Introduction
Acute mesenteric ischaemia (AMI) is a time- critical surgical emergency,1 where early diagnosis and management can prevent bowel infarction, multiorgan failure and death.2–4 It is defined as a sudden inadequacy of arterial supply or venous drainage to the bowel, leading to ischemia and cellular damage, with or without necrosis.5,6 AMI has an estimated incidence of ~1:1000 hospital admissions.1,4,7–9
A number of pathophysiological mechanisms can lead to mesenteric ischaemia.4 “Occlusive” mesenteric ischaemia is due to arterial or venous thrombosis or embolism. “Non-occlusive” is due to acute circulatory failure, usually in the critically unwell patient.10,11 Non-occlusive mesenteric ischaemia (NOMI) can also occur in the setting of critical illness secondary to the use of vasoactive drugs because of splanchnic vasoconstriction.5 Each of these processes cause a gut-derived systemic inflammatory response syndrome (SIRS) or mesenteric ischaemic necrosis, leading to severe metabolic derangement and culminating in multiple organ dysfunction requiring critical care intervention.12,13
Early imaging with computerised tomography (with arterial and portal venous phase)6 is important for diagnosis and instigating a timely management plan. The optimal management of AMI depends on the underlying pathophysiology and whether the affected bowel is ischaemic or infarcted. Treatment of AMI focuses on reperfusion and/or resection of non-viable bowel.14 As in any critically unwell patient, adequate resuscitation of haemodynamic parameters is important to optimise end-organ perfusion and prevent the development of multiorgan failure.
Given the extent of sepsis response, AMI management usually requires critical care support. Vasoactive drugs are often required in this setting to optimise haemodynamic status, with the aim of improving supply to the end organs as well as optimising the perfusion of blood to the adjacent intestine segments to the area of ischaemia.15 However, the choice of vasoactive agents is unclear for patients with AMI. This is a result of the various mechanism of action of these medications and differing levels of associated splanchnic vasoconstriction. Some agents, such as noradrenaline and adrenaline, can be effective in improving systemic vascular resistance and thus, maintain the perfusion pressure to the brain and heart. However, they can also be associated with profound splanchnic vasoconstriction which could exacerbate bowel ischaemia by precipitating NOMI.1,16 Other drugs are perceived to have less of an effect on splanchnic vasculature and could theoretically improve perfusion to the primary area of pathology but may impact on perfusion pressure for other organs.
The mortality rate is variable but often high, especially when detected late or accompanied by metabolic derangement.4,7 This variability in mortality may be secondary to differences in local practice5,17,18 and between clinicians. It may also reflect the lack of evidence-based guidelines available for these conditions.19–23 Vasoactive agents vary in their mechanisms of action, and balance of vasoconstriction, inotropy, and splanchnic vascular dilatation. It is not known whether one may be more beneficial than others in the setting of AMI. This primary aim of this systematic review is to evaluate the current evidence comparing mortality outcomes for vasoactive drugs in AMI. Our PROSPERO summary is illustrated in Figure 1.

Figure 1. Summary of study objectives.
Methods
Protocol and registration
This review has been prepared in line with the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) statement. The protocol for this systematic review was developed and registered to PROSPERO prior to the analysis of search results (CRD42020212291, 11/11/2020).
Eligibility criteria
This systematic review aims to identify randomised clinical trials (RCTs) comparing mortality rates associated with different vasoactive agents in AMI. The target population were any patients with AMI admitted into a critical care environment. Vasoactive drugs included were noradrenaline, adrenaline, dopexamine, dobutamine, dopamine, levosimendan, vasopressin, ephedrine or phenylephrine. Comparators were either no vasoactive drug or any other vasoactive drug. The primary outcome was mortality. Secondary outcomes were survival, length of preserved bowel, time to anastomosis, length of critical care admission, and overall length of hospital stay. All published works were searched regardless of date of publication or language.
Information sources
Searched sources were Ovid Medline, EMBASE, Cochrane CENTRAL and the Cochrane Database of Systematic Review.
Search strategy
The literature search was conducted by the library department of the Royal College of Surgeons of England. The Patient, Intervention, Control and Outcome (PICO) framework was used and is outlined in Figure 1. Electronic databases of Ovid Medline, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL) and Cochrane Database of Systematic Reviews (CDSR) were searched for relevant studies. The search was completed on the 5th of November 2020 and included all relevant studies since 1946 including non-English studies and case reports. Search strategies included certain drugs, such as noradrenaline, adrenaline, dopexamine, dobutamine, dopamine, levosimendan, vasopressin, ephedrine or phenylephrine were specified. However, freedom of the searchers to add more terms if required was allowed. A combination of keywords and controlled vocabulary was adapted for each database. Search strategies are outlined in Figures 2, 3 and 4.

Figure 2. Search strategy – Medline.
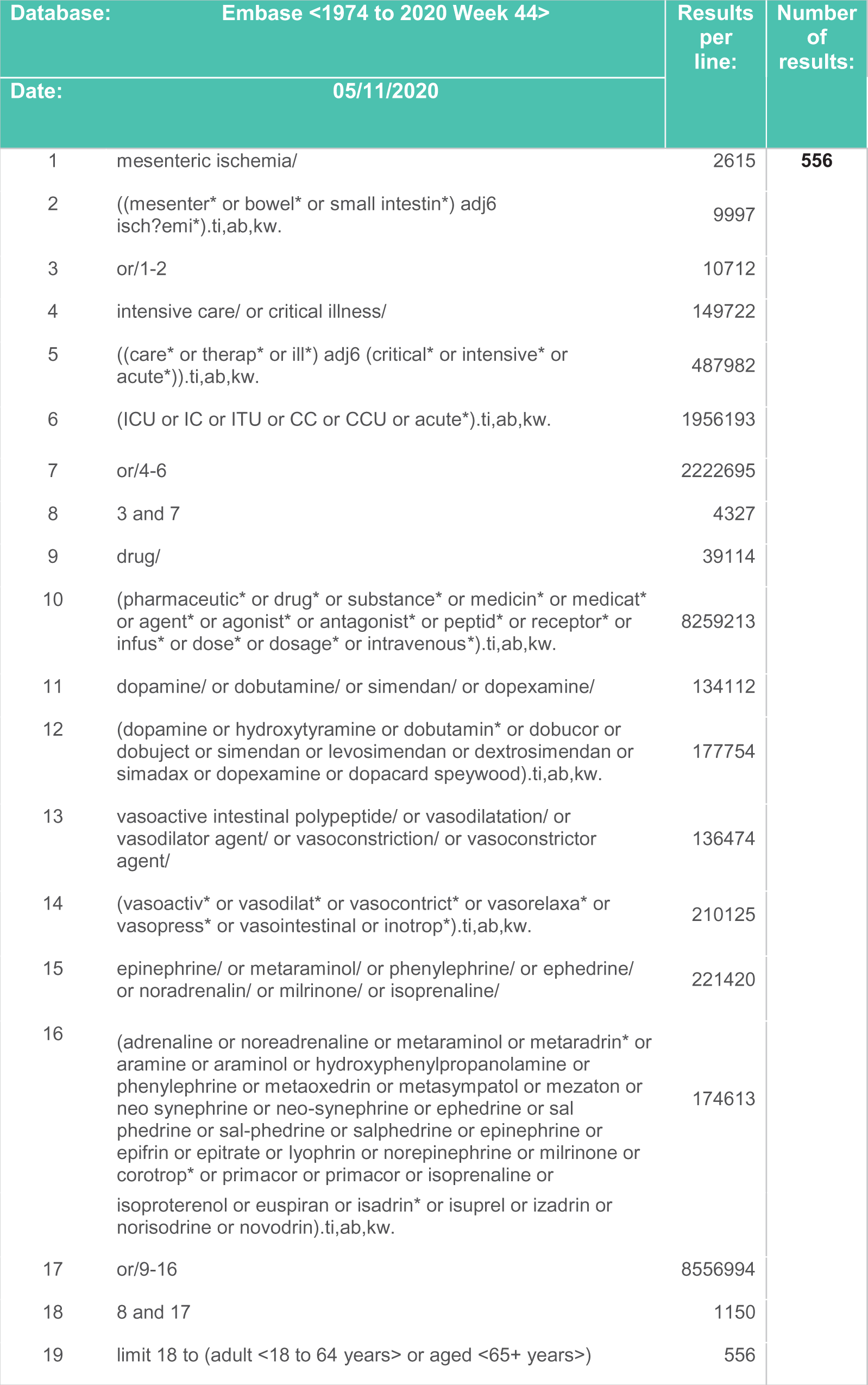
Figure 3. Search strategy – EMBASE.
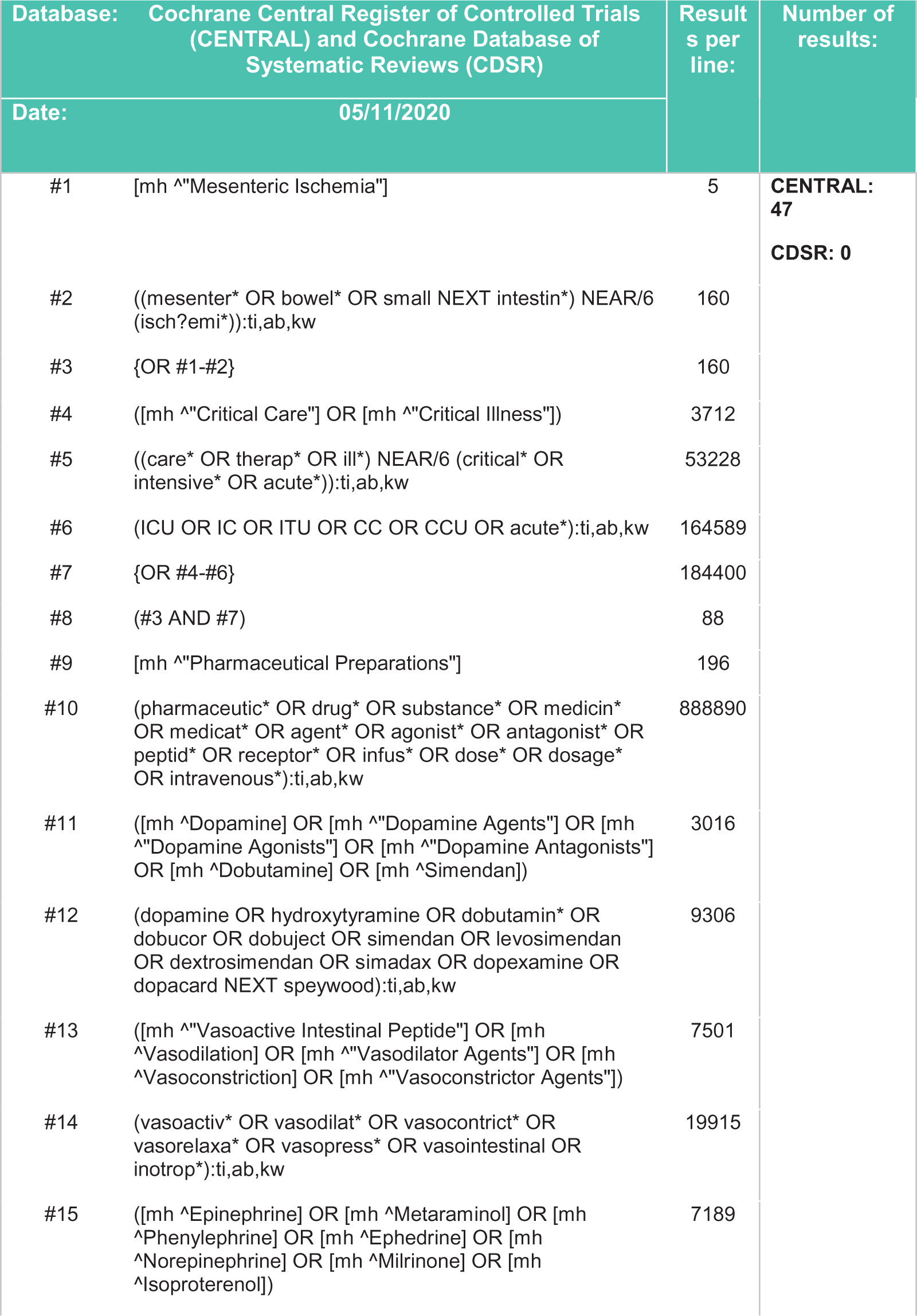

Figure 4. Search strategy - Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR).
Selection process
Studies were screened for relevant phrases of inclusion, where papers were identified as randomised control trials, performed on non-animal subjects, on the subject relating to the usage of vasoactive drugs in AMI. Papers were screened by four reviewers (CB, PO, EM, PF) independently. The process was facilitated using the Rayyan Platform, allowing for independent and anonymous review and analysis of searched literature. Any discrepancies underwent further review until a consensus was reached.
Data collection process
Data was collected from the reports identified by the literature search using the Rayyan platform. This platform facilitated independent and anonymous collection of data.https://www.rayyan.ai/
Data items
The Data items we sought were the following: the country of study, the study design, the intervention type (vasoactive medication), pathology and surgery (if any) subtype, the study size, the follow-up-time, the remaining length of small bowel, time to anastomosis, length of critical care stay, length of in hospital stay.
Risk of bias in individual studies
The reviewers were to assess selected studies using the Grading of Recommendations Assessment, Development and Evaluation (short GRADE) system. GRADE assesses study limitations/risk of bias, inconsistency, indirectness, impreciseness and publication bias. These criteria would have been applied on a study-by-study basis and an outcome level by two reviewers and where there was inconsistency, a third reviewer would assess and provide an outcome. We planned to perform a meta-analysis if the reported results permitted.
Data synthesis
In the case of a negative search without any eligible randomised controlled trials, the plan was to discuss the existing evidence regarding the problem (AMI) and how the intervention might work (use of vasoactive drugs).
Meta-bias(es)
The methods were to be reviewed externally to identify possible sources of potential bias.
Confidence in cumulative evidence
Strength was to be assessed using GRADE tools.
Results
This systematic review aimed to identify randomised control trials comparing mortality rates in relation to the use of different vasoactive drugs in AMI. Ovid Medline, EMBASE, Cochrane CENTRAL and the Cochrane Database of Systematic Review were systematically searched as outlined in Figures 1-4.
The initial search identified a total of 700 articles. After screening for duplicates, 614 articles remained and were reviewed for eligibility. Of these articles, 563 were not randomised control trials and thus immediately excluded. A further 22 trails were excluded as they were in animal studies. The remaining 29 RCT’s were reviewed, and their subject of focus in all cases was not related to mesenteric ischaemia. Thus, there were no eligible studies identified addressing the specific study question. Reasons for exclusion included: non-randomised controlled trials and studies, non-human trials, and studies which bore no semblance to the question of interest. In view of this, no quantitative analysis was performed. The PRISMA flowchart is outlined in Figure 5.
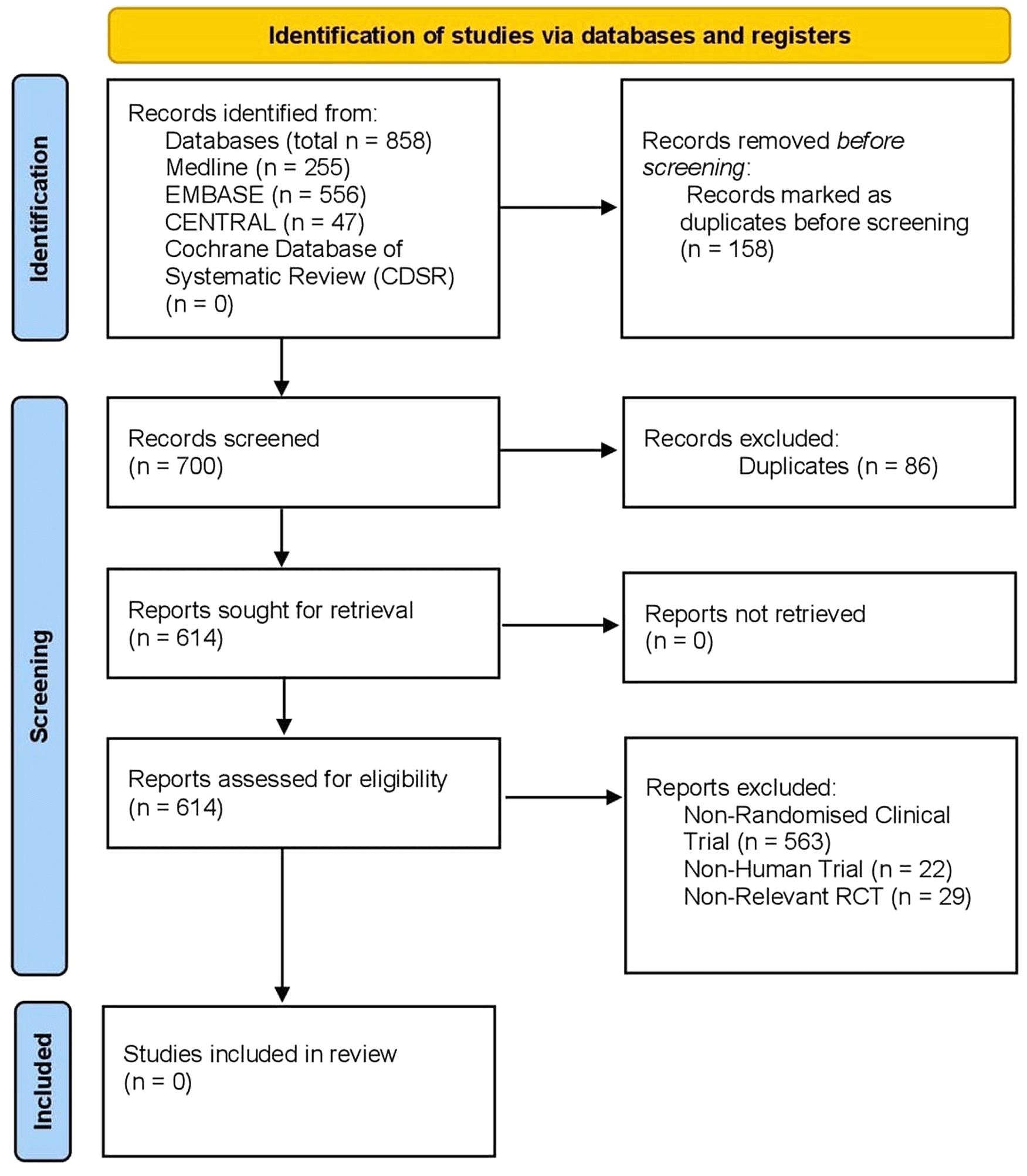
Figure 5. PRISMA flowchart.
Discussion
There is significant variation in the management of AMI, and this may reflect regional variations in mortality associated with this condition. The recent ACPGBI guidance24 on Emergency General Surgery highlighted the relative paucity of research in this field. However, a synthesis of specific aspects of AMI management, such as the choice of vasoactive medications and its influence on mortality was beyond its remit.
This systemic review aimed to ask that question and assessed the previous publications available. It is of interest that this work failed to identify any studies comparing the use of these drugs in AMI against outcomes. Although the focus of this paper is evaluating choice of vasoactive support and not comparing risk of associated ischaemia with agents, it worth considering both conditions pathophysiology’s for the evaluation of the most promising agent for a future RCT. Of the 29 excluded RCT’s, only 3 were studies comparing vasoactive agents. Although comparative studies, their subject base was deemed too different to be included for our subject matter. Hajjar et al compared vasopressin and noradrenaline in vasoplegic shock after cardiac surgery.25 Given the different physiological mechanism of shock, it is difficult to use this comparison for mesenteric ischaemia. Liu et al compared terlipressin and noradrenaline in septic shock, but identified no difference.26 Laterre et al compared serlipressin with placebo in another context of septic shock, and found no difference.27 Overall the comparative studies identified did not shed light on the optimal choice for mesenteric ischaemia.
In patients who present with symptoms, clinical findings or imaging suggestive of mesenteric arterial ischaemia, resuscitative measures including the avoidance of systemic hypoxia and intravenous fluids are crucial first steps to optimising blood pressure and end organ perfusion.6 Broad spectrum intravenous antibiotics should also be administered promptly due to the potential for bacterial complications in view of the breach of the mucosal barrier.6 Of the small cohort of patients deemed suitable, urgent revascularisation should be pursued to re-perfuse the ischaemic gut through liaison with the interventional radiologists and vascular surgeons. Most patients who are admitted to critical care units will require vasoactive drugs to optimise their blood pressure and cardiovascular status. However, the ideal drug which gives an appropriate increase in systemic arterial pressure without causing a decrease in or compromise of splanchnic perfusion remains to be elicited and the literature on this question is absent.
Catecholamines
Noradrenaline and adrenaline
In the context of AMI, splanchnic blood flow would seem a salient factor. Pharmacologically, noradrenaline is an endogenous catecholamine which primary has a direct alpha-1 effect, although there is a small degree of β-1 adrenergic agonism. It increases systemic vascular resistance (SVR), increasing afterload;- and causes venoconstriction increasing preload. It is weakly a positive inotrope through its β-1 effect. Some studies suggest that noradrenaline reduces hepato-splanchnic blood flow in septic and non-septic patients.28,29 Similarly, adrenaline, a sympathomimetic, was found to have a reductive effect on splanchnic blood flow.30
Dopamine and dopexamine
Dopamine is a catecholamine, a precursor to noradrenaline, and mediates inotropy via dopamine receptors and vasoconstriction via the alpha-adrenergic pathway. It shows a dose dependent change in action; causing splanchnic dilatation at low doses while increasing SVR at higher doses. Meier-Hellmann et al.31 reported an increase in hepato-splanchnic blood flow in septic patients given dopamine although Neviere and colleagues32 reported a decrease in gut mucosal perfusion. Maynard et al.33 suggested that dopexamine, a dopamine analogue which has vasodilatory effects, may improve gut microcirculation in septic shock; although subsequent investigators did not confirm these beneficial effects.
Dobutamine
Dobutamine, a synthetic catecholamine is a β1-selective adrenoceptor agonist which is utilised clinically as a positive inotrope in the treatment of acute heart failure and cardiogenic shock. Creteur and colleagues34 determined that a dobutamine infusion did improve both splanchnic oxygenation in septic animals and in septic patients. However, Bomberg et al.35 suggest that in pigs, dobutamine may improve arteriovenous shunting, but conversely may reduce jejunal mucosal perfusion.
Non-catecholamines
Vasopressin
One observational cohort study found that vasopressin, a potent non-catecholamine vasoconstrictor which acts on vasopressin receptors, improved small bowel perfusion and mortality in patients with non-occlusive mesenteric ischaemia (NOMI) who had undergone cardiopulmonary bypass for elective cardiac surgery.13 However, no further assessment of outcome in AMI appears to have been assessed.
Levosimendan and milrinone
Levosimendan is an inotrope which improves contractility by sensitizing cardiac muscle to calcium. It also produces vasodilation by opening ATP-sensitive K+ channels in vascular smooth muscle, although this is not yet demonstrated in the splanchnic circulation.
An experimental study on hypoxic, stressed new-born piglets showed milrinone, a phosphodiesterase inhibitor which produces an inotropic effect and vascular dilation, improves mesenteric perfusion.36
There is thus some basic scientific experimental data available on the effects of some vasoactive agents on the splanchnic circulation. However, there are a number of limitations to this, and none have been extrapolated into clinical trials investigating vasoactive medication in AMI, against other agents. In relation to our study question, most of the experimental data focuses on patients in a shocked state as a result of sepsis, or in elective settings such as planned cardiac surgery, rather than in AMI. None of the studies report any clear data in relation to mortality or morbidity, length of hospital or critical care stay associated with any vasoactive agents.
There may be a role for dobutamine, levosimendan, milrinone, dopamine or vasopressin or other vasoactive agents in improving splanchnic perfusion in mesenteric ischaemia, but further, more extensive, patient-based study is required to elucidate these theories and their clinical significance in relation to patient survival and morbidity.
Limitations
This study did not identify any qualifying randomised controlled trials in relation to the study question and therefore did not produce a quantitative analysis.
It should be considered why no randomised controlled trials have occurred in this field thus far. The acute presentation and potential early requirement for vasoactive support, coupled with initial uncertainty of diagnosis, may make comparative studies more difficult to perform.
Conclusions
This systematic review has identified a gap in literature and research relating to the choice of vasoactive agent in AMI. There are therefore actions we would recommend to aid identifying best practice for this condition. The results of this study would suggest that it is important to investigate current practice and clinician preference. The first step to this would therefore be a Delphi Study which is currently underway and can be found via this link: https://is.gd/vasoactive_agents_AMI. Following the survey is an optionable Delphi process.
RCTs where comparison of outcomes with different vasoactive agents is analysed could ultimately improve the care of the critically ill patient with mesenteric ischaemia and remains absent from any work relating to AMI.
Data availability
All data underlying the results are available as part of the article and no additional source data are required.
Reporting guidelines
Harvard Dataverse. PRISMA checklist and Review Data for: Vasoactive agents in acute mesenteric ischaemia in critical care. A systematic review. DOI: https://doi.org/10.7910/DVN/2GN0BS.37
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Acknowledgements
With thanks to Helen Fulbright, PhD, MA, PGDip LIS, BA (Hons), MCLIP, Information Specialist, Royal College of Surgeons of England Library and Archives Team, for conducting the literature searches.
References
- 1.
Tilsed JVT, Casamassima A, Kurihara H, et al.:
ESTES guidelines: acute mesenteric ischaemia [Internet].
Eur J Trauma Emerg Surg.
2016 [cited 2020 Sep 24]; 42: 253–270. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 2.
Heys SD, Brittenden J, Crofts TJ:
Acute mesenteric ischaemia: the continuing difficulty in early diagnosis.
Postgrad Med J.
1993 Jan; 69(807): 48–51. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 3.
Leone M, Bechis C, Baumstarck K, et al.:
Outcome of acute mesenteric ischemia in the intensive care unit: a retrospective, multicenter study of 780 cases.
Intensive Care Med.
2015; 41(4): 667–676. PubMed Abstract
| Publisher Full Text
- 4.
Cudnik MT, Darbha S, Jones J, et al.:
The Diagnosis of Acute Mesenteric Ischemia: A Systematic Review and Meta-analysis.
Acad Emerg Med.
2013; 20(11): 1087–1100. PubMed Abstract
| Publisher Full Text
- 5.
Oldenburg WA, Lau LL, Rodenberg TJ, et al.:
Acute Mesenteric Ischemia: A Clinical Review.
Arch Intern Med.
2004 May 24; 164(10): 1054–1062. PubMed Abstract
| Publisher Full Text
- 6.
Bala M, Kashuk J, Moore EE, et al.:
Acute mesenteric ischemia: guidelines of the World Society of Emergency Surgery.
World J Emerg Surg.
2017; 12: 38. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 7.
Heys SD, Brittenden J, Crofts TJ:
Acute mesenteric ischaemia: the continuing difficulty in early diagnosis.
Postgrad Med J.
1993. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 8.
Stoney RJ, Cunningham CG:
Acute mesenteric ischemia.
Surgery.
1993. PubMed Abstract
- 9.
Acosta S:
Epidemiology of mesenteric vascular disease: Clinical implications.
Semin Vasc Surg.
2010. PubMed Abstract
| Publisher Full Text
- 10.
Daviaud F, Grimaldi D, Dechartres A, et al.:
Timing and causes of death in septic shock.
Ann Intensive Care.
2015; 5: 16. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 11.
Guillaume A, Pili-Floury S, Chocron S, et al.:
Acute Mesenteric Ischemia Among Postcardiac Surgery Patients Presenting with Multiple Organ Failure.
SHOCK.
March 2017; 47(3): 296–302. PubMed Abstract
| Publisher Full Text
- 12.
Sertaridou E, Papaioannou V, Kolios G, et al.:
Gut failure in critical care: old school versus new school.
Ann Gastroenterol.
2015. PubMed Abstract
| Free Full Text
- 13.
Deitch EA:
Gut-origin sepsis: evolution of a concept.
Surgeon.
2012. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 14.
Chang RW, Chang JB, Longo WE:
Update in management of mesenteric ischemia [Internet].
World J Gastroenterol.
WJG Press;
2006 [cited 2020 Sep 24]; 12. : p. 3243–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 15.
Luther B, Mamopoulos A, Lehmann C, et al.:
The Ongoing Challenge of Acute Mesenteric Ischemia.
Visc Med.
2018 Jul 1 [cited 2020 Oct 1]; 34(3): 217–223. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 16.
Bomberg H, Groesdonk H, Raffel M, et al.:
Vasopressin as Therapy During Nonocclusive Mesenteric Ischemia.
Ann Thorac Surg.
2016; 102(3): 813–819. PubMed Abstract
| Publisher Full Text
- 17.
Crawford RS, et al.:
A Statewide Analysis of the Incidence and Outcomes of Acute Mesenteric Ischemia in Maryland from 2009 to 2013.
Front Surg.
2016. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 18.
Lemma AN, et al.:
Choice of First Emergency Room Affects the Fate of Patients With Acute Mesenteric Ischaemia: The Importance of Referral Patterns and Triage.
Eur J Vasc Endovasc Surg.
2019. PubMed Abstract
| Publisher Full Text
- 19.
Krejci V, Hiltebrand L, Sigurdsson G:
Effects of epinephrine, norepinephrine, and phenylephrine on microcirculatory blood flow in the gastrointestinal tract in sepsis.
Crit Care Med.
2006; 34(5): 1456–1463. PubMed Abstract
| Publisher Full Text
- 20.
Malay M, Ashton J, Dahl K, et al.:
Heterogeneity of the vasoconstrictor effect of vasopressin in septic shock.
Crit Care Med.
2004; 32(6): 1327–1331. PubMed Abstract
| Publisher Full Text
- 21.
Russell J, Walley K, Singer J, et al.:
Vasopressin versus norepinephrine infusion in patients with septic shock.
N Engl J Med.
2008; 358(9): 877–887. PubMed Abstract
| Publisher Full Text
- 22.
Auchet T, Regnier M, Girerd N, et al.:
Outcome of patients with septic shock and high-dose vasopressor therapy.
Ann Intensive Care.
2017; 7(1): 43. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 23.
Asfar P, Meziani F, Hamel J, et al.:
High versus low blood-pressure target in patients with septic shock.
N Engl J Med.
2014; 370(17): 1583–1593. PubMed Abstract
| Publisher Full Text
- 24.
ACPGBI Emergency General Surgery – A Consensus Statement [Internet]. [cited 2021 Apr 11]..
http
- 25.
Hajjar LA, Vincent JL, Barbosa Gomes Galas FR, et al.:
Vasopressin versus Norepinephrine in Patients with Vasoplegic Shock after Cardiac Surgery: The VANCS Randomized Controlled Trial.
Anesthesiology.
2017; 126(1): 85–93. PubMed Abstract
| Publisher Full Text
- 26.
Liu ZM, Chen J, Kou Q, et al.:
liuressin versus norepinephrine as infusion in patients with septic shock: a multicentre, randomised, double-blinded trial.
Intensive Care Med.
2018; 44(11): 1816–1825. PubMed Abstract
| Publisher Full Text
- 27.
Laterre P, Berry SM, Blemings A, et al.:
Effect of Selepressin vs Placebo on Ventilator- and Vasopressor-Free Days in Patients With Septic Shock: The SEPSIS-ACT Randomized Clinical Trial.
JAMA.
2019; 322(15): 1476–1485. Publisher Full Text
- 28.
Reinelt H, Radermacher P, Kiefer P, et al.:
Impact of exogenous beta-adrenergic receptor stimulation on hepatosplanchnic oxygen kinetics and metabolic activity in septic shock.
Crit Care Med.
1999 Feb; 27(2): 325–31. PubMed Abstract
| Publisher Full Text
- 29.
Duranteau J, Sitbon P, Teboul JL, et al.:
Effects of epinephrine, norepinephrine, or the combination of norepinephrine and dobutamine on gastric mucosa in septic shock.
Crit Care Med.
1999 May; 27(5): 893–900. PubMed Abstract
| Publisher Full Text
- 30.
Bangash MN, Kong ML, Pearse RM:
Use of inotropes and vasopressor agents in critically ill patients.
Br J Pharmacol.
2012 Apr; 165(7): 2015–33. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 31.
Meier-Hellmann A, Bredle D, Specht M, et al.:
The effects of low-dose dopamine on splanchnic blood flow and oxygen uptake in patients with septic shock.
Intensive Care Med.
1997; 23: 31–37. PubMed Abstract
| Publisher Full Text
- 32.
Nevière R, Mathieu D, Chagnon JL, et al.:
The contrasting effects of dobutamine and dopamine on gastric mucosal perfusion in septic patients.
Am J Respir Crit Care Med.
1996 Dec; 154(6 Pt 1): 1684–1688. PubMed Abstract
| Publisher Full Text
- 33.
Maynard ND, Bihari DJ, Dalton RN, et al.:
Increasing splanchnic blood flow in the critically III.
Chest.1995 Dec; 108(6): 1648–54. PubMed Abstract
| Publisher Full Text
- 34.
Creteur J, De Backer D, Vincent J:
A dobutamine test can disclose hepatosplanchnic hypoperfusion in septic patients.
Am J Respir Crit Care Med.
1999; 160(3): 839–845. PubMed Abstract
| Publisher Full Text
- 35.
Bomberg H, Bierbach B, Flache S, et al.:
Dobutamine versus Vasopressin After Mesenteric Ischemia.
J Surg Res.
2019 Mar 1; 235: 410–423. PubMed Abstract
| Publisher Full Text
- 36.
Manouchehri N, Bigam D, Churchill T, et al.:
Milrinone is preferred to levosimendan for mesenteric perfusion in hypoxia-reoxygenated newborn piglets treated with dopamine.
Pediatr Res.
2012; 71: 241–246. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 37.
Brennan CA, Osei-Bonsu P, McClenaghan RE, et al.:
Review Data for: Vasoactive agents in acute mesenteric ischaemia in critical care. A systematic review
Harvard Dataverse.
2021; V1. Publisher Full Text






Comments on this article Comments (0)