that exist in either saturated or unsaturated forms do not display any structural similarities to the synthetic 5αRis, but are also reported to have significant inhibitory effect against both 5αRs. All of these observations suggest a strong implication of various phytochemicals, especially PS, PP, and FA as potential CaP medicines targeting 5αR activity. In conclusion, plants represent a reservoir of novel phytochemicals that can further provide a promising line on the development of CaP therapeutic agents, especially in targeting the inhibition of 5αR enzymes.
No data are associated with this article.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Protein Biochemistry, Microbiology and Biotechnology
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Endocrine Cancer, Drug Discovery, Bioinformatics
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Partly
Are the conclusions drawn appropriate in the context of the current research literature?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Protein Biochemistry, Microbiology and Biotechnology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 06 Jul 21 |
read | |
|
Version 2 (revision) 14 Jun 21 |
read | |
|
Version 1 18 Mar 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)