Keywords
Arachnoid cyst, chronic subdural hematoma, headache, middle fossa, young adult
Arachnoid cyst, chronic subdural hematoma, headache, middle fossa, young adult
In this retrospective study, we reported a series of ten patients with concomitant chronic subdural hematomas and arachnoid cysts.
* We followed the suggestions and used the mentioned paper. Chronic subdural hematomas (cSDH) are the pathologies that are often seen in elderly patients, but uncommonly in young patients. Incidence in pediatric population is relatively rare. Patients having both a cSDH and an arachnoid cyst (AC) were determined in this study (10 patients, range:18-36 years). Young adults with ACs tend to be more susceptible to the development cSDH. Patients under the age of 18 were not included the study. Table 2 shows the comparison of the young adult patients who were between 18–40 years old and had chronic subdural hematomas; ten patients had arachnoid cysts and the twelve patients without arachnoid cysts.
* We discussed the reasons for cSDH (as due to arachnoid cysts or other reasons like shunts). The common predisposing factors of cSDH are diffuse brain atrophy and anticoagulant drugs in elderly population, but these factors are rare in pediatric population. cSDH is a rare complication in patients with intracranial AC. An increase in ICP could cause rupture of these bridging veins. The veins within the wall could be hurt because of decreased compliance. Rupture of an AC outer wall after head trauma is suggested to cause subdural effusion that could enlarge the cSDH. Especially in young adults, spontaneous tearing of the AC wall leads to leakage of CSF and blood into the subdural space. However, over drainage in patients with shunt operations could cause cSDH, the most common reason in pediatric patients.
* We provided information about arachnoid cysts or arachnoid defects in the spine that causes cSDH. The relevant literature was enlarged with recent articles.
See the authors' detailed response to the review by Jehuda Soleman
See the authors' detailed response to the review by Harsh Deora
See the authors' detailed response to the review by Roberto Colasanti
AC: arachnoid cyst
cSDH: chronic subdural hematoma
CSF: cerebrospinal fluid
CT: computed tomography
MRI: magnetic resonance imaging
Arachnoid cysts (ACs) are benign, intracranial extra-parenchymal cavities with a prevalence of 0.7% to 1,7% of the population.1,2 They are commonly observed in the middle fossa, mainly on the left side.3,4 Chronic subdural hematoma (cSDH) is a complex disease with an overall incidence of 1.7-20.6 per 100,000 persons per year and is more commonly encountered in the elderly population.5 Most ACs are asymptomatic, but they could become clinically obvious if the cyst grows and causes a cerebral parenchyma mass.1,6 cSDH is a rare complication in patients with intracranial AC.2,3 Approximately 2-4% of the patients admitted for AC were identified with cSDH.2-4 In this study, we present ten cases and aimed to investigate if ACs could predispose to cSDH in young adults. Although there are several previous manuscripts, our study is one of the most comprehensive assessments of the combination of ACs and cSDH.2-5,7-9 Since there is still a gap of knowledge about this subject today, we tried to contribute to the body of literature.
This study retrospectively reviwed the data of the patient files diagnosed with chronic subdural hematoma who were admitted to University of Health Sciences Izmir Bozyaka Education and Research Hospital Department of Neurosurgery between January 2013 and December 2019. Patients having both a chronic subdural hematoma and an arachnoid cysts were determined and included. We also included young adult cSDH patients under the age of 40 years for comparison. Patients under the age of 18 were not included the study. The same neurosurgeons evaluated all of the patients.
The patient data evaluated were age of patients and gender, location of the hematoma and arachnoid cyst, trauma history, symptoms at admission, cSDH maximum diameter, contiguity between AC and cSDH and treatment methods. Patients aged 18–40 years were accepted as young adults. Magnetic resonance images (MRIs) and computed tomographies (CTs) of patients were assessed. The maximum cSDH diameter measurements were performed on axial MRI slices. In six cases, we performed open cranitomy with the evacuation of the hematoma and arachnoid cyst.
Statistical Package for the Social Science 20.0 (IBM SPSS Statistics, RRID:SCR_019096) was used for analysis of parameters; JASP (RRID:SCR_015823) is an open access alternative which can perform the same function. Univariate analyses were performed using the Mann–Whitney U-test for non-normally distributed scale parameters. Median and ranges were used for description of scale parameters. All analysis were performed at 95% confidence interval and 0.05 significance level.
We treated 285 patients who were diagnosed with cSDHs at the University of Health Sciences Izmir Bozyaka Education and Research Hospital Department of Neurosurgery between January 2013 and December 2019. 22 (7.7%) of the 285 cSDH patients were aged under 40 years, and the other 263 patients (92.3 %) were more than 40 years old. 10 (45.5%) of the 22 young adult patients were diagnosed with both AC and cSDH. The mean age of these ten patients was 24.8 ± 3.9 years and the range was 19–36 years. Eight patients (80%) were male and two patients (20%) were female. Patients with both AC and cSDH had lower mean age than the patients having only a cSDH (p < 0.0001).
The characteristics of the ten patients with AC and concomitant cSDH were shown in the Table 1. In four patients, the onset of cSDH diagnosed after AC diagnosis (Cases 1,5,6 and 9) (Figures 1, 2, 3). Four patients did not have causative trauma history (Cases 1,3,5,8), two patients sufferred minor sports-related traumas (Cases 2,10). All patients had headache and only two patients had hemiparesis. AC locations were found in the middle fossa in eight patients (Cases 2,3,4,5,6,7,9,10) and two patients had the convexity of the Sylvian fissure location (Cases 1,8). The middle fossa located ACs were Galassi Tip I in seven patients and Galassi Tip II in one patient.10 All patients had cSDHs on the ipsilateral side of AC. None of the patients had cSDH on the contralateral side of AC. In eight patients, cSDH was close to ACs on neuroimaging studies, and the remaining two patients had a cSDH apart from an AC. Surgical intervention was performed in patients with a larger than 10 mm maximal diameter of cSDH. We evacuated the hematoma and arachnoid cyst following open craniotomy in six cases (Table 1: Cases 1,4,5,6,7,9). We also performed cyst fenestration and tried to remove the cyst membranes as completely as possible. The four patients who had smaller than 10 mm maximal cSDH diameter underwent conservative management (Table 1: Cases 2,3,8,10). They were followed by serial neuroimaging studies and it was noted that the hematoma disappered and the size of AC decreased over time without any neurological complication (Figure 4). All six operated patients recovered completely (Figures 1 and 3). cSDH recurrence was not reported during the period of follow-up of 5-60 months (median 12 months).
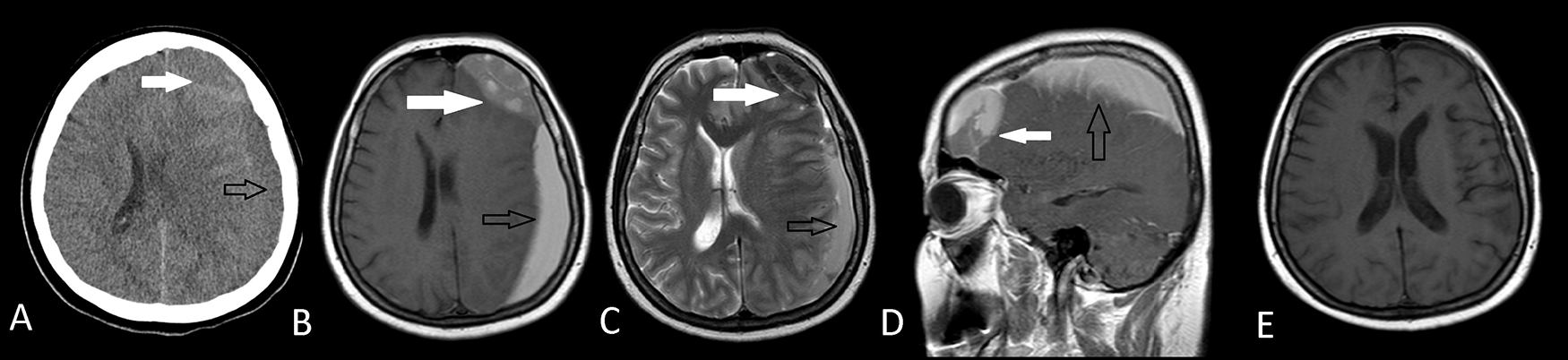
Arachnoid cyst had diagnosed before the onset of subdural hematoma and there was no history of a trauma. Postoperative axial T1W MRI (E) shows the total removal of both the arachnoid cyst and the subdural hematoma.
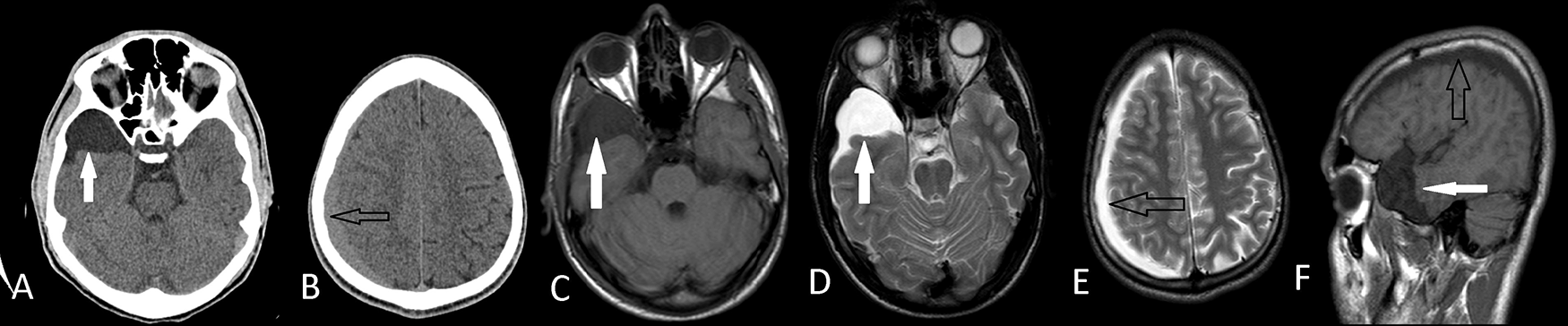
Axial T1W MRI(C), T2W MRI (D,E) and sagittal MRI (F) images shows that they are apart from each other. He had conservative treatment with a complete recovery.

Axial T1W MRI (C) shows ipsilateral subdural hematoma (black arrows). Postoperative axial T1W MRI (D,E) demonstrates that the hematoma and the arachnoid cyst was evacuated with a tiny fluid in the subdural place.
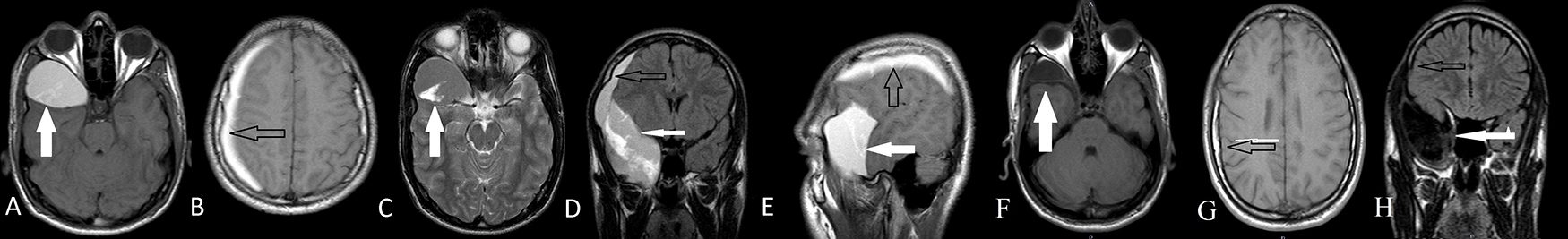
After two years follow-up, the patient’s AC size decreased as seen on axial T1W MRI (F), and cSDH disappeared thereafter the conservative treatment as seen on axial T1W (G), coronal MRI (H).
Table 2 shows the comparison of the young adult patients who were between 18–40 years old and had chronic subdural hematomas; ten patients having arachnoid cysts and the twelve patients without arachnoid cysts. The gender, frequency of trauma history, frequency of sports-related trauma, headache incidence, paresis incidence and outcomes distribution differences between patient groups were statistically insignificant (p > 0.05).
In this study, we present ten cases and aimed to investigate the correlation between the chronic subdural hematomas and the arachnoid cysts in young adults. We tried to contribute to the body of literature as there is still a lack of information on this subject today. Subdural hematomas are infrequently encountered complications of arachnoid cysts. Most of the studies about this subject in the literature analyzed the complicated ACs, from a total of patients with cSDH. Parsch et al.2 identified AC in 16 of 658 patients (2.4%) with cSDH. Wester and Helland3 reported that 4.6% (11 of 241) of their patients admitted for AC were identified with cSDH. Mori et al.4 reported an incidence of 2.2% (12 of 541) of AC in the cases with cSDH. In the case series of Wu et al.9 the incidence of AC-associated cSDH was 1.9% (5 of 266). cSDHs are the pathologies that often seen in elderly patients, but uncommonly in young patients.11 Incidence in pediatric population is relatively rare. When we compared the patients at the same period in our clinic, AC and concomitant cSDH patients had lower age mean than the patients with only cSDH. In our study, all ten patients were under age of 36. In our present study, which included 285 cSDH patients, 22 patients (7.7%) were under the age of 40 years. Previously reported rates were 2.4–8.8% and were consistent with the rate of this study.6,8 10 young adult patients under the age of 40 years with chronic subdural hematoma were found to have arachnoid cysts (45%). Approximately half of the 18–40-year-old patients had cSDH with AC. Considering that the prevalence of arachnoid cyst is 0.7% to 1.7% of the population,2,3 we can conclude that ACs may cause cSDH in young patients with or without trauma.
The common predisposing factors of cSDH are diffuse brain atrophy and anticoagulant drugs in elderly population, but these factors are rare in pediatric population. Arachnoid cysts or arachnoid defects in the spine also lead to chronic SDH.12 However, overdrainage in patients with shunt operation could cause cSDH, most common reason in pediatric patients.13,14 Various previous researches have conferred the risk of cSDH related with ACs.3,4,7,8,15,16 The exact mechanism is still unclear. However, theories suggest that 1) veins within the wall could be hurt because of decreased compliance 2) veins without structural support of cyst wall are vulnerable 3) a slit-valve mechanism is formed, lead to increased pressure within the AC and vein rupture.9,15-17 The compliance of the cyst is less than the normal brain. An increase in ICP causes rupture of these bridging veins. AC-related symptoms usually start with the cyst enlargement.9,15,18,19 In our study, in four patients without evident trauma, these theories explaining the spontaneous rupture of the AC into subdural space may be valid.
According to the ACs and cSDHs locations on neuroimaging studies; three types were defined: 1) a close cSDH to AC, 2) a separate cSDH from an AC on the ipsilateral side, 3) a contralateral side located cSDH to an AC.9,16 Wester et al.3 observed that there are small bridging veins between the dura mater and the outer membrane of the AC. They propounded that the bridging Sylvian veins may cause blood leakage into the subdural place. Rupture of an AC outer wall after head trauma is suggested to cause subdural effusion that could enlarge the cSDH.15,20 Especially in young adults, spontaneous tearing of the AC wall leads to leakage of CSF and blood into the subdural space.4 Page et al.15 declared that ACs are less flexible than the normal without AC brain with reduced intracranial buffering after trauma.3,15 Thus, hematoma could grow up on the ipsilateral hemispheric subdural space other than AC. The subdural hematoma in Cases 3 and 8 can be explained by this mechanism. The association between cSDH and AC could not be insidental. None of our cases had cSDH on the contralateral side of AC. In previous series, almost half of the 18–40-year-old patients with cSDHs had ACs.17,18 Young adults with arachnoid cysts tends to be more susceptible to the development chronic subdural hematomas.
Headache is one of the most common syptoms in the patients having both cSDH and AC. In our study, headache was observed in all patients and paresis was observed in two patients who were related with an accident. Headache may be due to increased intracranial pressure.21,22 Since the subarachnoid space is smaller in young patients than in the elderly, it is possible that they are more affected by increased intracranial pressure, in cSDH enlargement cases. In our study, sports-related cSDH was found in two of the young patients with ACs. Several case reports of cSDH associated with ACs have been reported after head injury in sports.23-26 Sports is an important factor of cSDHs in young patients. Mori et al.4 stated in a study that AC is a risk factor for chronic subdural hematoma in juveniles.
The surgical management of a cSDH related with an AC is a debated subject. Open craniotomy including the membranes of the AC and the cSDH removement, drainage of the hematoma using a burr hole, cyst fenestration or cystoperitoneal shunt could be chosen. The most common surgical method is open craniotomy alone.16,18 In their systematic review, Zuckermann et al. analysed 65 cases, and nearly half of all cases mentioned cyst fenestration after open craniotomy.16 Although some of the recent studies have argued against burr hole irrigation method, we prefered to remove part of the membranes with open craniotomy and to perform cyst fenestration as a safe procedure. Complex dissection interventions could be necessary in such cases in which a burr hole is insufficient in the management. A single burr-hole-mini craniotomy and hematoma evacuation followed by endoscopic inspection of the surgical cavity could be a preferred choise. Initial observation may be considered in patients with a thickness of 10 mm or less cSDH diameter without symtoms of intracranial hypertension. Middle meningeal artery embolization may be represented as a minimally-invazive alternative to surgery for new or recurrent chronic subdural hematomas, or as prophylaxis to reduce the risk of recurrence after surgery.27,28 Some studies suggest that corticosteroids might be beneficial in the treatment of cSDH; however, there is a lack of well-designed trials that support or refute the use of corticosteroids in cSDH.29 Recurrence can occur in 10%-20% of patients and is associated with several clinical and radiographic predictors. Postoperative cSDH volume and the Nakaguchi classification subtypes proved the most powerful predictors of recurrence and cure (separated or trabecular subtypes and postoperative cSDH volume ≥35.0 mL).30
It seems to be likely that presence of an arachnoid cyst in young adults is a predisposing factor for the formation of a chronic subdural hematoma with or without a head trauma. Headache is one of the most common symptom in the patients with both arachnoid cyst and chronic subdural hematoma. Coincidentally diagnosed arachnoid cyst patients must be followed up with periodical clinical examinations and neuroimaging studies. Young adults with an arachnoid cyst should be informed of the potential risk of developing a chronic subdural hematoma with forced physical exercises or even spontaneously.
This study was approved by the institutional ethics review committee at the University of Helth Sciences Izmir Bozyaka Education and Research Hospital (Date: 13,01,2021, Issue No: 07) in accordance with the World Medical Association Declaration of Helsinki and its most recent amendments. Formal consent was not obligatory for this research.
Open Science Framework: Underlying data for ‘Concomitant chronic subdural hematomas and arachnoid cysts in young adults’. https://doi.org/10.17605/OSF.IO/XM3GW.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neurosurgery
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neurosurgery, Pediatric Neurosurgery, Endoscopy, Vaskular Neurosurgery
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Deora H, Mishra A, Gupta R, Konar S, et al.: Paediatric chronic subdural haematoma: what are the predisposing factors and outcomes in management of these cases?. Childs Nerv Syst. 38 (1): 123-132 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Neurosurgery
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neurosurgery
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neurosurgery, Pediatric Neurosurgery, Endoscopy, Vaskular Neurosurgery
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neurosurgery
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 28 Apr 22 |
read | read | |
|
Version 2 (revision) 07 Feb 22 |
read | read | |
|
Version 1 26 May 21 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)