Keywords
Cellular senescence, human dermal fibroblasts, adipose-derived stem cells, platelet-rich plasma, protein retinoblastoma
This article is included in the Cell & Molecular Biology gateway.
This article is included in the Advances in Fibroblast Research collection.
Cellular senescence, human dermal fibroblasts, adipose-derived stem cells, platelet-rich plasma, protein retinoblastoma
Adipose-derived stem cells (ADSCs) are mesenchymal stem cells that reside in fat tissue and were first identified by Zuk et al.1 ADSCs with a unique secretome have a paracrine effect on surrounding cells giving rise to the theory that the efficacy of stem cell therapy is more due to the paracrine effect of cell secretion than the effect of cell differentiation.2 The paracrine effect of ADSCs is widely used in regenerative medicine such as tissue repair, wound healing, and as an anti-aging therapy.3 Conditioned media from adipose-derived stem cells (ADSC-CM) contains all the bioactive components of ADSCs, and is easy to store and safer during application.4 The cells are often cultured using a basal medium containing foetal bovine serum (FBS). FBS is important in the cell culture process. However, when it is used for regenerative medicine, this serum is less safe because it contains xenoproteins, which can cause rejection or transmission of infectious products. As a result, nowadays, the use of non-autologous cell culture media, like FBS in ADSCs for clinical applications is legally challenged. Thus, to reduce the use of animal products in ADSC cultures, a study was conducted using platelet-rich plasma (PRP) instead of FBS.25
The use of PRP is intended as a safer alternative supplement medium for ADSC culture. PRP is blood plasma that contains many platelets which are often used for wound healing.5 Platelets are a natural source of various growth factors such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), endothelial growth factor (EGF), and epidermal growth factor (EGF). They are efficient at triggering cell proliferation, differentiation, and tissue regeneration.6,7 There are many protocols for PRP preparation, from conventional blood centrifugation to other innovative methods, and the PRP can be activated by adding other materials or methods, for example, calcium, collagen, and/thrombin, or by glass contact or freeze-thaw cycles.8,9
Cellular senescence is an arrest of the cell cycle with a phenotypic change in which the cell will lose its replicative ability.10 In the past, cellular senescence was considered an irreversible process as protection against cancer, however, recent findings have shown its role in aging, tissue repair and development of senescent fibroblasts.11 In vitro, cells that experience senescence will undergo morphological changes such as cell flattening and enlargement, the apparition of vacuoles, and sometimes multiple nuclei; these contribute significantly to the aging process.12-14
Cells that experience senescence will lower the expression of genes involved in the cell cycle and several components of the extracellular matrix, and increase the expression of cell cycle inhibitor genes and matrix-degrading enzymes.15 Cell cycle withdrawal associated with cellular senescence is triggered by the p53-p21 and p16-Rb pathways.16,17 In senescent human dermal fibroblasts (HDFs), retinoblastoma (Rb) proteins accumulate in an active state; a hypo-phosphorylation state. In this condition, this protein is unable to phosphorylate in the middle or the end of the G1 phase and thus it is unable to enter the S phase.18 Research by Song et al. showed that ADSCs decreased p16 expression in senescent fibroblasts, thus it appears that ADSCs can reverse the aging process.19
Several extensive studies on PRP have been conducted, however, the biological mechanisms and clinical effects of PRP on HDFs are unclear. Besides, there has been no research on the combination of ADSC-CM and 10% PRP on Rb protein in senescent HDFs.
Our research has been done at the Biomedical and Parasitology laboratory, Faculty of Medicine, Universitas Brawijaya, Malang Indonesia. The HDFs as a culture source for fibroblast cells and ADSCs were isolated from a 25-year-old pregnant woman during an elective caesarean operation. The participant provided informed written consent prior to the study and all of the procedures in this research were approved by the Ethics Committee of the Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia (No.169/EC/KEPK-S3/05/2019). The HDF cells were an explant culture from human skin and were put into a 6-well plate until the explant attached. The cells were grown in a solution containing 20% FBS and Dulbecco’s Modified Eagle Medium (FBS-DMEM, ThermoFisher). The method used was a modified version of the method used by Takashima (1998), while the senescent HDF method used was that of Radiono et al. (2016).20,21 The HDFs were shifted into a senescent state by replacing the medium with 10% PRP in DMEM (ThermoFisher).
ADSCs from the pregnant woman were cultivated in 6-well plates and cultured in a complete culture medium (10% PRP in DMEM). The PRP preparation mentioned above was adopted from Amable et al.22 Briefly, whole blood was placed in tubes containing anticoagulants, and was centrifuged for 20 min (3,000 × g). After obtaining three layers in the whole blood, the upper layer was transferred into 10 ml empty sterile micro centrifuge tubes. The PRP was activated with 20mM CaCl2.
The expression of senescence-associated β-galactosidase (Sa-β-Gal) was analysed in HDFs using purchased Sa-β-Gal staining kit (MyBioSource, San Diego, CA, CAT# MBS168501) referring to manufacturer’s procedures. Concisely, the cells were fixed with 25% glutaraldehyde for 5 min, then they were washed with phosphate-buffered saline (PBS). Subsequently, the cells were incubated in cell staining solution overnight. The stained HDFs were washed with PBS and examined using an IX71 inverted microscope (Olympus) at 200× magnification.
The migration of HDF cells was investigated using a scratch assay. HDFs were grown to 80% confluence in 24-well plates and scraped with a 200 μl pipette tip. Next, the media were replaced to reduce the debris. HDF cells were photographed at 0 and 72h thereafter. After that, the HDFs were washed with PBS and detached by trypsin. The cell suspension was collected and centrifuged for 5 min (1000×g). The measurement of Rb protein levels was done using an enzyme-linked immunosorbent assay kit (ELISA) according to the protocol issued by the manufacturer’s instruction (MyBioSource, Cat#MBS2509425, RRID: AB_10568804). In brief, 100 μl of standard or sample were added to each well and incubated for 90 min at 37°C. The liquid was replaced with the provided biotinylated monoclonal antibody (60 min, 37°C) and followed by washing 3 times. The biotinylated detection antibody is specific to Human Rb1 and Avidin-Horseradish Peroxidase (HRP) conjugate. A 100 μl HRP conjugate was added (30 min, 37°C). After washing 5 times, 90 μl of substrate reagent was added and incubated for 15 min at 37°C. The reaction was stopped with 50 μl stop solution and the optical density (OD) was directly determined at 450 nm.
The data obtained was analysed using SPSS software version 25 (IBM). The normality test was carried out by the Shapiro Wilk test. If the P-value >0.05, then the normality assumption was fulfilled so an independent t-test was carried out. However, if the normality test was not fulfilled, the Mann-Whitney test was performed.
The 10% PRP - DMEM media was used to culture ADSCs and these media were used to shift the senescence state in HDFs. HDF explant culture began to grow on the seventh day. After the seventh passage, the HDF culture medium was replaced with 0.5% FBS for 72 hours to allow HDF cells to undergo senescence. The HDF media was then removed and the cells were transfected with the ADSCs - 10% PRP for another 72 h and subsequently examined by ELISA to determine the Rb protein level.
The scratch test showed that the senescent HDF cell group that received the ADSCs – 10% PRP medium proliferated faster than the control group (Figure 1). The Sa-β-Gal investigation showed that cells with ADSCs – 10% PRP medium had a lower number of senescent cells than the control group (Figure 2).
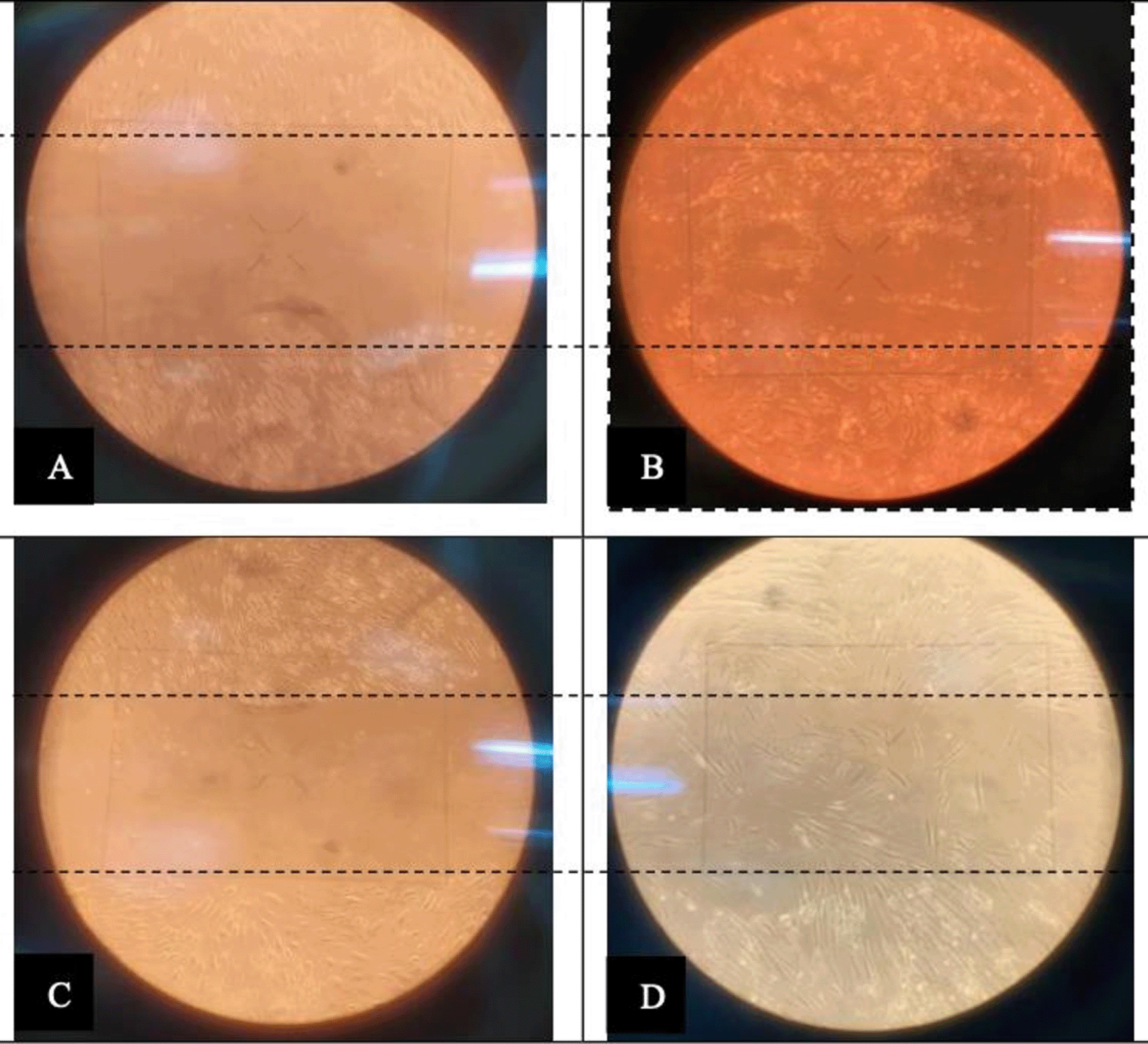
Scratch test of HDFs in the control group on day 0 (A) and after 72 hours (B). Scratch test of HDFs in the ADSCs – 10% PRP group on day 0 (C) and after 72 hours (D).
ADSCs: adipose-derived stem cells; HDF: human dermal fibroblasts; PRP: platelet-rich plasma.

ADSCs, adipose-derived stem cells; PRP, platelet-rich plasma.
Based on the Shapiro Wilk normality test, the P-value obtained for both groups was > 0.05, thus the independent t-test was carried out and gave a statistically significant result (P < 0.001) (Table 1,38).
Besides being easy to develop in vitro, ADSCs have similar capabilities to bone marrow stem cells, but they can be obtained easily from subcutaneous adipose tissue without invasive action, can maintain concentrations and proliferation consistently, and represent a more biologically relevant model for studying aging mechanisms when compared to other stem cells.23,24
PRP is a fraction of autologous blood plasma with a high platelet concentration.26 PRP also produces various cytokines, chemokines, and growth factors that can trigger recruitment, adhesion, proliferation, and maintain the differentiation of ADSCs.25,27
Research by Jia et al. (2017), which examined the effect of PRP in an in vitro photo-aging model, showed that PRP reduced the number of aging cells by 1% based on Sa-β-Gal positive cell tests. Furthermore, PRP prevents cell cycle arrest caused by irradiation by reducing the expression of p53 and p21, and decreasing the expression of matrix metalloproteinase (MMP) -1, -3, and -9 but not MMP-2 at the mRNA level.28
Research conducted by Kocaoemer et al. (2007) stated that 10% of non-autologous PRP activated by shock freezing or thrombin after being cultured for 11 days will increase ADSC proliferation faster than 10% FBS.29 Meanwhile, Pham et al. (2013) found that 15% PRP is the optimal concentration that will stimulate ADSC proliferation.30 Also, using the supernatant of the 10% activated PRP increases the proliferation of mesenchymal stem cells compared to other supplementary media such as whole blood, non-activated PRP, and non-activated platelet-poor plasma.31
Higher PRP concentrations will interfere with cell growth in vitro because the platelets will release protein complexes that negatively affect the action of the PRP growth factor.32 Besides, the concentration of proteolytic enzymes such as collagenase, cathepsin, elastase, and acid phosphatase is very high in PRP; therefore, it causes inhibition of cell growth.33
Stessuk et al. (2016) studied the combination of PRP with ADSC-CM in vitro and found significant proliferation stimulation in fibroblasts cultured with 25% PRP combined with ADSC-CM after 48 hours, while keratinocyte proliferation also increased insignificantly after 24 hours.34 Park et al. (2011) found that 10% PRP stimulates fibroblast cell migration and proliferation within 24 hours, presumably because PRP releases the PDGF-AA growth factor. However, PRP did not have a significant effect on the activity of MMP-1, MMP-2, and MMP-9 on HDFs cells. This is appropriate because fibroblast cells will be strongly reactive to PDGF, b-FGF, and EGF.35
Liu et al. (2014) examined the ability of PRP to improve cellular conditions from the senescence process and subsequently be able to slow down aging in animals. The research showed that PRP can change the expression of senescence markers such as p16, p19, and p53 on transgenic mice. In addition, PRP also can trigger cell growth, proliferation, and colony formation, and increase osteogenesis, reduce adipogenesis, and fight oxidative stress in stem cells from aged mice.36
Cho et al. (2012) examined the effects of PRP on cell migration, proliferation, and expression of various cell cycle regulatory proteins in HDFs cells. From this study, it was found that low concentrations of PRP (0.05 – 0.5%) would increase the expression of cyclin-A in HDFs cells, and 5% PRP would enhance the expression of proteins involved in the cell cycle, for example, Rb protein, cyclin-E, cyclin-A, and Cdk2. It seems that PRP through various growth factors will promote the progression of the cell cycle back into the G1 phase by regulating the expression level and activation of cyclin-Cdks.37
In summary, this study found an increase in Rb protein expression on senescent HDFs which were given a combination of ADSCs and 10% PRP. However, further studies are still needed to determine the effect of the combination of ADSCs and PRP on other senescence markers.
Figshare: Supplementary Data - The combination of ADSCs and 10% PRP increase Rb protein expression on senescent human dermal fibroblasts, https://doi.org/10.6084/m9.figshare.14716314.v1.38
This project contains the following underlying data:
- The combination of ADSCs and 10% PRP increase Rb protein expression on senescent human dermal fibroblasts.xlsx
- Statistic Result The combination of ADSCs and 10% PRP increase Rb protein expression on senescent human dermal fibroblasts.docx
- Picture 1A. Scratch test of HDFs in the control group on day 0.jpeg
- Picture 1B. Scratch test of HDFs in the control group after 72 h.jpeg
- Picture 1C. Scratch test of HDFs in the ADSCs – 10% PRP group on day 0.jpeg
- Picture 1D. Scratch test of HDFs in the ADSCs – 10% PRP group after 72 h.jpeg
- Picture 2A. SaBGal staining of fibroblasts in the control group.jpeg
- Picture 2B. SaBGal staining of senescent fibroblasts treated with ADSCs – 10% PRP.jpeg
- Figure 1.jpeg
- Figure 2.jpeg
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like to thank RSIA Melati Husada Malang, Indonesia, for providing the fat and dermal tissue. The authors would like to thank Dr. Tinny Endang Hernowati, SpPK (K), for her kindness and support during the research, may she Rest In Peace.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
No
References
1. Cory G: Scratch-wound assay.Methods Mol Biol. 2011; 769: 25-30 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Yeast and human aging and cancer, cell cycle regulation, and ubiquitin signalling.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Campisi J: Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors.Cell. 2005; 120 (4): 513-22 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical dermatology, venereology, skin cancer, biomolecular, dermatopathology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 30 Jun 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
SciScore: 6/10
Document Identifier: 102249
SciScore Report
Below you will find your SciScore report containing three tables. Your score is calculated based on adherence to scientific rigor criteria (Table 1) and identification of key biological resources (Table 2).
Table 3 contains statistical tests and oligonucleotides but is not scored. If SciScore makes any mistakes, please contact us to help us learn and improve.
Table 1: Rigor Adherence Table
Ethics
Consent: The participant provided informed written consent prior to the study and all of the procedures in this research were approved by the Ethics Committee of the Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia (No.169/EC/KEPK-S3/05/2019).
IRB: The participant provided informed written consent prior to the study and all of the procedures in this research were approved by the Ethics Committee of the Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia (No.169/EC/KEPK-S3/05/2019).
Inclusion and Exclusion Criteria
not detected.
Attrition
not detected.
Sex as a biological variable
The HDFs as a culture source for fibroblast cells and ADSCs were isolated from a 25-year-old pregnant woman during an elective caesarean operation.
Subject Demographics
Age: The HDFs as a culture source for fibroblast cells and ADSCs were isolated from a 25-year-old pregnant woman during an elective caesarean operation.
Randomization
not detected.
Blinding
not detected.
Power Analysis
not detected.
Replication
not required.
Table 2: Key Resources Table
Antibodies
The measurement of Rb protein levels was done using an enzyme-linked immunosorbent assay kit (ELISA) according to the protocol issued by the manufacturer’s instruction (MyBioSource, Cat#MBS2509425, RRID: AB_10568804).
RRID Verified: (MyBioSource Cat# MBS120046, RRID:AB_10568804)(link )
Software and Algorithms
The data obtained was analysed using SPSS software version 25 (IBM). SPSS
RRID Suggestion: (SPSS, RRID:SCR_002865)( link)
Table 3 Other Entities Detected Your Sentences Recognized Entity
Statistical Tests
The normality test was carried out by the Shapiro Wilk test.
Entity detected: Wilk test
If the P-value >0.05, then the normality assumption was fulfilled so an independent t-test was carried out.
Entity detected: t-test
However, if the normality test was not fulfilled, the Mann-Whitney test was performed.
Entity detected: Mann-Whitney test
Please note:
SciScore is an automated tool that is designed to assist expert reviewers by finding and presenting formulaic information scattered throughout a paper in a standard, easy to digest format. SciScore is not a substitute for expert review. SciScore also checks for the presence and correctness of several unique identifiers, including RRIDs (research resource identifiers) in the manuscript, detects sentences that appear to be missing RRIDs, and can even suggest RRIDs under certain circumstances. All RRID suggestions should be verified; only the author can know whether the suggestions are correct.
For a full description of scored criteria and tips for improving your score, please see https://www.scicrunch.com/sciscorereport-faq
SciScore: 6/10
Document Identifier: 102249
SciScore Report
Below you will find your SciScore report containing three tables. Your score is calculated based on adherence to scientific rigor criteria (Table 1) and identification of key biological resources (Table 2).
Table 3 contains statistical tests and oligonucleotides but is not scored. If SciScore makes any mistakes, please contact us to help us learn and improve.
Table 1: Rigor Adherence Table
Ethics
Consent: The participant provided informed written consent prior to the study and all of the procedures in this research were approved by the Ethics Committee of the Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia (No.169/EC/KEPK-S3/05/2019).
IRB: The participant provided informed written consent prior to the study and all of the procedures in this research were approved by the Ethics Committee of the Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia (No.169/EC/KEPK-S3/05/2019).
Inclusion and Exclusion Criteria
not detected.
Attrition
not detected.
Sex as a biological variable
The HDFs as a culture source for fibroblast cells and ADSCs were isolated from a 25-year-old pregnant woman during an elective caesarean operation.
Subject Demographics
Age: The HDFs as a culture source for fibroblast cells and ADSCs were isolated from a 25-year-old pregnant woman during an elective caesarean operation.
Randomization
not detected.
Blinding
not detected.
Power Analysis
not detected.
Replication
not required.
Table 2: Key Resources Table
Antibodies
The measurement of Rb protein levels was done using an enzyme-linked immunosorbent assay kit (ELISA) according to the protocol issued by the manufacturer’s instruction (MyBioSource, Cat#MBS2509425, RRID: AB_10568804).
RRID Verified: (MyBioSource Cat# MBS120046, RRID:AB_10568804)(link )
Software and Algorithms
The data obtained was analysed using SPSS software version 25 (IBM). SPSS
RRID Suggestion: (SPSS, RRID:SCR_002865)( link)
Table 3 Other Entities Detected Your Sentences Recognized Entity
Statistical Tests
The normality test was carried out by the Shapiro Wilk test.
Entity detected: Wilk test
If the P-value >0.05, then the normality assumption was fulfilled so an independent t-test was carried out.
Entity detected: t-test
However, if the normality test was not fulfilled, the Mann-Whitney test was performed.
Entity detected: Mann-Whitney test
Please note:
SciScore is an automated tool that is designed to assist expert reviewers by finding and presenting formulaic information scattered throughout a paper in a standard, easy to digest format. SciScore is not a substitute for expert review. SciScore also checks for the presence and correctness of several unique identifiers, including RRIDs (research resource identifiers) in the manuscript, detects sentences that appear to be missing RRIDs, and can even suggest RRIDs under certain circumstances. All RRID suggestions should be verified; only the author can know whether the suggestions are correct.
For a full description of scored criteria and tips for improving your score, please see https://www.scicrunch.com/sciscorereport-faq