Keywords
acute abdomen, gastrointestinal tuberculosis, tuberculosis, tuberculous peritonitis, neoplasm, cancer
This article is included in the Oncology gateway.
acute abdomen, gastrointestinal tuberculosis, tuberculosis, tuberculous peritonitis, neoplasm, cancer
This new version is a little more extended version giving more details about the case description and discussion according to the reviewers’ comments.
In the Abstract, we added a sentence making transition from tuberculosis in general to abdominal tuberculosis.
In Evolution of Case 1 and Case 2, we structured the text into three paragraphs in order to make this paragraph easier to read.
In Discussion, we have rewritten two paragraphs in order to relate pertinent literature with our presented cases. In addition, we highlighted the fact that tuberculosis is the great mimicker and diagnosing properly tuberculosis could be economically beneficial.
We added two new References regarding misdiagnosis of cancer as tuberculosis (the opposite as in our cases) and cost and cost-effectiveness analysis of diagnosis of tuberculosis.
Finally, we changed some improperly used words in Abstract, Evolution and Discussion.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Tuberculosis (TB) is the most widespread infectious disease and one of the leading causes of death worldwide.1-3 The proportion of patients with extra-pulmonary tuberculosis has risen during the last decades all over the world.1,2 Tuberculosis in Peru ranks fifteenth among the causes of death, and predominantly affects the poorest social strata.2 Abdominal tuberculosis is defined as infection of the peritoneum, omentum, mesentery, lymphatics nodes, and hollow or solid abdominal organs such as the liver, spleen, and pancreas with Mycobacterium tuberculosis.4-7 Abdominal TB accounts for about 5% of all TB cases and 15% of extrapulmonary TB forms and is an increasingly common disease that can have bizarre presentations.8-11
Herein, we present two cases of abdominal TB mimicking at the presentation an acute abdomen related to surgical aspect of appendicitis, “carcinomatosis”, and septic shock. This report aims to suggest to clinicians the need of including TB in the differential diagnosis of pulmonary and extra-pulmonary malignancies.
A 32-year-old man presented to the Emergency Department (ED) of Hospital Regional Lambayeque in Peru on July 21, 2019. He had been diagnosed with diabetes mellitus (type 2) two years before, but he did not follow treatment because of financial reasons. He denied risky sexual behavior, smoking, alcoholism, or any previous infectious disease. Furthermore, he denied weight loss, cough, hemoptysis, sweating, diarrhea or constipation. Thirty days before admission, he reported mild colicky hypogastric pain, without irradiation. One week before admission, abdominal pain turned to severe, diffuse, and persistent; and anorexia, general malaise, and premature abdominal fullness after meals were added. Three days before admission, fever and vomiting started.
At admission, remarkable basic hematology and biochemistry were leukocytosis (15 200/μL), lymphopenia (608/μL), hypoalbuminemia (2.9 g/dL), and a glycated hemoglobin of 6.8 mg/dL. Serologies for human immunodeficiency virus (HIV), human T-lymphotropic virus type 1 (HTLV-1), hepatitis B and C, and rapid plasma reagin/Venereal Disease Research Laboratory (RPR/VDRL), were negative. A chest x-ray (CxR) was normal. An abdominal ultrasound showed free fluid (200 ml) in the pelvic cavity associated with inflammatory signs of mesenteric fat in the right iliac fossa. Acute appendicitis was diagnosed and laparotomy was scheduled. The surgical report described: “multiple and widespread adhesions, linitis plastica, carcinomatosis, hard tumors (0.3-0.5 cm) in the peritoneum, short bowels, and cecum, and multiple abscess in both iliac fossae with purulent and foul-smelling secretion”.
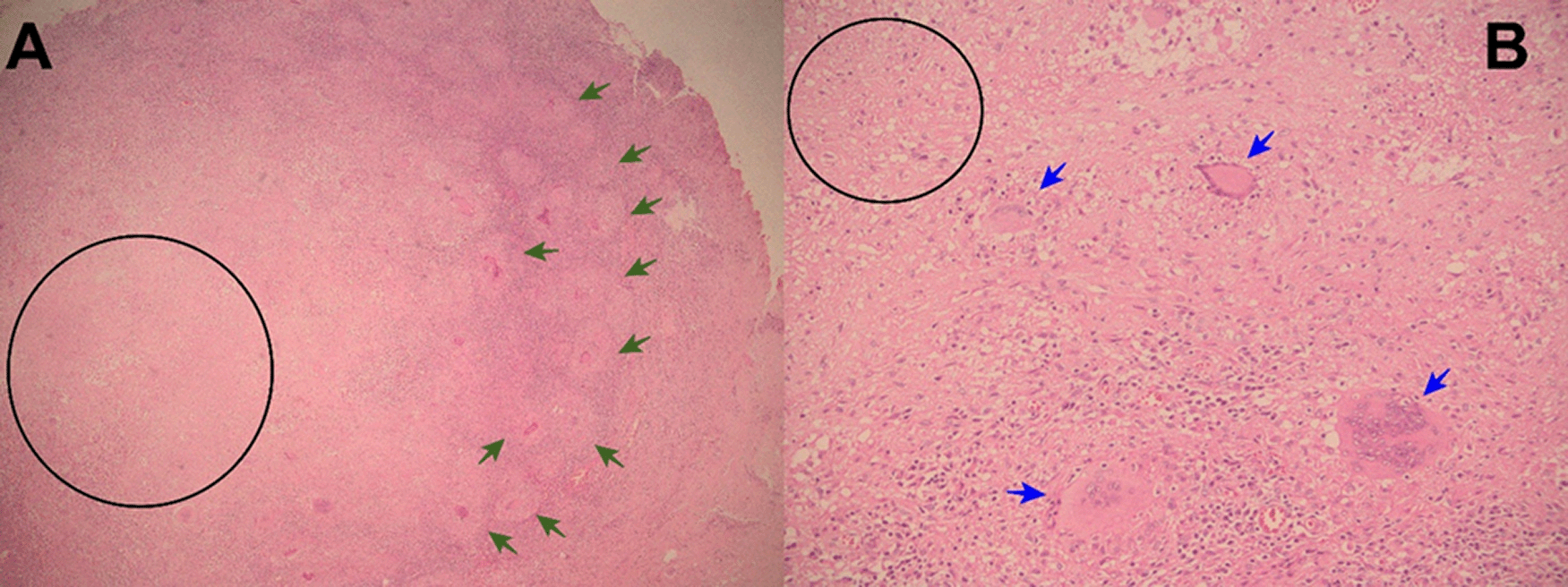
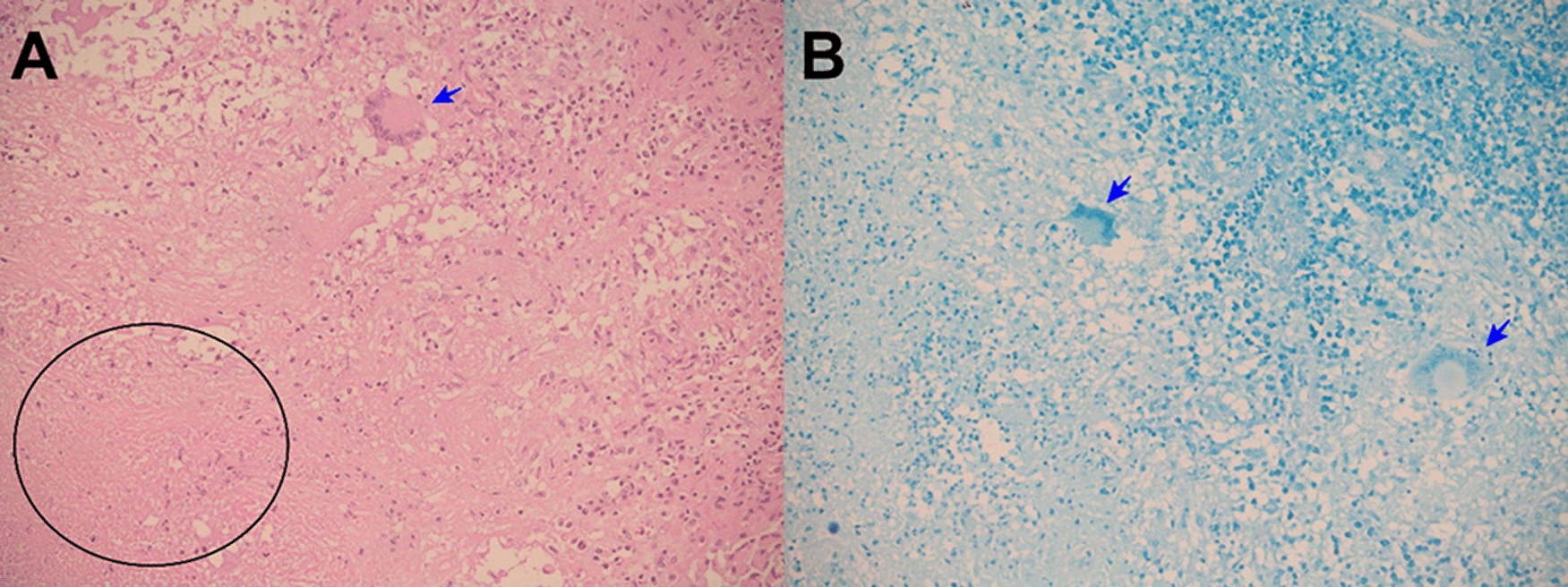
Evolution. One hour after surgery, because of clinical signs of hemodynamic shock, the patient was transferred to the intensive care unit (ICU). At ICU admission the patient was febrile (38.5°C), with hypotension (arterial blood pressure 70/30 mmHg), tachycardia (116 beats per minute), tachypnea (26 breathes per minute) without hypoxia (peripheric saturation 96% in ambient-air). Severe dehydration with cold and mottled skin with prolonged capillary refill time, with sleepiness but respondent to verbal stimulus; the abdomen was distended and painful, with reduced intestinal peristalsis, and the wound (8 cm in length) of the recent surgery was macroscopically in order and percutaneous drainages in both iliac fossa were in place showing serohematic secretion. A broad-spectrum antibiotic therapy (meropenem and metronidazole) was started, together with supportive treatment including vasopressors (noradrenaline) and fluid replacement. After a week of the above-mentioned treatment, the clinical condition of the patient was improved and at the seventh day after the surgery, the patient was transferred from the ICU to another hospital division. Then, the result of the histopathological exams of samples collected during surgery showed: “chronic granulomatous inflammation, granulomas, extensive caseous necrosis and Langhans giant multinucleated cells; Ziehl-Neelsen (ZN) stain for Acid Fast Bacilli (AFB) negative”, and a diagnosis of abdominal TB was made (Figures 1-3). Based on this result quadruple anti-tuberculous treatment (isoniazid 5 mg/kg, rifampicin 10 mg/kg, ethambutol 20 mg/kg, pyrazinamide 25 mg/kg) was initiated in concordance with Peruvian and international guidelines.11,12 Subsequently, the diagnosis was confirmed by a polymerase-chain reaction (PCR) for Mycobacterium tuberculosis in peritoneal fluid. The tuberculin skin test (Mantoux or PPD test) was negative. Serial ZN smears for AFB in urine, sputum, secretion of both pelvic drains, were negative; adenosine deaminase test in peritoneal fluid 292.7 U/L (reference value < 40 U/L). Blood cultures, and cultures for common pathogens, fungi, and M. tuberculosis in urine and peritoneal fluid were negative. Before discharge, the patient was evaluated by an oncologist who suggested some other exams: serum tumor markers (CEA, CA 125, AFP, CA19.9, β-HCG), esophagogastroduodenoscopy, and colonoscopy with ileoscopy, all of which were within normal ranges. In addition, contrast-enhanced chest-abdomen-pelvis computed tomography (CT) and magnetic resonance imaging (MRI) showed “multiple nodular implants in spleen, gut, and peritoneal cavity; but no hepatosplenomegaly or abnormal lymph nodes enlargement” (Figure 4). One week after the start of the antituberculosis treatment, the patient was discharged from hospital because of clinical improvement.

A 20-year-old man presented to the ED of Hospital Regional Lambayeque on July 18, 2020. He denied any previous disease, but had been incarcerated for almost two years before admission. He presented for six months before admission with fever, productive cough, abdominal pain, nausea and weight loss. One week before admission, he initiated with colicky abdominal pain, nausea, vomiting, and anorexia for three days. One day before admission, abdominal pain turned into intense and diffuse, and fever, persistent vomiting, and absence of passage of flatus and feces were added.
Physical examination at admission. The patient was emaciated and mildly dehydrated; blood pressure: 100/60 mmHg, respiratory rate: 20 breathes per minute, heart rate 136 beats per minute, temperature: 37.9 °C, and oxygen saturation: 95% at room air. Absent breathe sounds and dullness in both lung bases. Tachycardia. Distended and painful abdomen, bowel sounds diminished in frequency, McBurney’s and Blumberg's signs were positive. Awake and oriented patient, no focal deficit, no meningeal signs.
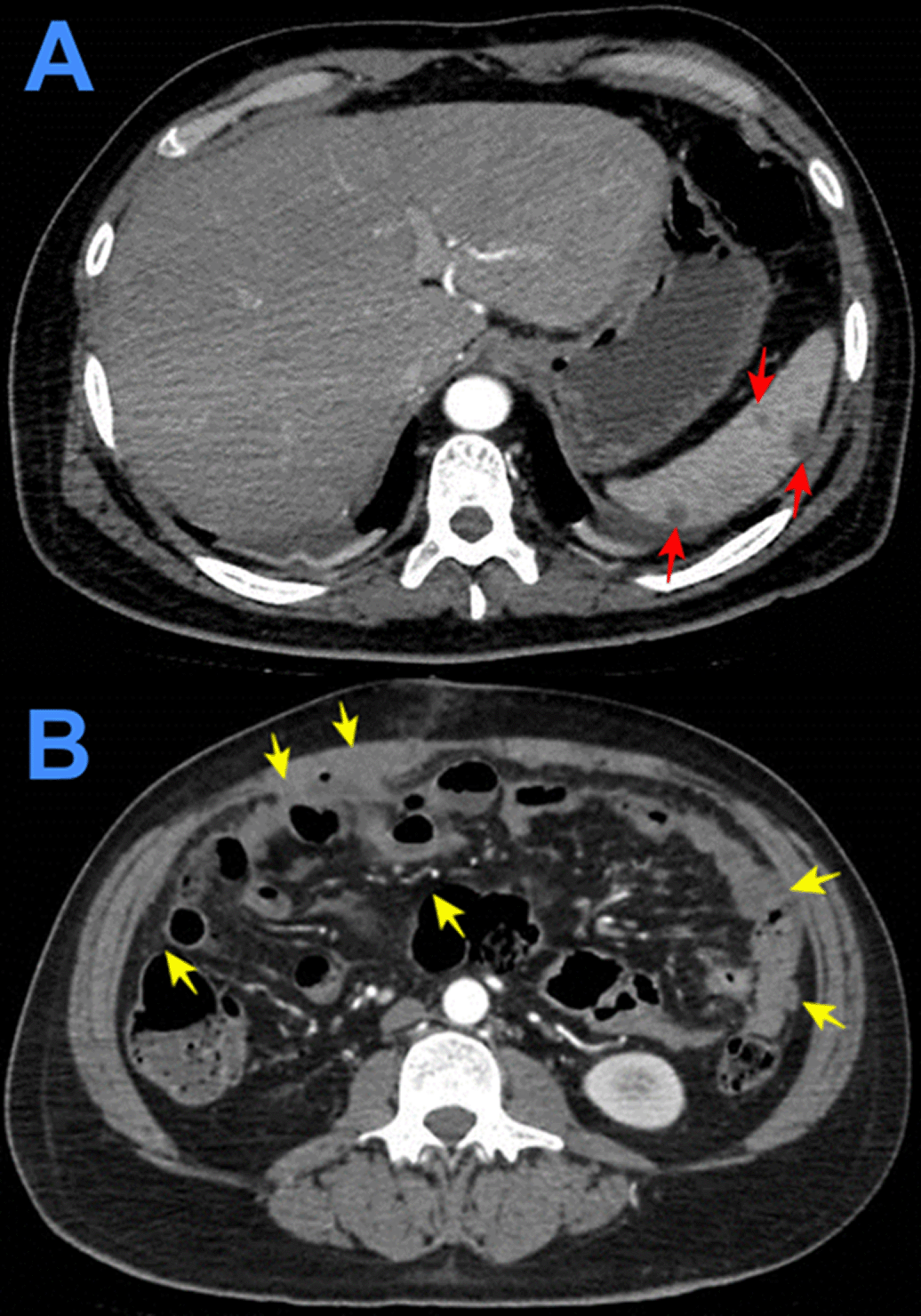
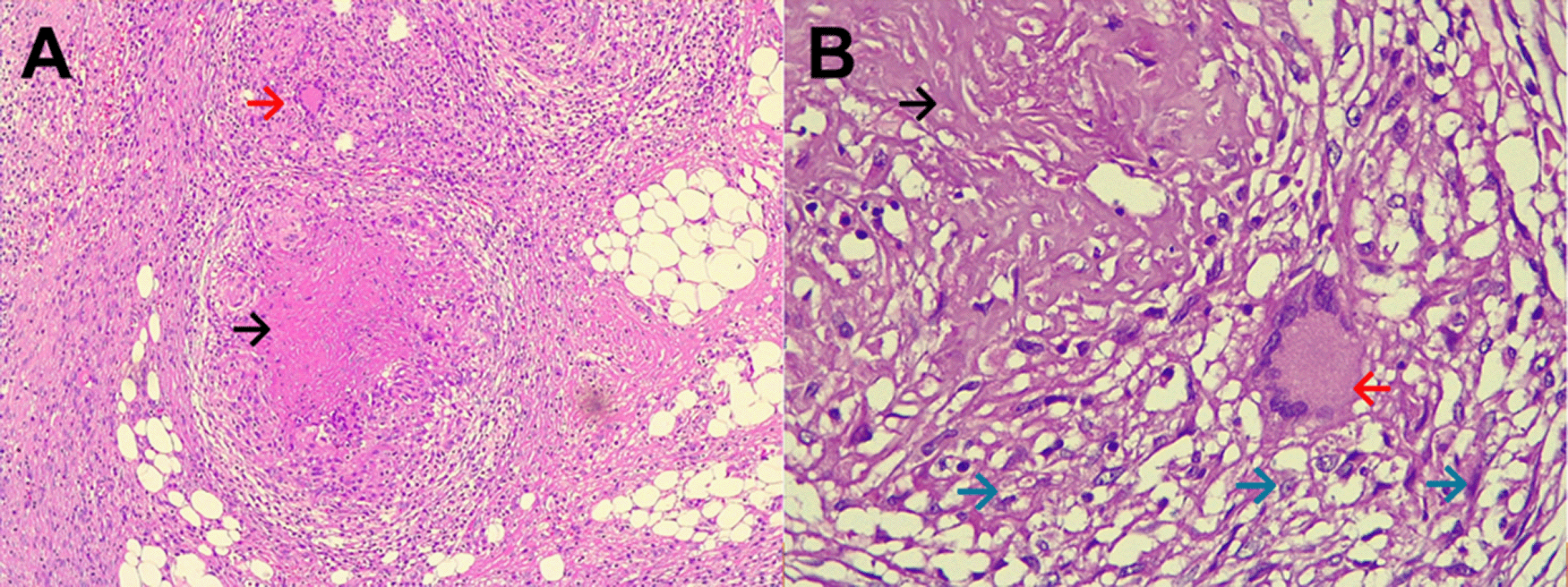
Evolution. After admission, acute abdomen due to appendicitis was diagnosed. Symptomatic treatment was initiated and preoperative exams and plain chest and abdominal x-ray were ordered, which showed significant bilateral pleural effusion and ascites. Three hours after admission, the patient was transferred to the operating room. During exploratory laparotomy “multiple cerebroid nodules scattered through parietal and visceral peritoneum, small bowel, colon and epiploon; and plenty of ascitic fluid were observed”. The postoperative diagnosis was “carcinomatosis”. After surgery, he was transferred to the ICU, and continued treatment with saline, noradrenaline, imipenem, metronidazole, sedation with midazolam, analgesia with fentanyl, and ventilatory support. Based on clinical data, risk factors, intraoperative findings, and chest x ray imaging, TB was suspected. Then, stains and cultures of multiple samples for Mycobacterium tuberculosis (MTB) and a contrast-enhanced tomographic scan of thorax, abdomen and pelvis were ordered (Figure 5). Three days after surgery thoracocentesis was performed. Five days after surgery RT-PCR resulted positive for MTB in pleural fluid and quadruple anti-tuberculous treatment for MTB was initiated (the same scheme as Case 1). Ten days after surgery histopathology of epiploon was obtained, showing extensive caseous necrosis with granulomas and Langhans multinucleated giant cells. The patient died seven days after initiation of anti-tuberculous treatment due to refractory septic shock (Figure 6). A serial ZN smear for AFB in bronchial secretion, blood cultures, and urine culture, were negative. Serologies for HIV, HTLV-1, hepatitis B and C, and RPR/VDRL, were also negative.
Abdominal TB generally presents in four main clinical forms according to the abdominal involvement: enteritis (gastrointestinal), peritonitis, lymphadenitis, and visceral (solid organs) tuberculosis. Most of abdominal TB cases have a combination of these basic forms.5,12,13 Tuberculous enteritis most frequently involves the ileocecal region (80-90% of cases).5,13,14 In our patients we demonstrated enteric and peritoneal involvement.
Abdominal TB occurs by: 1) hematogenous or lymphatic dissemination from a primary lung focus or miliary disease; 2) reactivation from a latent infectious focus; 3) contiguous spread from mesenteric or celiac lymphatic nodes or adjacent organs (i.e. retrograde dissemination from fallopian tubes); 4) direct mucosal invasion by ingestion of tuberculous mycobacteria (ingestion of unpasteurized milk or undercooked meat). Secondary dissemination from a miliary or lung focus occurs in 10-50% of patients, and generally presents with massive involvement of the gastrointestinal tract.5,13-15
Approximately 15-25% of patients with abdominal TB have concomitant active pulmonary TB,1,13-15 more than 50% of patients with abdominal TB patients have some alteration in CxR suggestive of TB,6,13-15 and one third of abdominal TB have evidence of extraperitoneal TB.4,6 This high prevalence of extrapulmonary TB suggests that abdominal TB is a part of disseminated TB. Hematogenous dissemination from a primary lung focus is probably the most important mechanism of peritoneal involvement.6,15 In our patients, we did not find active pulmonary tuberculosis.
Abdominal TB represents a diagnostic challenge and requires a high index of suspicion. Clinical manifestations of abdominal TB are variable and unspecific and can include fever, weight loss, abdominal pain and/or distension, ascites, hepatomegaly, diarrhea, intestinal obstruction, and abdominal mass.5,15 Tuberculosis can be confused with other diseases. The differential diagnosis of abdominal tuberculosis includes abdominal cancers, sarcoidosis and Crohn´s disease. These entities can imitate clinical, radiologic, endoscopic, and even laparoscopic features of entero-peritoneal tuberculosis. Thus, it is of paramount importance to take biopsies.5,15-18 Direct biopsy of suspicious lesions by laparoscopy or laparotomy is the most certain method to obtain diagnostic samples.4-6
Histopathology (necrotizing or caseous granulomas and/or AFB) is highly suggestive and virtually diagnostic of tuberculosis, but it is not specific or pathognomonic. However, in the right clinical and epidemiological context, typical findings can support a presumptive diagnosis of TB1,6,14-16 as occurred in our patients. Hepatic biopsy has a sensitivity of 68% for caseating granulomas in hepatic biopsies.15,18 But, among patients with colonic tuberculosis, only 32.6% have caseous granulomas, and 5% have AFB in colonic biopsies.18
A definitive diagnosis of abdominal TB is made by demonstration of Mycobacterium tuberculosis (in peritoneal fluid or involved organ) by culture and/or PCR. The sensitivity of AFB smear and mycobacterial culture for ascitic fluid is low (<2% and <20%, respectively); whereas, the sensitivity of AFB smear and mycobacterial culture for biopsy specimens is in general <50%.1,16 PCR is more sensitive and specific for diagnosis of TB than AFB smear or mycobacterial culture, but the diagnostic yield of PCR varies depending on the tissue type. The sensitivity and specificity are high for peritoneal fluid and pancreatic and hepatic tissue, but intestinal tissue may be associated with false-positive PCR results.1,15
Abdominal tuberculosis is the great imitator or mimicker of abdominopelvic pathology.4,8-10 There exist a few reports of cases initially misdiagnosed as cancer that subsequently were proved to be tuberculosis.8-10 In a study with 170 patients referred to the National Institute of Neoplastic Diseases of Peru because of a presumed diagnosis of cancer, the final diagnosis was extrapulmonary TB in 77.7% of cases, and pulmonary TB in the 22.3% remaining. Among these 170 patients, three patients were admitted with diagnosis of gastrointestinal cancer and 17 with ovarian cancer; and entero-peritoneal TB was finally diagnosed in nine, and genitourinary TB in seven patients. Despite extrapulmonary TB accountings for up to 20-25% of TB cases in non-HIV infected patients, it is the most common localization that could mimic cancer, probably due to the fact that solid lesions such as tuberculomas —more common in not severely immunocompromised patients— tend to be more frequently confused with malignancies. Besides, in 63.5% of patients the extension of the “neoplastic” lesions would suggest an advanced-stage disease.8 Misdiagnosis of tuberculosis as cancer is a major concern. Even worse, assuming a case of abdominal tuberculosis as an advanced-stage cancer would reduce the chance of performing more exhaustive studies to get a definitive diagnosis which could have profound implications in the management and prognosis of these patients. Consequently, most of these patients who are considered erroneously to have advanced cancer are offered only palliative care.8 Conversely, there exist reports of cancers mistakenly diagnosed and treated as tuberculosis. Underdiagnosis or misdiagnosis of cancer is reported to be as high as 44%.15,19
Prompt diagnosis and treatment can reduce morbidity and mortality, and additionally can limit tuberculosis dissemination especially if there exist concomitant pulmonary disease. Almost all abdominal TB cases offered proper treatment result in complete remission and prognosis is excellent. Urgent surgery is only necessary in a minority of cases, if there is obstruction, ischemia, perforation, massive bleeding, or secondary peritonitis.4,15,16 Furthermore, strengthening health systems to improve the value of tuberculosis diagnostics in low and middle-income countries is potentially highly cost-effective.20
These cases are enlightening and worthy of reflection because of several reasons: 1) the rare presentation of the clinical picture as an acute surgical abdomen, septic shock, and “carcinomatosis”; 2) the absence of other causes of immunosuppression, except for diabetes in one case; 3) although, histopathology did not establish a definitive diagnosis, it did provide important diagnostic clues to support a presumptive diagnosis and initiation of empiric antituberculosis treatment, until TB was confirmed subsequently by RT-PCR.
In conclusion, these cases exemplify that we must employ our clinical judgement in conjunction with all diagnostic tools available in order to make an accurate diagnosis within the shortest possible time in patients with a clinical picture suggestive of malignancy. We must be aware that TB can mimic cancer, but the opposite is also true. In consequence, clinicians should always consider TB among the differential diagnosis of malignancy, especially in high endemicity regions or countries with high rates of immigrants.
All data underlying the results are available as part of the article and no additional source data are required.
Informed Consent: The patients provided written consent to the publication of this clinical practice article and pictures. This study was approved by the Ethics and Research Committee of Hospital Regional Lambayeque (approval number: 0321-095-20 CEI).
Declaration of Helsinky: The Authors have respected the Declaration of Helsinki as a statement of ethical principles for medical research involving human subjects, including research on identifiable human material and data.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the cases’ history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the conclusion balanced and justified on the basis of the findings?
Yes
References
1. Foster N, Cunnama L, McCarthy K, Ramma L, et al.: Strengthening health systems to improve the value of tuberculosis diagnostics in South Africa: A cost and cost-effectiveness analysis.PLoS One. 2021; 16 (5): e0251547 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Medicine clinical and research
Is the background of the cases’ history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the conclusion balanced and justified on the basis of the findings?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Surgical and public health research.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 12 Jul 21 |
||
|
Version 1 07 May 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)