Keywords
Cigarette Smoke, Candida albicans, Biofilm , RNA-aptamer, ALS3 and HWP1.
Cigarette Smoke, Candida albicans, Biofilm , RNA-aptamer, ALS3 and HWP1.
In this revised version, we made corrections to some typos. Also, as suggested by the reviewer, the concentrations of the used aptamer have been changed to 1 ng/μL and 10 ng/μL instead of 1% and 10%, respectively.
See the authors' detailed response to the review by Zamirah Zainal-Abidin
See the authors' detailed response to the review by Samira Shirooie
Candida albicans is a normally harmless inhabitant of the oral cavity. However, unlike other fungal pathogens that exist primarily in either yeast or hyphal forms, C. albicans is an opportunistic pathogen. The fungus’s behaviour correlates with its ability to grow in distinct morphogenic states, including budding yeast or blastospores, pseudo hyphae, and true hyphae1–3. This morphogenic transition from yeast to hypha form is important for the pathogenesis of C. albicans, is dependent on how the fungus cell responds to the environmental cues4. As shown in the literature, cigarette smoke is one factor that can aid and accelerate this transformation5–7, and there is a clear association between oral candidiasis and smoking habit8. In this regard, the presence of nicotine in cigarette smoke promotes C. albicans growth rate and adhesion, in which, a higher level of expression of genes related to adherence, such as ALS3 and HWP1 have been reported9.
Smoking is a social habit in many countries10, and it is an important public health problem, including in Indonesia11. However, despite the extensive research exploring the deleterious role of a smoking habit on oral microorganisms, there is little information about the effect of non-conventional tobacco, i.e., clove cigarettes, on the virulence attributes of C. albicans. By searching the literature, we found that, like conventional cigarettes, the clove cigarette, commonly known as kretek, is the most popular12 in Indonesia. It is also a source of numerous toxicants, and they have a potential implication in the oral ecosystem13, which may also influence oral Candida pathogenicity14. The unique aspect of kretek is the dried clove buds it contains15 which have never been identified in a conventional cigarette. The involvement of clove cigarette smoke condensate (CCSC) on the biofilm formation of C. albicans remains obscure. Given that cigarette smoke contains many toxicants13, it is important to explore the involvement of CCSC on the growth and morphogenesis of C. albicans, as this fungus is the most implicated oral pathogen in the clinical setting.
During the last several years, aptamers, either single strain DNA or RNA, and against different microorganism species, have become the focus of growing interest. In an earlier study, we reported an anti- Candida activity of an RNA-aptamer (Ca-apt1) against the fungus while growing as biofilm16. Here, we evaluated the aptamer's beneficial properties against C. albicans biofilm formation induced by cigarette smoke. Particularly, the aptamer's ability to restrict the transition of fungus phenotype from yeast to hyphal form.
In this study, we used a clinical isolate that we collected previously from the denture surface of denture wearer subject17 and selected by using CHROMAgar18, while the reference strain (C. albicans ATCC 10231), which was used as a targeted aptamer ligand16, was needed for the evaluation and validation of this experimental study. Briefly, all microorganisms were taken from stock cultures frozen in 15% glycerol at -80°C and sub-cultured onto yeast peptone agar plates (1% yeast extract, 2% peptone, 2% glucose;YPD) or when indicated in yeast nitrogen base medium, supplemented with 50 mM glucose (YNB).
The synthetic oligonucleotide used in this study was RNA-aptamer (Ca-apt 1) that was obtained from the systemic evolution of ligands by exponential enrichment (SELEX) method16.
In this study, condensed smoke cigarettes (CSC) were generated from Indonesia's non-filtered clove cigarette (kretek) and imported non-clove cigarettes (Figure 1) that we purchased from a local tobacco outlet. The CSC, either from a clove cigarette (CCSC) or a non-clove cigarette (NCSC), was prepared by smoking five cigarettes that were smoked to an in-house smoking device5 and concentrated in 100 mL of 0.09% Sodium Chloride solution. The resulting condensate smoke solution was further sterilized by filtration through a Millipore filter (0.22 µm). The influence of CCSC/ NCSC-containing YNB (pH was adjusted to 7.2) on the viability of aptamer-precoated C. albicans in the preformed biofilm was assessed colorimetric (MTT) assay and kept at 4°C until use.
To determine that the inhibition effect of the tested aptamer in the early biofilm development stage was independent from the biofilm biomass maturation, we separated biofilm development into adhesion and growth phases. Initially, by analysing the mRNA expression of adhesion-associated genes (ALS3 and HWP1), we wanted to confirm if the inhibition event was due to the lessened ability of C. albicans to switch its phenotype from yeast to hypha form. To do this, the test aptamer was first preincubated with C. albicans yeast cells before the fungus was inoculated into microplate wells. Further, we did an analysis of mRNA expression of adhesion-associated genes (ALS3 and HWP1) after 90 min incubation time. The transcription level of these genes was also measured after C. albicans was exposed with either cigarette smoke, but without precoating aptamer. This was done to evaluate CSC's involvement in the transition C. albicans morphogenetic, from yeast or blastospore to hyphae form. All the procedures used as described in a previous study17. Briefly, RNA isolation, purification, and reverse transcription of cDNA were conducted using TRIzol™ Reagent (Invitrogen Life Technologies, Carlbad, California, United States) followed by reverse transcription using the TaqMan® Reverse Transcription Reagents (Applied Biosystems, Waltham, Massachusetts, USA). The resulting cDNA (1 µg) was amplified by qPCR with specific primers used in our previous study19. The qPCR analysis was performed in ABI StepOnePlus™ Real-Time PCR Systems (Applied Biosystems) with Platinum™ SYBR™ Green qPCR SuperMix-UDG (Invitrogen). The qPCR cycling conditions consisted of a 10-minute initial denaturation at 95°C followed by 40 PCR cycles of 15 seconds at 95°C and one minute at 60°C. The formula of fold change 2-ΔΔCt was used to calculate the relative mRNA expression, which was compared with that of the housekeeping gene, 18S rRNA. The mRNAs gene (ALS3 and HWP1) expressed by C. albicans without bound aptamer or without CSC exposure were used as a control, set at one.
To test the effectivity of CSC, with and without precoating aptamer, on C. albicans biofilm formation, we used a biofilm assay that was performed as previously described19. Briefly, 100 µL containing 1.8 X 105 yeast cells of C. albicans (counted by using a haemocytometer), from overnight culture in YPD broth was aliquot into 96-well microtiter plates containing mixture of 70% of fresh yeast nitrogen base (YNB; sigma-Aldrich) and 30% condensate smoke (vol/vol). The pH of the mixture was adjusted to neutrality (7.0) using 1M NaOH. This was done to define that the pH of the mixture does not play a role in modulating biofilm formation.
The aptamer was prepared by separating it in two different concentrations (1ng/µL and 10 ng/µL) in buffer16, prior to being added into separate wells, and the plates were incubated at 37°C in 5% CO2 in air for 90 min with gentle shaking. Candida albicans biofilm with mix medium added instead of aptamer was used as a negative control. To promote biofilm formation, the adhered cells were washed twice with sterile Phosphate Buffer Saline (PBS), C. albicans were further grown in a medium (150 µL) containing growth medium without aptamer. The culture period was further lengthened to 48-h. The extent of biofilm formation estimated using the semi-quantitative MTT (3-(4,5-dimethylthiazol-2,5-diphenyltetrazolum bromide) reagent (Sigma-Aldrich, St. Louis, MO, USA). The absorbance was measured spectrophotometrically at 450 nm with 620 nm as the reference wavelength for this assay. The results expressed as OD450/620, and were correlated with cellular metabolic activities within the biofilm. The assay was done in triplicate, repeated two times independently. Moreover, the percentage of fungal damage was calculated based on data obtained in MTT assay, using the formula: 100X [(1- ODCandidaCSC+apt / ODCandida alone)]. Biofilm formation on the bottom of microtiter well plates was qualitatively observed using an inverted light microscope.
Following incubation for 90 min or 48-h, C. albicans cultures were observed microscopically and its morphology images were analysed qualitatively.
Statistical analysis was performed with GraphPad PrismTM (version 9.00) software (GraphPad Software, Inc., San Diego, California, USA). The two-way analysis of variance (Two-way ANOVA) with Geisser-Greenhouse correction was used to verify the significant level of response between and within groups comparison. Each experiment was carried out in triplicate wells and repeated at least twice, independently. A P < 0.05 value was considered statistically significant.
Written informed consent was obtained from participants for use of their data.
The samples were collected, after informed consent was obtained from all the participants17, in accordance with the approved protocol of the Bioethics Committee of the Faculty of Dentistry, Universitas Indonesia (protocol number 020950818). The protocol conformed to the criteria of the Helsinki Declaration and the good clinical practical guidelines of the International Council on Harmonization.
To understand the underlying relationship between precoating aptamer (Ca-apt1) and C. albicans morphogenesis at molecular level, we firstly compared the expression profile of two adhesion-associated genes (ALS3 and HWP1). To do this, the test aptamer was preincubated with C. albicans yeast cells before the biofilm was allowed to form. Further, we combined quantitative analysis of mRNA expression of these genes and qualitative microscopic images to describe the adhesion event. The result showed, that after 90 min, the expression of ALS3 by the adherence cells were proportionally reduced by ≈ 8% and ≈ 4%, for aptamer concentration 1 ng/µL and 10 ng/µL, respectively. A similar trend in the expression of adhesion-associated genes was noted in either C. albicans strain used. We noted that the reduction of HWP1 expression was higher than ALS3 under different concentrations of Ca-apt1. In either C. albicans strain, this gene downregulated was > 50% (Figure 2A and B). Further, the microscopic images show that at the attachment phase (90 min), the biofilm consisted of both yeast cells and blastospore that adhered to the surface, and those cells adhered to other cells attached to the surface (Figure 2C–E).
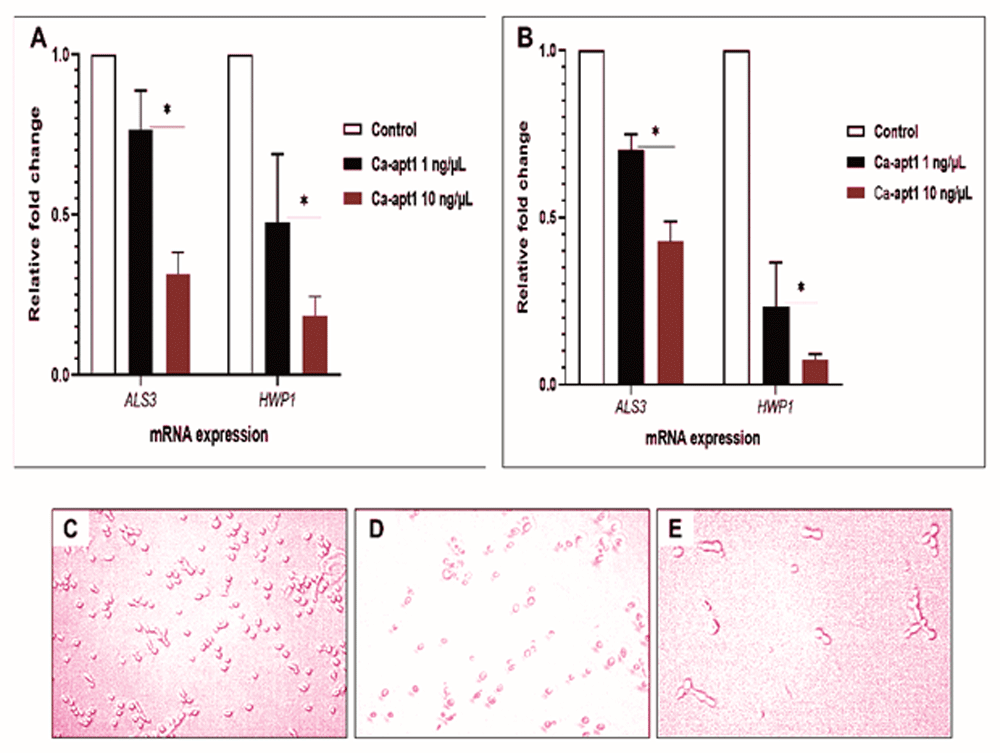
The upper panel shows the effect of precoating aptamer on ALS3 and HWP1 expressed by C. albicans in preformed biofilm (90 min incubation time) were analysed by qPCR. The mRNA expression levels were normalized relative to the control (yeast cells without aptamer precoating), which was set to one for each gene to determine the fold change in expression of genes in C. albicans clinical isolate (A) and the ATCC 10231 (B). The results are expressed as the mean and standard deviation (SD) of triplicate experiments and repeated two times independently. *Significantly higher downregulation in the expression of mRNA (P < 0.05) in the presence of precoating aptamer. The lower panel is representative of micrograph of C. albicans yeast cell (that only shown in clinical isolate) without precoating aptamer (C), and the reduced germinated cells after incubating for 90 min with precoating aptamer 1 ng/μL (D) and 10 ng/μL (E). All images were captured by using light microscopic at X 200 magnification.
Next, we tested the mRNA expression of ALS3 and HWP1 on the biofilm formation promoted by either cigarette smoke condensate (CSC) after 48-h incubation time without precoating aptamer. As shown in Figure 3A and B, by comparing CCSC and NCSC, we observed that Candida adhesion was due to the presence of CCSC that subsequently increased the transcription level of hypha-associated gene (HWP1), compared to the biofilm induced by NCSC-added growth medium (p< 0.05). For the adhesion-related gene (ALS3), the transcription level was comparable when induced by either CCSC or NCSC-treated growth medium or growth medium only (control/ unexposed C. albicans). We noted that both C. albicans strain (clinical isolate and ATCC) showed a similar pattern. Figure 3C–E show the result provided by the microscopic analysis. After a 48-h time period, the hyphal form was more abundant in CCSC-treated growth medium than those in NCSC-treated biofilm.
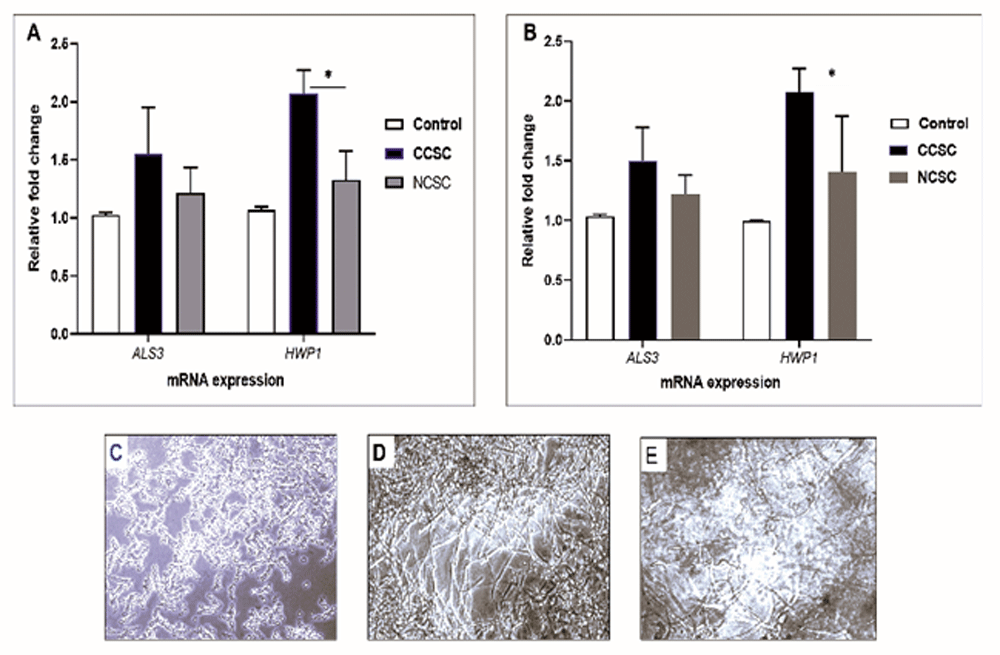
The qPCR analysis of ALS3 and HWP1 genes is shown after the preformed biofilm (90 min incubation) was treated by growth medium supplemented with different CSC (CCSC or NCSC). The mRNA level of each gene was normalized to that of 18S rRNA, while the expression level of the control (untreated biofilm) was set to one for each gene to determine the fold change in the expression of each targeted gene in clinical isolate (A) and the ATCC strain (B) of C. albicans. *Significantly higher upregulation of mRNA expression of HWP1 induced by CCSC than NCSC (P < 0.05). Data are expressed as the mean and standard deviation (SD) of triplicates from two separate experiments. The CCSC and NCSC are clove and non-clove cigarettes smoke condensate, respectively. The lower panel shows the 48-h biofilm formation that only shown in clinical isolate; the control (C) and those biofilms treated with CCSC (D) and NCSC (E), respectively. All the biofilms are visualized by using light microscopic images at X 200 magnification.
Next, we tested the impact of different concentrations of precoating aptamer (1ng/μL and 10 ng/μL) on C. albicans biofilm formation. For this, biofilms were developed in CCSC/NCSC-containing YNB (pH was adjusted to 7.2), and the quantification of biofilm cell (MTT assay) was evaluated after the biofilms reach the maturation time (48-h). This method was done, because we wanted to determine whether the precoating aptamer could adversely affect C. albicans biofilm formation after treating with the biofilm enhancers (CCSC or NCSC). Our data found that in comparison to the control, 1ng/ μL aptamer concentration of precoated yeast cells was sufficient to reduce biofilm cell, by about 10%, and 40 %, as observed in CCSC and NCSC-treated biofilm, respectively. At this maturation state, the viable cells detected within this biofilm were significantly reduced (p< 0.05), because of the increased aptamer concentration by 10% in the pre-adherence phase of the biofilm (Figure 4A–B). Further, the effect of CCSC or NCSC on the transition of yeast to hypha morphology in pre-adhered cells was visualized microscopically. We observed that when the fungus cells were precoated with aptamer without exposed with CCSC or NCSC and incubated for 48-h, the pre-formed biofilms were dominated by blastospores, as compared to the control cells (uncoated cells), which showed that the adhered cells were dominated by dense hyphae form (Figure 5 A–B). This qualitative effect was also dependent on the concentration of the aptamer tested (Figure 5C–F).
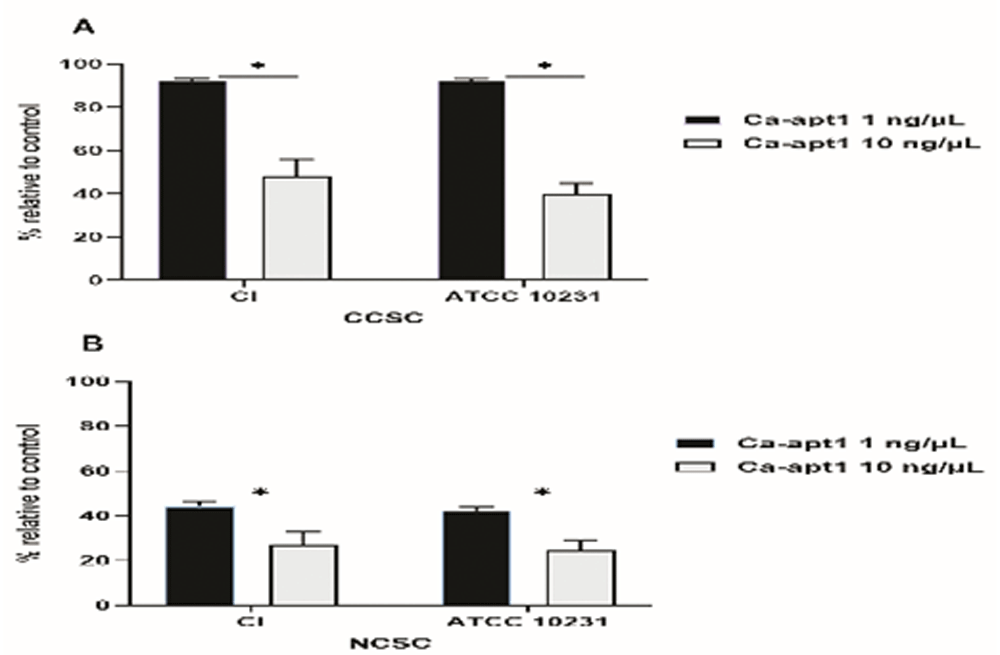
The effect of CCSC (A) and NCSC (B) exposure on biofilm formation of C. albicans, which had been precoated with different concentration of Ca-apt1, evaluated after incubation for 48-h. The relative biofilm formation compared to biofilm without the precoating aptamer (control) was calculated. Data represent the mean and standard deviation (SD) of three biofilms grown on two separate occasions. Asterisks denote statistically significant differences between the effect of cigarette smoke condensate determined by MTT assay, p < 0.05. The CCSC and NCSC are clove and non-clove cigarette smoke condensate, respectively.
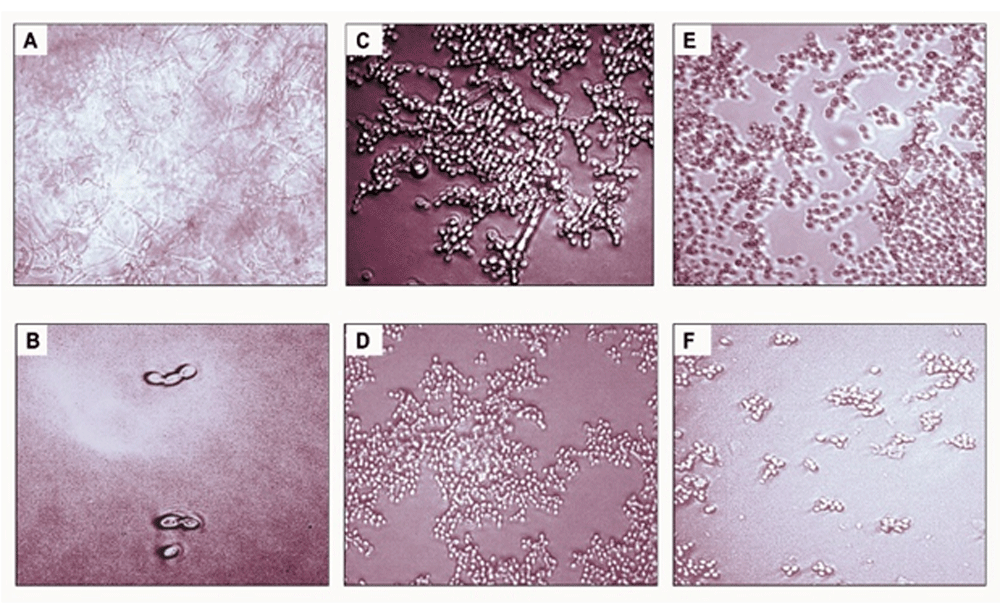
The pictures show biofilm of C. albicans derived from clinical isolate, without either precoating aptamer or added CSC (A), C. albicans with precoated aptamer but untreated with either CSC/ CCSC or NCSC (B), C. albicans precoated with 1 ng/μL aptamer, CCSC (C) and NCSC (E), and 10% aptamer with CCSC (D) and NCSC (F). All microscopic image show at X 200 magnification.
The current study attempted to get further insight into clove cigarettes' effect on non-mammalian eukaryotes cells, taking the fungus C. albicans as a model system. Initially, as a means to determine that the restriction effect of C. albicans to switch its phenotype from yeast to hypha form was due to the presence of the precoating aptamer on yeast, we separated biofilm development into adhesion and growth phases. The result of the transcription assay during the initial stage of biofilm formation indicated that the aptamer involvement in reducing the expression of adhesion and hypha-associated gene (ALS3 and HWP3)20, and the transcription profile was in line with the increased concentration of the aptamer, but not on C. albicans strains. This result suggests that the aptamer (Ca-apt1) might bind to a common chemical structure on the fungal yeast cell, probably glucans and chitins21 that form the basic cell wall scaffold22. We presume that this aptamer may interfere with the adherence mechanism where these molecules are involved22.
Our study is different from most in vitro studies, which use an antibody that recognizes cell-wall proteins, that are commonly related to the host immunological status23,24. In this study, the precoating aptamer (Ca-apt1) does not directly recognize the cell wall proteins as an antibody would. In this way, we assumed that based on the ALS3/HWP1 profiles explained above, the obvious virulence factors (morphology transformation from yeast to hypha) were more likely to be the main effect of precoating Ca-apt1. This assumption was supported by microscopic observation that at the attachment phase (90 min time point) the morphology of C. albicans consisted of both yeast cells and blastospore only. This result confirmed the transcription assay that the aptamer involvement in reducing adhesion and hypha-associated gene (ALS3 and HWP3)20 was in line with the increased concentration, but not on C. albicans strains. Likewise, this qualitative data exhibited a significant potentiate of Ca-apt1 in modulating biofilm-associated genes, as well as C. albicans morphogenesis, at the early step of biofilm formation.
Although the pattern was similar, in general, the biofilm formation (90 min time point) treated with CCSC resulted in a higher viable cell than its NCSC counterpart, as we observed that after being treated for 48-h by CCSC or NCSC. Different cellular response of C. albicans was noted, both by analysing the gene (ALS3 and HWP1) profiles and observing microscopically. At this maturation biofilm state, the induction of hypha-associated gene (HWP1) was significantly different, but not for the gene's adhesion (ALS3). We noted that the transcription level of HWP1 by either C. albicans strain was higher than ALS3 only when the preformed biofilm was treated with CCSC. This result indicates that unlike ALS3, the transcription level of HWP1 was dependent on the source of the condensate smoke used. The effect of condensate smoke on C. albicans cells viability in biofilm development does not relate to C. albicans strain. We assumed that different tobacco components, specifically the principal component alkaloid of tobacco (nicotine), between kretek and conventional cigarettes25,26, may have a different effect on C. albicans when the fungus was growing in distinct environmental conditions that trigger the hyphal growth. Additionally, the HWP1 product (Hwp1) is a hypha-association protein commonly expressed on germ tube27,28. Indeed, this result can be considered after 90 min, when germ tube was induced29. The upregulation of HWP1 when the biofilm is maturing (48-h) is a survival pathway used by C. albicans to resist, or be tolerant against, the effect of chemical toxic-containing CCSC, by modulating the hypha form.
It has been reported that, like NCSC, CCSC comprises a high number of dangerous alkaloid chemical compounds, the most abundant of these being nicotine25. A previous report also showed that the CCSC and NCSC used in this study deliver significant quantities of carbon monoxide (CO), tar and presumably other toxic components of tobacco smoke. Their smoke chemistry were found to be similar30. The other chemical compound in the gas phase of cigarette smoke is nitrogen (N2)31. The nicotine in smoke yields from CCSC is equally delivered as by NCSC32. However, the nitrogen content in CCSC is lower than found in conventional cigarettes/NCSC30. The most distinguished particle-phase, which is only found in CCSC, is eugenol15. Eugenol is a phenylpropanoid compound reported to have antimicrobial activity against planktonic cells of C. albicans and sessile cells within C. albicans biofilms33.
In contrast to the eugenol, the presence of nicotine in cigarette smoke promotes a high level of adhesins leading to the increased adherence of C. albicans34 on a solid surface, as found in this study. Hence, it has the potential to increase biofilm formation by C. albicans. Considering that eugenol impairs the growth of C. albicans, we assume that under the experimental condition set in this study, C. albicans sensed eugenol in CCSC, and the presence of nitrogen helped it to grow. Since we grew the fungus in a hypha conditioning medium, the result obtained in this study indicates that by comparing with NCSC, the lower nitrogen in CCSC is the likely reason for the augmentation of C. albicans biofilm formation9,35 enhanced by the CCSC. As we evaluated microscopically, more hypha in 48-h biofilm was observed in response to the presence of CCSC than NCSC.
As shown in the literature, cigarette smoke exposure (CSC) has a potential adverse health effect on the oral ecosystem36, and the growth rate of C. albicans increases when the fungus is grown in the presence of non-clove cigarette smoke condensate/NCSC5. Indeed, cigarette smoke is an important predisposing factor for oral candidiasis35. Although kretek/clove cigarette smoke condensate (CCSC) and NCSC contains many toxicants, they have some different chemical constituents30–32, which may lead to the differences in Candida cell behaviour that we observed in this study.
We further address whether the precoating aptamer could adversely affect the preformed biofilm formation's viability when treated with CCSC or NCSC. The experiment was done to confirm that, on the one hand, experimental C. albicans biofilm formation promoted by cigarette smoke (CCSC and NCSC) could be induced. On the other, that the precoating aptamer is needed for the success of this model. In this way, the discrepancy between CCSC and NCSC on C. albicans susceptibility was measured using a MTT reduction assay. This is a reliable test for an indirect method to quantify biofilm cell numbers37,38. It has been demonstrated that when growing as a biofilm, the metabolic activity of C. albicans increases over time39. However, another factor (the aptamer) was added in the current study. Here, we observed that the control group (C. albicans of untreated condensate cigarette smoke/ CSC, without precoating aptamer (Ca-apt1) remained growing as a biofilm throughout the experimental time period set in this study. This result may suggest that C. albicans used the tobacco compound as a nutritional source, as aromatic hydrocarbons in cigarette smoke can be converted by the fungus35.
Further, we found that after treating with NCSC for 48-h, the lowest concentration of tested aptamer (1ng/µL) was enough to reduce the cell viability > 50%. This indicates that the effectivity of NCSC as an accelerator of biofilm formation5 was significantly suppressed. Hence, it affirms the anti-adhesion effect of the aptamer on biofilm development accelerated by conventional cigarette smoke5,7. Surprisingly, aptamer-precoated C. albicans behaves differently upon exposure to growth medium-containing CCSC. We noted that at one ng/µL of the aptamer, significant cell growth changes were not observed compared to the control (uncoated aptamer). However, at 10 ng/µL, the aptamer could inhibit the biofilm cell growth until C. albicans reached a steady state at a 48-h time period, more and less at a similar rate to those cells exposed by NCSC. The result was supported by the light microscopy data, in which we observed that when the fungus was exposed with the minimal concentration (1 ng/µL) of Ca-apt1, the composition of 48-h-old biofilms consisted of pseudo hypha, almost similar to the control (C. albicans without pre-coated aptamer). We also observed that when the concentration of the tested aptamer was increased by 10-fold, it prevented the cell's viability and successful germination of the adherence cells, resulting in scant biofilms, and more yeast cells were observed microscopically in treated groups (CCSC and NCSC exposure). For NCSC, we observed that regardless of the tested aptamer's concentration in precoating step, the NCSC-treated biofilms were predominantly in the blastospore or yeast form compared to the control. After 48-h exposure, many of these biofilm cells were unbudded and had an unelongated morphology. At the end of the experiment time, both C. albicans strains, which belonged to the treated groups (C. albicans with precoating aptamer), showed a similar phenotype response to either CCSC or NCSC exposure. Our result contradicted a previous report that demonstrated that the clinical isolate showed less biofilm growth activity than the laboratory strain40. We reasoned that the similar trend found in either C. albicans strain used in this study is probably because the clinical isolate and the ATCC strain used in this study have a similar karyotype as it has been previously reported41.
Regardless of the mechanism involved, this study demonstrated that both CCSC and NCSC have a potentiate to enhance biofilm formation by C. albicans. By exposing either cigarette smoke prepared from clove and non-clove cigarettes, C. albicans, either clinical isolate or the reference strain, shows a similar trend in its capacity to form biofilm. However, the pattern of biofilm formation was different. Besides, this study clearly indicates that the aptamer (Ca-apt1) had an impact on the degree of cell adhesion and its concentration affected the profile of biofilm formation at the maturity phase. However, the underlying mechanisms remain to be investigated at molecular level. The underlying mechanism is particularly important for pathogenic fungus, such as C. albicans, during exposure to tobacco smoke.
Figshare: Underlying data for ‘The discrepancy between Clove and Non-Clove Cigarette Smoke-Promoted Candida albicans Biofilm Formation with precoating RNA-aptamer’, DOI 10.17605/OSF.IO/WF2KB42
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
License: CC0 1.0 Universal
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology, Oral Microbiology
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology, Oral Microbiology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology, Oral Microbiology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 29 Jul 21 |
read | |
|
Version 2 (revision) 12 Jul 21 |
read | read |
|
Version 1 11 May 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)