Keywords
adjunctive alpha-blocker, ureteral stone, ureteroscopy
adjunctive alpha-blocker, ureteral stone, ureteroscopy
The new version clarifies that alpha-blockers exert their effects on the distal part of the ureter muscle. Due to the fact that kidney and bladder stones can form in a number of sites, the author and reviewer felt it was critical to express this openly. The author expects that by defining the location of efficacy for alpha-blockers, which was the distal ureter, this study may make future implementation easier.
See the authors' detailed response to the review by Ferry Safriadi
In the last decade, urolithiasis has become a common condition, and the number continues to increase. The prevalence of urinary tract stones globally currently ranges from 2–20% with a recurrence rate of around 50%. The increase in urinary tract stones incidence was also followed by the rise in the frequency of urinary tract endoscopy, one of which was retrograde or antegrade ureterorenoscopy, which was indicated to treat ureteral stones and kidney stones1. Compared to the extra-corporeal shock-wave lithotripsy (ESWL) procedure, (URS) is more preferred, as it has been proven to achieve higher success rates in a single operation2,3.
In recent literature, the adjunctive alpha-blocker is recommended to facilitate distal ureteric stone expulsion, decrease postoperative complications, improve stents tolerability, and reduce colic episodes to reduce the necessity for secondary procedure retreatment1.
The present study aims to investigate the efficacy of adjunctive alpha-blockers for improving the success rate of the URS procedure for urolithiasis. By conducting this review and analysis, a definite conclusion regarding the effectiveness can be achieved. Thus, clinicians can decide the necessity of adjunctive alpha-blockers.
This review was done according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statements4. This study attempted to improve alpha-blocker therapy effectiveness in post-URS urolithiasis patients, with success rate parameters. Thus, this meta-analysis included studies which compared alpha-blockers as adjunctive therapy, versus either a placebo or no drug at all, in post-URS patients. No restrictions on the type of URS were performed in patients. There were also no restrictions on the kind of alpha-blocker given to patients. The success rate was then defined as the stone-free rate and overall postoperative complication rate.
We performed an article search on four electronic databases (MEDLINE/Pubmed, CENTRAL/Cochrane, CINAHL/EBSCOHost, and EMBASE/Elsevier). The investigation was carried out from 14 August to 9 September 2020. PICOS were used to trace studies and identify the suitability of any we found5. We used specific keywords, adjusted to each search engine specification, in the form of (postoperative OR adjunctive) AND (alpha-blocker OR tamsulosin OR alpha-adrenergic antagonists OR Alpha-adrenoreceptor antagonists OR doxazosin OR terazosin OR alfuzosin OR prazosin) AND (ureteroscopy OR URS OR ureterorenoscopy OR retrograde intrarenal surgery) AND ureteral stone. We also looked at a reference list of several reviews to expand the search coverage of the study.
Study selection was carried out independently and duplicated by each author, referring to inclusion and exclusion criteria. The inclusion criteria in this study included: 1) RCT or quasi-RCT studies that were compatible with PICOS; 2) English/Indonesian written articles; 3) Full-text articles available; 4) The output assessed were, at least, one of postoperative stone-free rate or overall complication rate; and 5) Published between 1 January 2000 and 31 December 2020.
There were no restrictions on the type of URS and alpha-blockers given to patients. The exclusion criteria for this study included review articles, case reports, case series, editorial letters, studies on animals, and/or studies in the process of peer review (has not been published yet).
The decision to study eligibility was determined by each author independently. Any disagreement was resolved by discussion.
Data extraction was carried out by each author independently and in duplication. We extracted the study's primary characteristics, including the first author, location, sample size, and publication year.
Following the dependent variables in this meta-analysis, we also extracted patient baseline data and postoperative data, including a stone-free rate and overall complication rate. We also noted the type of alpha-blocker and the duration of alpha-blocker administration.
This study explored the efficacy of adjunctive alpha-blockers in increasing URS's success rate in urolithiasis patients, divided into the stone-free rate and overall complication rate, in the form of relative risk (RR). We used a 2×2 contingency table to obtain each study's RRs and pooled the overall RRs using the Review Manager 5.3 application6. Analysis using the DerSimonian and Laird random-effects model was performed when high heterogeneity was found.
To see the precision and examine publication bias, this meta-analysis utilized funnel plots, which were also produced via Review Manager 5.36.
We searched five electronic databases, using specific keywords tailored to each database, to improve search sensitivity and specificity. The total records retrieved were 520 studies, and 131 studies were then excluded as there were duplications. From 389 studies screened, 376 studies were further excluded because of unrelated topics and objectives, resulting in 13 studies to be assessed for eligibility. Another five studies were later excluded due to unsuitable study design (n = 2) and review articles (n = 3). We obtained eight studies that were included in the qualitative and quantitative synthesis. The summary of study identification and selection according to the PRISMA Statement flow diagram are shown in Figure 1 and Table 1.
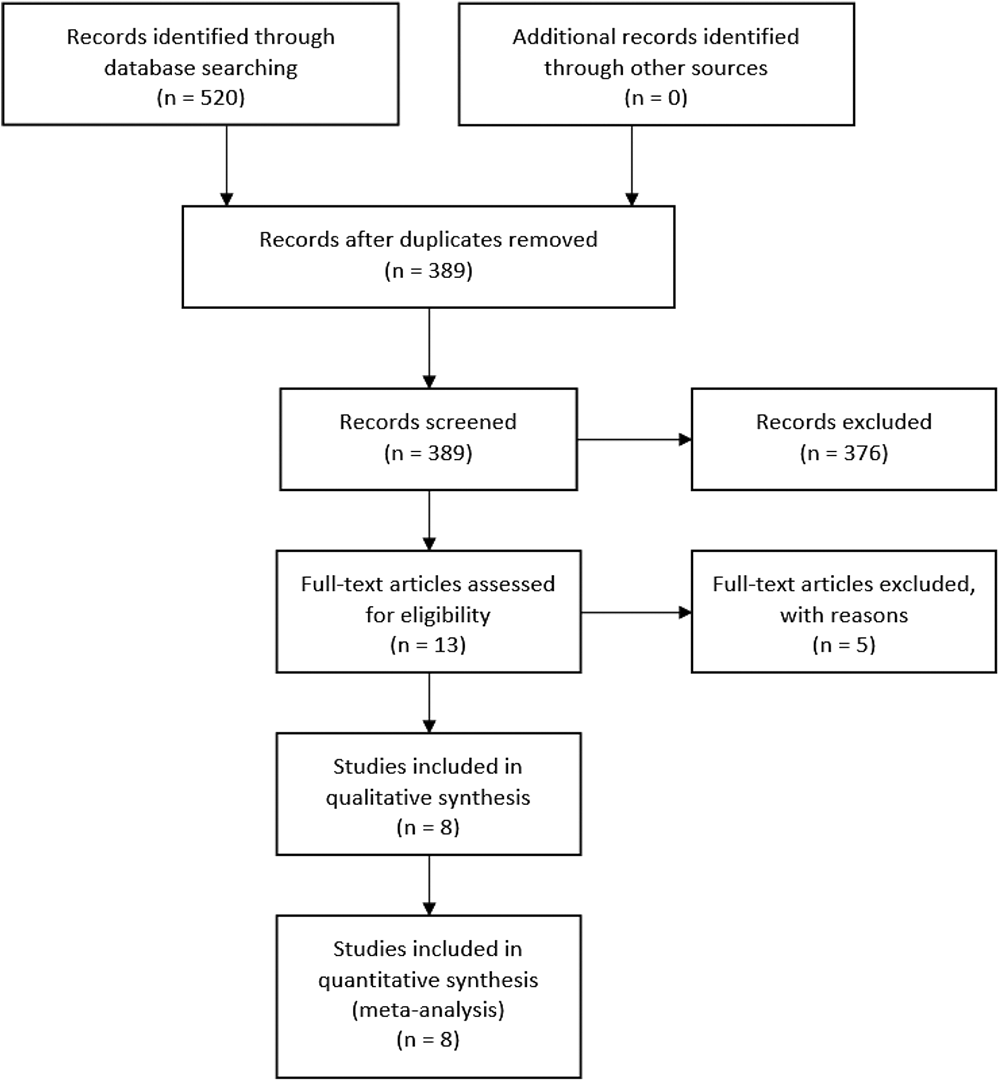
Initial database searching yielded 520 items, 389 of which were left after duplicate screening. Abstract screening excluded 376 more items. Eight items survived full-text assessment and were included in both the qualitative and quantitative synthesis.
Three of the eight studies we included in this study were multicenter, prospective, randomized trials7–9. While the other five were a single-center, prospective, randomized trial1,3,10,11. However, the numbers of patients enrolled in the pilot studies did not differ significantly between studies. Overall, the total number of patients included in this meta-analysis was 913 patients.
Seven of the eight studies gave alpha-blockers before URS, three of those gave Tamsulosin 0.4 mg once daily for seven days before surgery1,8,10. Two studies administered alpha-blockers only once, the day before surgery3,9. One study gave Silodosin 8 mg once daily for ten days before surgery10, and one other study gave Tamsulosin 0.4 mg once daily for 14 days postoperatively11. Bhattar et al.12 also looked at Tamsulosin independently and in combinations. Data on the characteristics of the included studies are shown in Table 2.
| Author | Regimen | Timing | N | Age* | Duration | Design | Stone size* (mm) | Stone density* (HFU) |
|---|---|---|---|---|---|---|---|---|
| Ahmed et al. 2017.2 | Tamsulosin 0.4 mg qd | 1 week preoperatively, discontinued after surgery | 81/84 | 35.7±11.0/37.6±11.2 | 8 weeks | Prospective, randomized, multicentre study | 13.37±2.73/12.73±2.39 | 815.35±341.6/778.36±269.2 |
| Bayar et al. 2019.7 | Tamsulosin 0.4 mg qd | 1 week preoperatively, discontinued after surgery | 61/63 | 42.1±11.4/39±14.6 | 4 weeks | Prospective, randomized, multicentre study | NR | NR |
| Ketabchi et al. 2014.6 | Tamsulosin 0.4 mg qd | 1 day preoperatively, discontinued after surgery | 52/50 | 24±6.5/27±8.8 | 2 weeks | Prospective, randomized, single-centre study | 6.6 ± 2.3/6.2 ± 3.2 | NR |
| Aydin et al. 2018.8 | Silodosin 8 mg qd | 1 day (A) and 3 days (B) preoperatively, discontinued after surgery | 50/47/50 | 37 (22–65)/43 (22–78)/37.5 (20–69) | 4 weeks | Prospective, randomized, multicentre study | NR | NR |
| Mohey et al. 2018.9 | Silodosin 8 mg qd | 10 days preoperatively, discontinued after surgery | 62/65 | 38.27±9.37/39.67±9.54 | 4 weeks | Prospective, randomized, single-centre study | 12.6±1.25/12.9±1.29 | 907.66±208.52/898.97±212.04 |
| Abdelazis et al. 2017.1 | Tamsulosin 0.4 mg qd | 1 week preoperatively, discontinued after surgery | 51/47 | 35/38 | 2 weeks | Prospective, randomized, single-centre study | 6.6±2.3/6.2±3.2 | NR |
| John et al. 2010.10 | Tamsulosin 0.4 mg qd | Initiated postoperatively for 2 weeks | 40/38 | 36.9 (21-57)/42.2 (26-61) | 4 weeks | Prospective, randomized, single-centre study | 1.3 (1-2)/1.2 (1-1.8) | NR |
| Bhattar et al. 2018.11 | Tamsulosin 0.4 mg qd | 10 days preoperatively, discontinued after surgery | 36/36 | 35.42±11.4/37.1±10.9 | 4 weeks | Prospective, randomized, single-centre study | 10.2±2.1/11.3±2.8 | NR |
| Ahmed et al. 2017.2 | 43.4±12.3/49.6±13.6 | 1.2±0.3/1.4±0.6 | 2.68 †/3.17 †† | 0.34 | 0.09 | 0.34 | 0.61 | NR |
| Bayar et al. 2019.7 | 33±9.4/30.9±6.6 | NR | 2.62 | 0.51 | NR | NR | NR | NR |
| Ketabchi et al. 2014.6 | NR | NR | 7.00 | NR | NR | NR | 0.51 | 0.16 |
| Aydin et al. (A) 2018.8 | 30 (10–45)/30 (15–75) | NR | 1.00 | 0.31 | NR | NR | NR | NR |
| Aydin et al. (B) 2018.18 | 30 (10–50)/30 (15–75) | NR | 5.15 | 0.07 | NR | NR | NR | NR |
| Mohey et al. 2018.9 | 41.61±4.67/46.85±4.6 | NR | 5.75 | 0.28 | 0.20 | 0.25 | NR | NR |
| Abdelazis et al. 2015.1 | 52.0±14.9/71.0±17.3 | 1.2±0.6/1.7±0.9 | 2.34 | 0.62 | 0.92 | 0.45 | 0.36 | NR |
| John et al. 2010.10 | NR | NR | 2.08 | NR | NR | NR | NR | 0.24 |
| Bhattar et al. 2018.11 | 34.41±6.76/45.20±6.963 | 2.12±0.33/2.56±0.93 | 2.67 | NR | 0.27 | 0.21 | NR | NR |
Of the eight studies included in this meta-analysis, five had a high risk of bias because assessor outcome blinding was not performed. One study clearly stated no blinding in patients who were given intervention or control11. Selection bias in some studies was also considered high, because no allocation concealment was performed. In general, the quality of the studies included in this meta-analysis varied from low to high.
The bias assessment was carried out independently by each author and in duplication, in which all authors assessed all articles. Disagreements between the authors were resolved by discussion or consensus. Figure 2 visualizes the summary of bias risk.
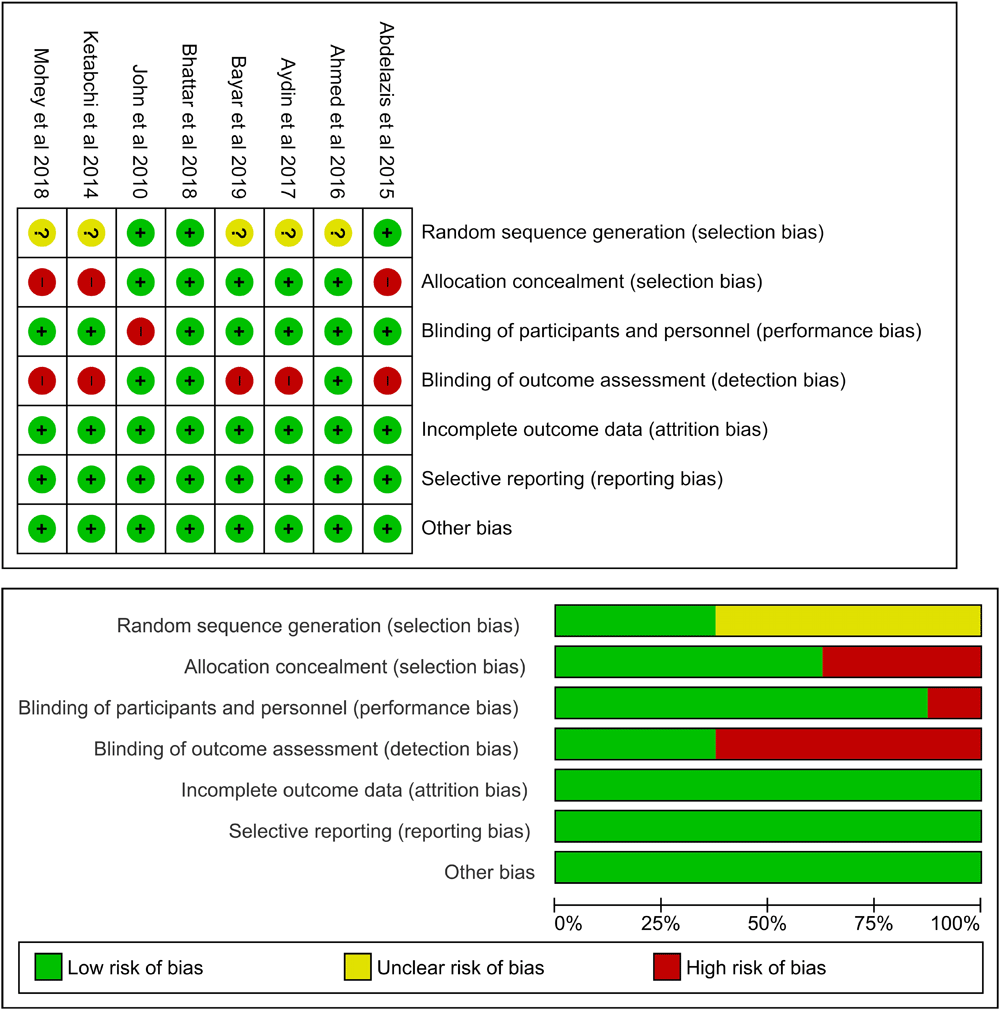
Selection bias from allocation concealment is present in three studies and not enough information was given to assess random sequence generation. Detection bias was detected in five studies. Only one study presented with performance bias. No attrition or reporting bias were detected.
The stone-free rate was found to be higher in patients given adjunctive alpha-blockers in most of the studies. A study by Aydin et al. 20179, found no difference at all in the stone-free rates of patients with and without adjunctive alpha-blockers one day before URS surgery. However, this study also compared alpha-blocker administration three days before surgery with a placebo and found a significant difference between the placebo and alpha-blocker groups.
Based on the results of the meta-analysis in Figure 3, it could be seen that the pooled RR favors the experimental group (adjunctive alpha-blocker), with a value of 1.17 (95% CI, 1.11–1.24). This indicates that a significantly higher stone-free rate was found in the adjunctive alpha-blocker patient.
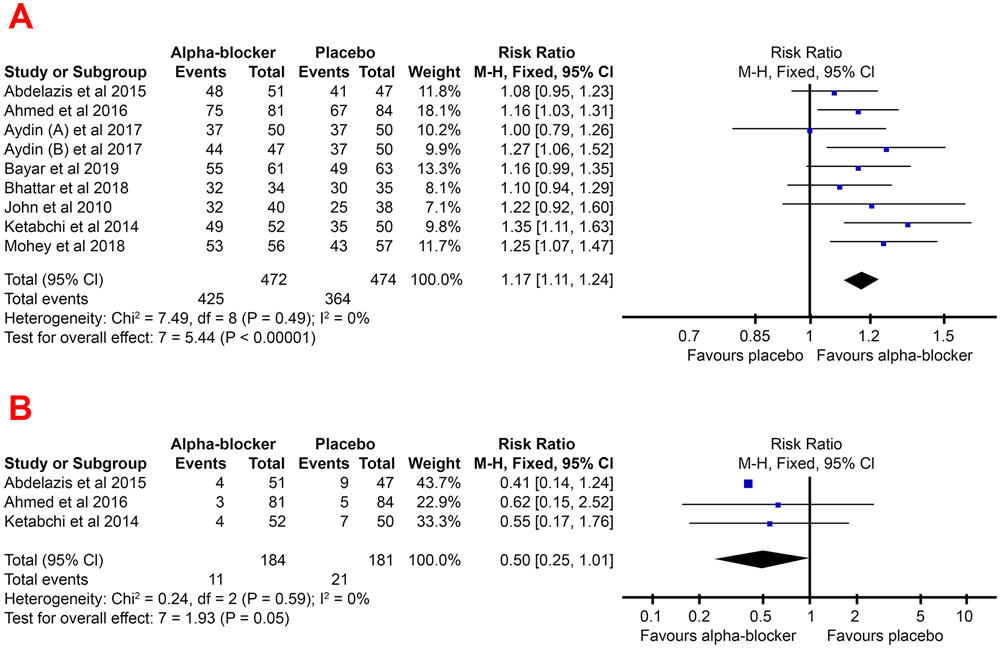
Forest plot of stone free rate (A) and readmission (B) pooled RR for the group treated with alpha-blocker compared to group treated with placebo -blocker compared to control group.
The lower risk of readmissions due to initial URS failure also supported a higher stone-free rate in the alpha-blocker group. The risk of readmission to URS in the alpha-blocker group tends to be lower than the placebo group, with a pooled RR of 0.50 (95% CI, 0.25–1.01).
In all studies, the complication rate was higher in the control group, either general complication or overall complication, hematuria, or mucosal injury. The alpha-blockers group had a significantly lower risk of complications than the placebo group, with a pooled RR of 0.41 (95% CI, 0.27–0.61). In this meta-analysis, the heterogeneity was recorded at only 0%; thus, we used a fixed-effect model to pool the effect estimate.
Several studies break down their patients' complications into several types of complications, such as hematuria, mucosal injury, and colic episode. For each of these complications, the alpha-blocker group was shown to have a lower risk of developing these complications. For all three types of complications, the alpha-blocker group had a significantly lower risk, with the pooled RR for hematuria, mucosal injury, and a colic episode of 0.42 (95% CI, 0.22–0.79), 0.31 (95% CI, 0.13–0.73), and 0.21 (95% CI, 0.06–0.69), respectively. These are summarized in Figure 4.
According to this systematic review and meta-analysis, postoperative alpha-blocker is associated with URS procedure's success rate for urolithiasis2,13. From the forest plot, it was discovered that patients having postoperative alpha-blockers are more likely to be stone-free with the RR=1.17 (95% CI, 1.11–1.24). This means that patients consuming postoperative alpha-blockers are 1.17 times more likely to be stone-free. The risk ratio ranges from 1.11–1.24, which are both greater than 1. Thus, it can be concluded that postoperative alpha-blocker is effective in increasing the stone-free rate after ureteroscopy. The study with the highest weight is by Ahmed et al. 20172, with a weight of 72.2%, followed by a study by Bayar et al. 20198, with a weight of 27.8%. This result is similar to a study done by Alsaikhan et al. in 202014, albeit using different parameters. They did a systematic review and meta-analysis about preoperative alpha-blockers usage for ureteroscopy for ureteric stones with parameters including risk reduction in need for intraoperative ureteral dilatation, stone-free status at four weeks post-operatively, and at final follow-up, the likeliness of urologists to reach the stone with the ureteroscope, operative time, and length of hospital stay. They study the preoperative use of alpha-blockers, meanwhile this study analyzed the usage of alpha-blockers as an adjunctive both preoperatively and postoperatively. The study result showed that at four weeks post-operatively and at final follow-up, patients have increased stone-free status with RR 1.17 (95% CI: 1.08 to 1.26), p < 0.0001 and 1.18 (95% CI: 1.11 to 1.24), p < 0.00001 respectively. It is important to note that some studies that were assessed by this study and Alsaikhan et al. 2020 are different14.
Tamsulosin, an alpha-1A blocker, has been shown to improve the distal ureteral stone expulsion rate and minimize the probability of colic episodes in patients during watchful waiting15,16. It relaxes the muscle of the distal ureteral wall, aiding gravel clearance after URS or ESWL procedure. Furthermore, a relaxed ureter allows the instrument forwarding to become easier. In patients with Tamsulosin, the ureteral orifices were dilated, easily identified, and provided a more accessible entrance for the ureteroscope8,17. Tamsulosin also lessens the amplitude of ureteral contractions and shortens the duration between contractions15,18.
Postoperative complications outcome is also affected by the administration of postoperative alpha-blockers19,20. It was found that endoscopic treatments without the administration of adjunctive alpha-blockers are associated with a higher probability of complications2,21. This was shown by the forest plot, where the adjunctive alpha-blocker gives a protective effect from postoperative complications with RR = 0.41 (95% CI, 0.27–0.61). Therefore, postoperative alpha-blocker administration reduces the odds of postoperative complications. For this outcome, a study by Mohey et al. 201810, weighs 19.3%, while a study by Ahmed et al. 20172 weighs 47.8%. Extra benefits, such as shorter hospital stay, lesser hospital bills, milder postoperative complications, and better symptomatic improvement were obtained by simultaneous administration of Tamsulosin18,20.
Postoperative alpha-blocker improves the incidence of colic episodes. This was shown by RR=0.21 (95% CI, 0.06–0.69). Numerous reports have demonstrated alpha-adrenoreceptors on the ureteral wall, with the distal ureter's highest density1,2,15. Variable distribution of alpha receptor subtypes were found in the proximal, middle, and distal ureter. Alpha-blockers are now commonly prescribed for ureteral colic in various hospitals15,22.
This systematic review and meta-analysis have several strengths. Firstly, this systematic review and meta-analysis included a relatively broad scope of population. This review then assesses the primary outcome and considers other additional outcomes, which is also essential in clinical practices, albeit not widely studied. Low risk of bias in included studies, utilization of guidelines, no heterogeneity between studies, symmetrical funnel plots as shown in Figure 5, and high specificity also strengthen this study.
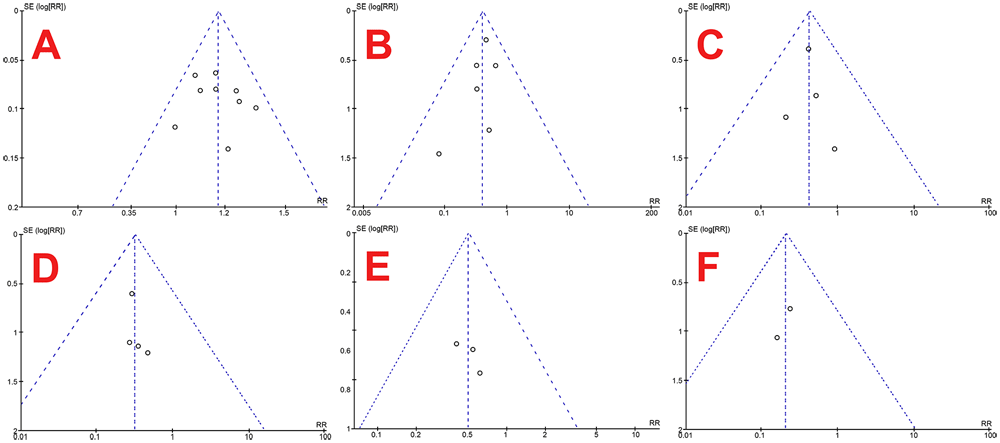
Funnel plots of stone-free rate (A), overall complication rate (B), hematuria (C), mucosal injury (D), readmission rate (E) and colic episodes (F).
Aside from the strengths, this systematic review and meta-analysis has a limitation. This study's limitation is that there were only eight studies eligible for review, and there were only two studies for each outcome, which makes its representability somewhat questionable.
In conclusion, our study shows that the administration of adjunctive alpha-blockers improves the URS procedure's success rate for ureteral calculi in terms of increasing stone-free rate, reducing postoperative complications, and minimizing colic episodes. This is because the alpha-blocker relaxes and reduces the ureteral wall's contractions, allowing easier stone clearance. Alpha-blockers have been shown to enhance the rate of distal ureteral stone ejection. The use of -blockers in the conservative therapy of distal ureteral stones is advised in the appropriate clinical context.
Open Science Framework: PRISMA checklist and flow chart for 'The efficacy of adjunctive alpha-blocker on ureteroscopy procedure for ureteral stones: a systematic review/meta-analysis'
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
The authors acknowledge that no contributions to this review originated outside the listed authors.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Endourology and oncology urology
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Urology
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Endourology and oncology urology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 12 Apr 22 |
read | |
|
Version 1 28 May 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)