Keywords
aneurysmal subarachnoid hemorrhage, Nimodipine, Papaverine, delayed cerebral ischemia, general anesthesia, conscious sedation, functional outcome, hypoperfusion
aneurysmal subarachnoid hemorrhage, Nimodipine, Papaverine, delayed cerebral ischemia, general anesthesia, conscious sedation, functional outcome, hypoperfusion
To address key issues in the reviewer comments, text was added to the methods section of the paper clarifying how the choice of anesthesia was made in each patient. Additionally, WFNS, Hunt & Hess, BNI and Fisher Scores were statistically analyzed. The corresponding p-values were added to Table 1.
See the authors' detailed response to the review by Giovanna Brandi and Stefan Yu Bögli
See the authors' detailed response to the review by Roland Roelz
Cerebral vasospasms (CVS) and delayed cerebral ischemia still remain among the leading causes of morbidity and mortality in survivors of aneurysmal subarachnoid hemorrhage (aSAH). Up to 40% of aSAH patients experience symptomatic CVS, resulting in disability in up to 50% thereof.1 CVS, a narrowing of cerebral arteries thought to be caused by blood breakdown products, mostly develop between 5 to 14 days after aSAH.2 So far, there is no therapy known, which was shown in randomized trials to improve cerebral perfusion and thus to avoid brain ischemia and infarction in symptomatic patients. Commonly used rescue treatments for symptomatic CVS include induced hypertension, and in refractory CVS angioplasty or intra-arterial application of vasodilators, e.g. nimodipine or papaverine.1,3,4 Both have been shown in case series to improve neurological outcome in said patients.5–7
In recent years, several studies investigated the best method of anesthesia for endovascular treatment in acute ischemic stroke.8–20 While initially in mostly retrospective studies, data showed conscious sedation (CS) to be superior,8,9 a recent meta-analysis showed no significant difference in outcomes for CS and general anesthesia (GA)17 if only randomized controlled trials were considered.15,16,18 To the best of our knowledge, no studies comparing CS and GA in endovascular treatments for refractory CVS after aSAH have been performed.
The aim of this study is to compare six-month outcomes for choice of sedation in patients treated with endovascular vasodilators for CVS after aSAH.
This is a single-center retrospective case-control study analyzing clinical outcomes in patients with symptomatic CVS after aSAH treated with endovascular vasodilators at the University Hospital Bern, Bern, Switzerland.
The University Hospital Bern conducts a prospective database for patients treated with aSAH. This database was retrospectively searched for patients hospitalized between September 2011 and October 2019. Only patients aged >18 and <85 years were included. Inclusion criteria were: 1) aSAH of all severities (World Federation of Neurosurgeons (WFNS) score I–V), 2) secured aneurysm either by endovascular or surgical treatment, 3) refractory CVS treated by intra-arterial admission of either nimodipine and/or papaverine. Exclusion criteria were: 1) incomplete data, 2) loss of follow up, 3) continuous intra-arterial nimodipine treatment, 4) re-rupture of aneurysm during the hospital stay.
Patients were divided into three treatment groups: patients who underwent treatment with endovascular vasodilators in CS only (“CS”), in GA only (“GA”), or patients who received intra-arterial treatments in CS and GA (“both”). Choice of anesthesia modality was made by the treating physician on an individual basis. However, according to institutional guidelines, GA was preferred in patients with impaired consciousness (GCS ≤ 8) or insufficient swallowing.
All data was acquired from patient records and the institutional electronic Patient Data Management System (CentricityTM Critical Care, General Electric Company, GE Healthcare, United States of America). Vital signs are automatically recorded and the bedside team additionally enters clinical scores and administered drugs into the system.
The primary endpoint of this study was functional outcome at six months, analyzed by the modified Rankin Scale (mRS). Secondary outcome parameters included mRS at discharge and National Institutes of Health Stroke Scale (NIHSS) assessed 24 hours before the (first) intra-arterial vasodilator treatment (t1), directly before (t2), directly after (last) treatment (t3), 24 hours after (last) treatment (t4) and consecutively at discharge from the hospital (t5) and after six months (t6). Further parameters consisted of interventional parameters such as duration of intervention, choice of vasodilator (nimodipine or papaverine), number of treated arteries and vasodilator dosage.
Patient characteristics such as age, sex, aneurysm location and treatment and Barrow Neurological Institute (BNI), Fisher, Hunt & Hess and WFNS scores were obtained from institutional patient records.
The statistical analysis was performed using SPSS Statistics 21.0 (IBM, Armonk, NY, USA). The Shapiro-Wilk normality test was used to test for normal distribution.
Univariate Analysis of Variance (ANOVA) test was used to compare “CS”, “GA” and “both” groups for differences in age as well as BNI, Fisher, Hunt & Hess and WFNS scores. Differences in sex and aneurysm treatment were explored with Pearson Chi Squared analysis.
For mRS at discharge and six months, outcomes were divided in functionally independent (mRS 0-2) and functionally dependent (mRS 3-6). A Chi Squared test was used to test for significant group differences between CS, GA and both. An additional subgroup analysis was performed using a Chi Squared test with “CS” and “GA” groups divided into single versus multiple interventions, resulting in five groups (“single CS”, “single GA”, “multiple CS”, “multiple GA”, “both”).
For the analysis of NIHSS, a 3 × 6 analysis of variance (ANOVA) for repeated measures with post hoc Bonferroni correction for multiple comparisons was conducted. The factors were (i) treatment (“CS”, “GA” and “both”) and (ii) time (t1 – t6).
Interventional parameters were analyzed for each intervention separately and therefore compared between those performed in CS and GA. For the duration of the intervention, a Welch's two sample t-test was performed. The choice of vasodilator was analyzed by Pearson Chi Squared test. Vasodilator dosage as well as number of treated arteries were analyzed with an independent samples t-test.
Data are presented as mean with standard deviation (SD) in brackets and in figures as mean with +1 SD as error bars. A p-value of p < 0.05 was considered statistically significant.
This study was carried out in accordance with the recommendations of the local ethics committee (Kantonale Ethikkommission Bern, Switzerland). All subjects gave written general consent in accordance with the Declaration of Helsinki. The protocol was approved by the local ethics committee (Kantonale Ethikkommission Bern, Switzerland).
In total, 109 patients with refractory CVS treated by intra-arterial admission of either nimodipine and/or papaverine between September 2011 and October 2019 at the University Hospital Bern, Bern, Switzerland were included. Of those, 11 patients had to be excluded. Reasons for exclusion were incomplete data (n = 2), re-rupture of aneurysm during the hospital stay (n = 2), continuous intra-arterial nimodipine treatment (n = 1) and loss of follow-up at six months (n = 6). The final study population consisted of 98 patients, 23 patients in the “CS” group, 53 patients in the “GA” group and 22 patients in the “both” group. In the “CS” group, 16 patients received a single intervention (“single CS”) and seven patients received up to five interventions (“multiple CS”). In the GA group, 26 patients received one intervention (“single GA”) and 27 patients received 2-10 treatments (“multiple GA”). As per definition, all patients in the “both” group received more than one and up to 10 interventions.
Table 1 shows patient characteristics of the three anesthesia groups. Overall mean age was 54.7 years (range 24-81). All groups showed higher percentages of female patients. Age, sex, aneurysm treatment modality did not significantly differ between the three groups.
| CS | GA | Both | Total | p-value | |
|---|---|---|---|---|---|
| Number of patients | 23 | 53 | 22 | 98 | |
| Mean age (years, range) | 57.0 (32 – 74) | 54.0 (24 – 81) | 53.7 (36 – 72) | 54.7 (24 – 81) | 0.550# |
| Sex | 0.219+ | ||||
| Female | 20 (87%) | 40 (75%) | 21 (91%) | 80 (82%) | |
| Male | 3 (13%) | 13 (25%) | 2 (9%) | 18 (18%) | |
| Admission WFNS Score | 0.023*# | ||||
| I | 12 (52%) | 15 (28%) | 8 (36%) | 35 (36%) | |
| II | 1 (4%) | 7 (13%) | 9 (41%) | 17 (17%) | |
| III | 3 (13%) | 5 (9%) | 1 (5%) | 9 (9%) | |
| IV | 4 (17%) | 14 (26%) | 4 (18%) | 22 (22%) | |
| V | 3 (13%) | 12 (23%) | 0 (0%) | 15 (15%) | |
| Hunt & Hess Score | 0.023*# | ||||
| 1 | 3 (13%) | 6 (11%) | 4 (18%) | 13 (13%) | |
| 2 | 11 (48%) | 16 (30%) | 13 (59%) | 40 (40%) | |
| 3 | 3 (13%) | 7 (13%) | 2 (9%) | 12 (12%) | |
| 4 | 0 (0%) | 7 (13%) | 1 (5%) | 8 (8%) | |
| 5 | 6 (26%) | 17 (32%) | 2 (9%) | 25 (26%) | |
| BNI Score | 0.999# | ||||
| 1 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| 2 | 7 (30%) | 15 (28%) | 8 (36%) | 30 (31%) | |
| 3 | 8 (35%) | 22 (42%) | 7 (32%) | 37 (38%) | |
| 4 | 5 (22%) | 8 (15%) | 2 (9%) | 15 (15%) | |
| 5 | 3 (13%) | 8 (15%) | 5 (23%) | 16 (16%) | |
| Fisher score | 0.675# | ||||
| 1 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| 2 | 2 (9%) | 0 (0%) | 0 (0%) | 2 (2%) | |
| 3 | 14 (61%) | 38 (72%) | 18 (82%) | 70 (71%) | |
| 4 | 7 (30%) | 15 (28%) | 4 (18%) | 26 (27%) | |
| Aneurysm location | |||||
| Choroideal artery | 0 (0%) | 1 (2%) | 0 (0%) | 1 (1%) | |
| ACA | 1 (4%) | 0 0%) | 0 (0%) | 1 (1%) | |
| ACOM | 9 (39%) | 18 (34%) | 5 (23%) | 32 (33%) | |
| Basilar | 2 (9%) | 4 (7%) | 2 (9%) | 8 (8%) | |
| ICA | 1 (4%) | 6 (11%) | 1 (5%) | 8 (8%) | |
| MCA | 5 (21%) | 9 (17%) | 6 (27%) | 20 (20%) | |
| PCA | 1 (4%) | 0 (0%) | 0 (0%) | 1 (1%) | |
| PCOM | 1 (4%) | 10 (19%) | 7 (32%) | 18 (18%) | |
| A.pericallosa (A2) | 2 (9%) | 0 (0%) | 0 (0%) | 2 (2%) | |
| PICA | 0 (0%) | 3 (6%) | 0 (0%) | 3 (3%) | |
| Superior cerebellar artery | 1 (4%) | 1 (2%) | 0 (0%) | 2 (2%) | |
| Vertebral | 0 (0%) | 1 (2%) | 1 (5%) | 2 (2%) | |
| Aneurysm treatment | 0.916+ | ||||
| Clipping | 5 (21%) | 10 (19%) | 4 (18%) | 19 (19%) | |
| Coiling | 18 (78%) | 42 (79%) | 18 (82%) | 78 (80%) | |
| Flow diverter | 0 (0%) | 1 (2%) | 0 (0%) | 1 (1%) |
mRS at six months is displayed in Figure 1a. There was a tendency for a slightly higher percentage of functionally independent patients (mRS 0-2) in the “CS” group (78.3%) at six months. However, this difference did not prove to be statistically significant (p = 0.109). The subgroup analysis comparing single and multiple intra-arterial interventions separately for each anesthesia modality is displayed in Table 2. This analysis also revealed no significant difference in functional outcome at six months between “single CS”, “single GA”, “multiple CS”, “multiple GA” and “both” groups (p = 0.089).
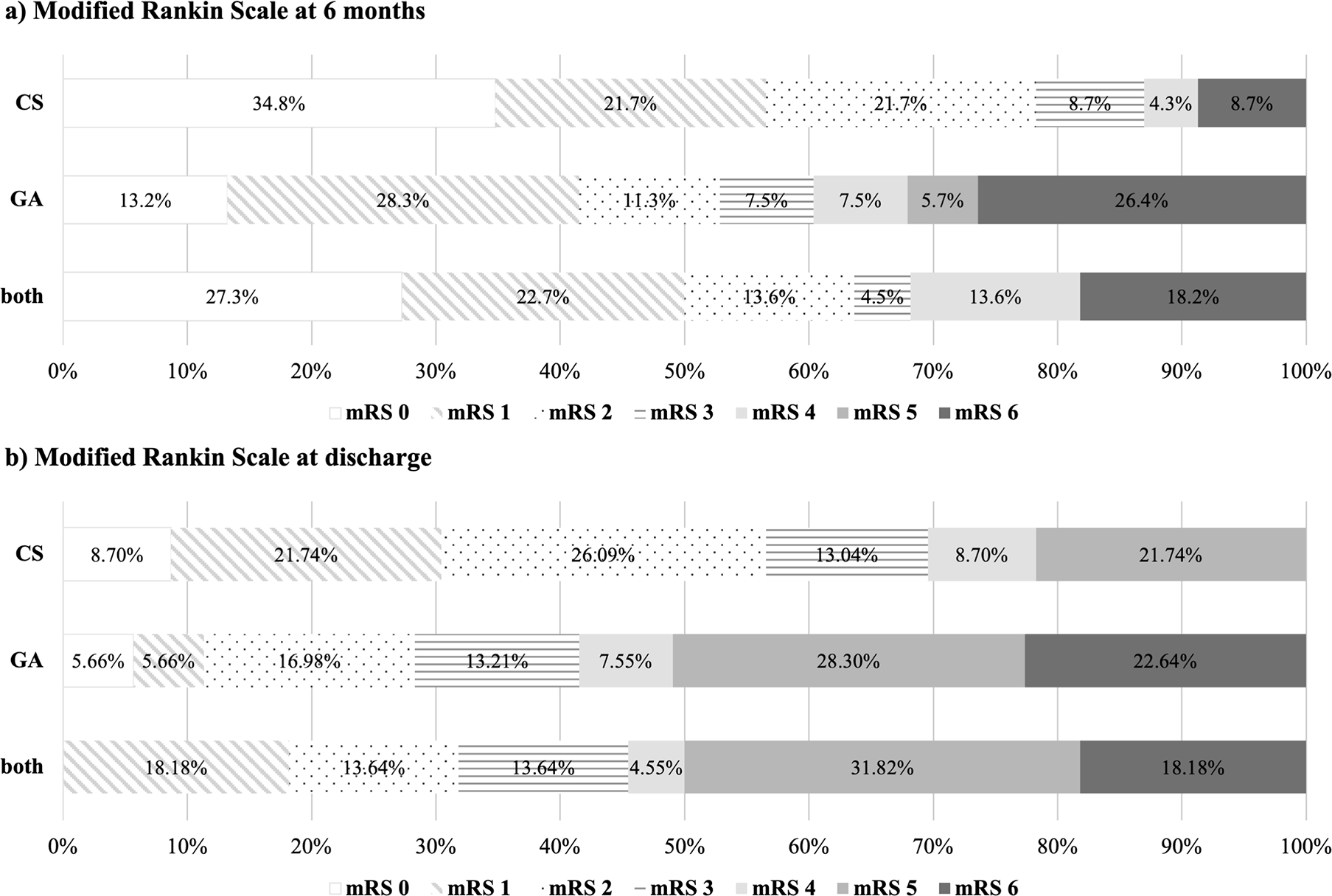
Numbers represent the percentages for each mRS category per group. a) “CS”, “GA” and “both” at six-month follow up. b) “CS”, “GA” and “both” at discharge from hospital.
| single CS | multiple CS | single GA | multiple GA | both | p-value | ||
|---|---|---|---|---|---|---|---|
| mRS 6 months | mRS 0-2 | 11 (68.8%) | 7 (100%) | 16 (61.5%) | 12 (44.4%) | 14 (63.6%) | 0.089+ |
| mRS 3-6 | 5 (31.3%) | 0 (0%) | 10 (38.5%) | 15 (55.6%) | 8 (36.4%) | ||
| mRS discharge | mRS 0-2 | 9 (56.3%) | 4 (57.1%) | 9 (34.6%) | 6 (22.2%) | 7 (31.8%) | 0.156+ |
| mRS 3-6 | 7 (43.8%) | 3 (42.9%) | 17 (65.4%) | 21 (77.8%) | 15 (68.2%) |
Figure 1b shows mRS at discharge from hospital. This analysis displays no significant difference between “CS”, “GA” and “both” groups (p = 0.056). The subgroup analysis for single and multiple interventions separately also revealed no statistical significance (p = 0.156), as listed in Table 2.
The NIHSS time course analysis is presented in Figure 2. ANOVA for repeated measures displayed a significant interaction of “time*treatment” (p = 0.008) and of “time” (p < 0.001). Post-hoc analysis revealed that significant group differences only occur when comparing “GA” to the two other groups. All of these significant differences were between t1 and t5, meaning between 24 hours before (first) intervention and discharge from the hospital. At the six-month follow up appointment, “CS”, “GA” and “both” did not differ significantly regarding NIHSS.
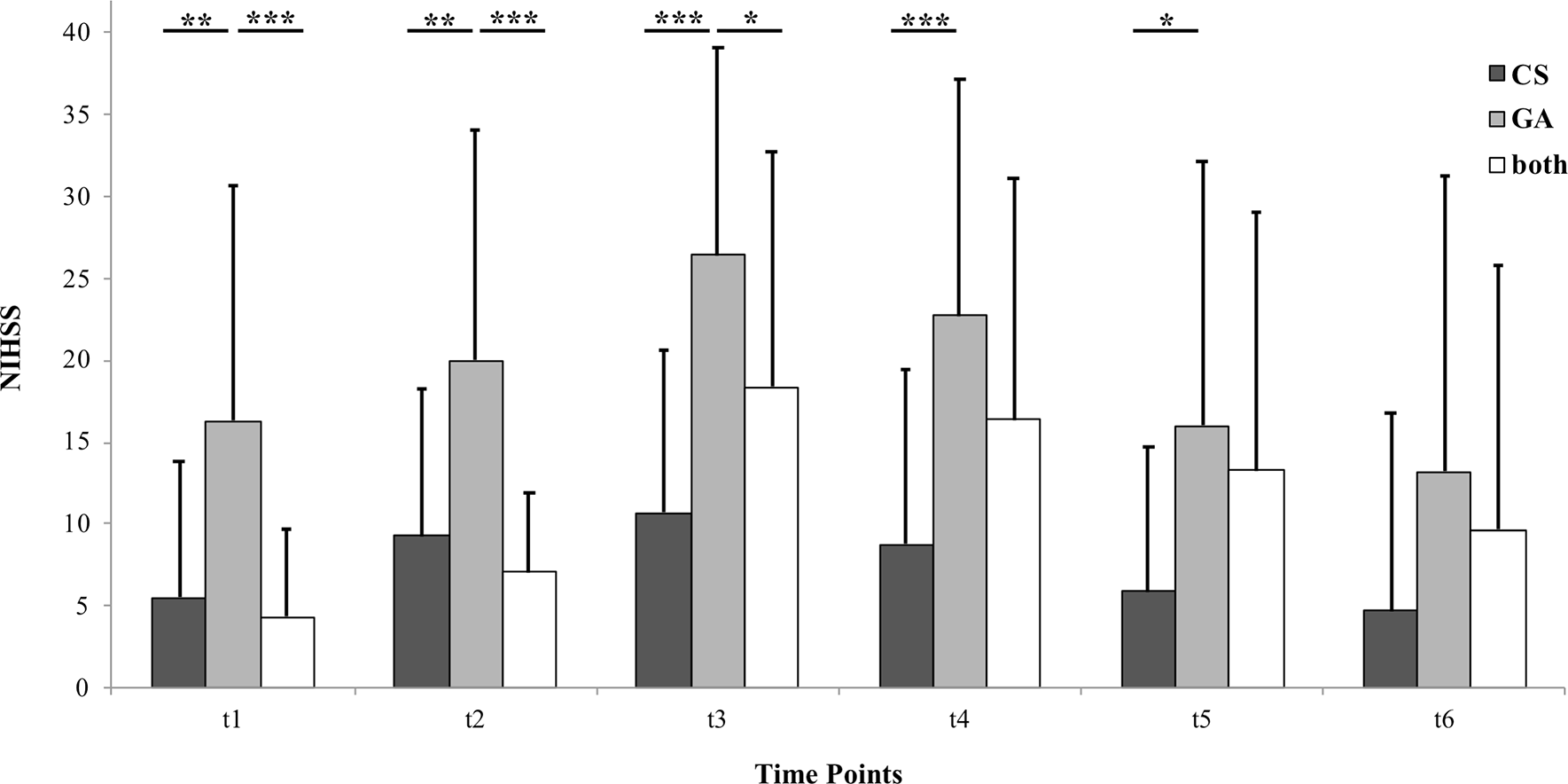
Patients were treated in conscious sedation (“CS”), general anesthesia (“GA”) or a combination of both (“both”). Each bar represents a different group. Each cluster represents a different time point. Time points are 24 hours before (first) intra-arterial vasodilator intervention (t1), immediately before (t2), immediately after (t3), 24 hours after (last) intervention (t4), at discharge from the hospital (t5) and at six-month follow up (t6). Data are presented as mean + 1 Standard Deviation (SD) as error bars. Significant inter-group differences are highlighted with asterisks. One asterisk represents p < 0.05, two asterisks represent p < 0.01 and three asterisks represent p < 0.001.
Interventional parameters are displayed in Table 3. Overall, a total of 237 intra-arterial vasodilator treatments were performed, 65 of which were performed in CS and 172 in GA. Mean duration of intervention was significantly longer if performed in GA (p = 0.002). A total of four interventions had to be excluded from further analysis because of missing data about medication (n = 3) and number of treated arteries (n = 1). Neither choice and dosage of intra-arterial vasodilator, nor number of treated arteries showed significant differences between groups.
| CS | GA | p-value | Total | |
|---|---|---|---|---|
| Number of interventions | 65 | 172 | 237 | |
| Duration of intervention (min.) | 80 (31) | 96 (46) | 0.002**+ | 92 (44) |
| Medication (number of interventions) | 0.517x | |||
| Nimodipine | 59 | 147 | 206 | |
| Papaverine | 3 | 15 | 18 | |
| Nimodipine and papaverine | 2 | 7 | 9 | |
| Medication dosage (mg) | ||||
| Nimodipine | 4.8 (1.7) | 4.8 (1.7) | 0.928# | 4.8 (1.7) |
| Papaverine | 200.0 (69) | 224.2 (100) | 0.696# | 212.1 (94) |
| Nimodipine and papaverine | 2.5 (0.07) and 120.0 (0) | 3.5 (2) and 123.9 (85) | 0.507# and 0.953# | 3.0 (1.8) and 121.9 (74) |
| Number of treated arteries | 1.8 (0.6) | 2 (0.8) | 0.057# | 1.9 (0.8) |
This retrospective study found no significant differences in functionally independent outcomes (mRS 0-2) six months after aSAH in patients who were treated with intra-arterial vasodilators in CS, GA or a combination of both. While NIHSS was significantly higher in patients undergoing endovascular therapy in GA compared to patients of the “CS” or “both” group in the time window 24 hours before intervention up to discharge from the hospital, this difference was no longer found at six months.
To the best of our knowledge, the effect of anesthesia modality (CS versus GA) on functional outcome has not yet been studied for intra-arterial admission of either nimodipine and/or papaverine in patients with refractory CVS after aSAH. Albeit, there have been several research papers published regarding anesthesia in aneurysm treatment.21–23 Most articles describe both CS and GA to be generally safe for treatment of unruptured aneurysms or aSAH with no clear recommendation for either one.
Additionally, multiple studies have had similar research questions in relation to the choice of anesthesia during endovascular therapy of acute ischemic stroke. Abou-Chebl et al. (2010) showed in their analysis of the “North American SOLITAIRE Stent-Retriever Acute Stroke” (NASA) registry, that patients treated in GA experienced poorer neurologic outcome at 90 days and higher mortality rates than patients treated in CS.8 Berkhemer et al. (2016) reported similar results in a post-hoc analysis of a prospective trial.15 Correspondingly, a recent analysis of the “Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3” (DEFUSE 3) trial by Powers et al. (2019) showed higher rates of functional independence (mRS 0-2) and a lower NIHSS score at 24 hours for patients treated in CS. At discharge they did not find a statistically significant difference in NIHSS scores anymore.11
In contrast, most recent randomized controlled trials found a non-inferiority of GA when compared to CS. Hendén et al. (2017) reported no difference in outcome at three months or NIHSS after 24 hours in their Anesthesia During Stroke (AnStroke) Trial.16 Schönenberger et al. (2016) and Simonsen et al. (2018) report, that GA produced better 3-month outcomes, with the former even finding this result to be significant.18,19 Finally, a recent meta-analysis determined no significant difference between GA and CS if only randomized controlled trials were considered.17
Our results are in line with these recent randomized controlled trials published for endovascular treatment in acute ischemic stroke. Similar to Schönenberger et al. (2016), Hendén et al. (2017), Simonsen et al. (2018) and Kim et al. (2019), we also found no significant difference in functional independency (mRS 0-2) at discharge or six months.16–19
Similar considerations regarding choice of anesthesia hold true in intra-arterial vasodilator therapy after aSAH and treatment for acute ischemic stroke alike. Disadvantages of GA may be a delay in treatment, hemodynamic changes and complications associated with intubation such as an increased risk for pneumonia.8 Disadvantages of CS may be procedural discomfort for patients, more difficult interventions because of patients’ movements, emergency conversion to GA or increased risk for aspiration.8 Many of the conceived disadvantages of GA have also been analyzed in prospective studies concerning ischemic stroke treatment. For example, Berkhemer et al. (2016) as well as Hendén et al. (2017) found no treatment delay in the GA group.15,16 Schönenberger et al. (2016) reported no difference in feasibility, safety and intra-interventional complication between the groups.18 They did however discover more postprocedural complications after GA such as delayed extubation and pneumonia. Different authors mention the possibility of blood pressure drops and decreased cerebral blood flow as possible additional complications of GA, which could potentially worsen outcomes because of an increase of the ischemic area.15 Others argue that GA on its own has a neuroprotective effect by lowering the neuronal oxygen need.24,25 Overall, the similar functional outcome in our study as well as in the above cited studies in ischemic stroke suggest that these factors are of minor relevance.
The major limitations of this study are its retrospective design and the single-center approach. A potential bias could lie in the choice of sedation for treatment. Even before intra-arterial vasodilator treatment, patients who would go on to receive treatments in GA showed significantly higher NIHSS scores when compared to the “CS” and “both” groups. This indicates that patients in clinically and neurologically worse conditions were more likely to be treated in GA. However, this bias was not reflected in our results, as we did not find a significant difference in mRS scores between the anesthesia groups at discharge or at six months and also no significant difference in NIHSS scores at six months. The “GA” group were therefore in an initially worse state but still managed to reach similar outcomes in the long-term clinical course. This suggests to an even greater degree, that GA will not negatively affect long term outcome in these patients.
Furthermore, some subgroups consisted of a small number of patients. The results of this study will have to be replicated by a larger prospective trial in the future.
Our preliminary results indicate that choice of anesthesia method does not negatively affect six-month outcome in aSAH patients who undergo intra-arterial vasodilator treatment for CVS. Treating physicians should therefore decide between CS and GA individually based on patient characteristics and circumstances.
Dryad: Functional Outcome after intraarterial vasodilator therapy in CS vs GA, https://doi.org/10.5061/dryad.g4f4qrfq5.26
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neurocritical care
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neurocritical care
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neurosurgery
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 02 Aug 21 |
read | |
|
Version 1 25 May 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)