Keywords
innate immunity, toll like receptors, interleukin-1 receptor-associated kinase-4, case report
innate immunity, toll like receptors, interleukin-1 receptor-associated kinase-4, case report
A few details regarding the assay used to evaluate cytokine response to TLR agonists were added. Additional discussion regarding the role of the CD62L shedding assay as a potential screening test was added. Additional discussion focusing on explaining why immunoglobulin prophylaxis has a role in IRAK4 deficiency has been added. A brief discussion regarding the importance of screening other family members for the same pathogenic variants was added. Lastly, a brief comment on the long-term prognosis of patients with IRAK4 deficiency was also added.
See the authors' detailed response to the review by David Hagin
See the authors' detailed response to the review by Shanmuganathan Chandrakasan
Pseudomonas aeruginosa (P. aeruginosa) is an opportunistic aerobic Gram-negative bacterial pathogen associated with a variety of genitourinary (UTI), pulmonary as well as skin and soft tissue infections (SSTI) in hospitalized pediatric patients often in association with significant morbidity1. Rarely, P. aeruginosa can be associated with severe and life-threatening infections among children without previously recognized associated risk factors2. In these cases, it is vital to consider the possibility of an underlying inborn error of immunity. Invasive P. aeruginosa infections have been described in the setting of inborn errors of immunity including antibody deficiencies (agammaglobulinemia) Bruton (Bruton agammaglobulinemia), combined immunodeficiency disorders (severe combined immunodeficiency, ataxia telangiectasia), defects of phagocytes (chronic granulomatous disease, leukocyte adhesion deficiency), defects in actin-polymerization (Wiskott-Aldrich syndrome, MKL1-deficiency), chronic neutropenia and innate immunity including defects in canonical NFKB-signaling (e.g., NEMO/NFKBIA) as well as those that impair the downstream signaling of toll-like receptors (TLRs), such as defects in interleukin-1 receptor-associated kinase (IRAK)-4 and myeloid differentiation factor 88 (MyD88)2,3. We describe the presence of an invasive soft tissue and central nervous system infection with P. aeruginosa in a previously healthy two-year-old female which prompted evaluation for an inborn error of immunity.
A previously healthy two-year-old Hispanic female was evaluated for left hip and knee pain associated with a fever and a refusal to ambulate. She had been given cephalexin for presumed insect bites on her back and legs. Her past medical history was non-contributory. She was fully immunized. Her family history was negative for consanguinity and inborn errors of immunity. She was afebrile and her physical examination demonstrated a 3 cm circular erythematous lesion on her mid left back which was non-indurated. Her left knee was minimally swollen, warm to touch, and non-erythematous. Her hips and knees remained in flexion with demonstrated resistance to knee extension. A nodular 1.5 cm mass in her popliteal fossa was also documented with tenderness upon palpation and she was unable to walk. Her laboratory investigation demonstrated a white blood cell count of 17.2 × 103/uL (normal range: 3.9-13.7), Hgb 10.4 g/dL (normal range:10.2-15.4), platelets 319 × 103 (normal range: 150-450), 64% neutrophils (normal range: 25-72), 26% lymphocytes (normal range: 24-71), 9% monocytes (normal range: 0-14), 0.6% eosinophils (normal range: 2-10), 0.3% basophils (normal range: 0-2), erythrocyte sedimentation rate (ESR) 126 mm/hr (normal range: 0-20), and C-reactive protein (CRP) 68.8 mg/L (normal range: 0-10). A blood culture was negative. Magnetic resonance (MR) imaging of her lower extremity demonstrated myositis and fasciitis involving the soft tissues of the distal left thigh, but no abscess (Figure 1A–C). Likely pathogens included methicillin sensitive Staphylococcus aureus, methicillin resistant Staphylococcus aureus, and group A Streptococcus.
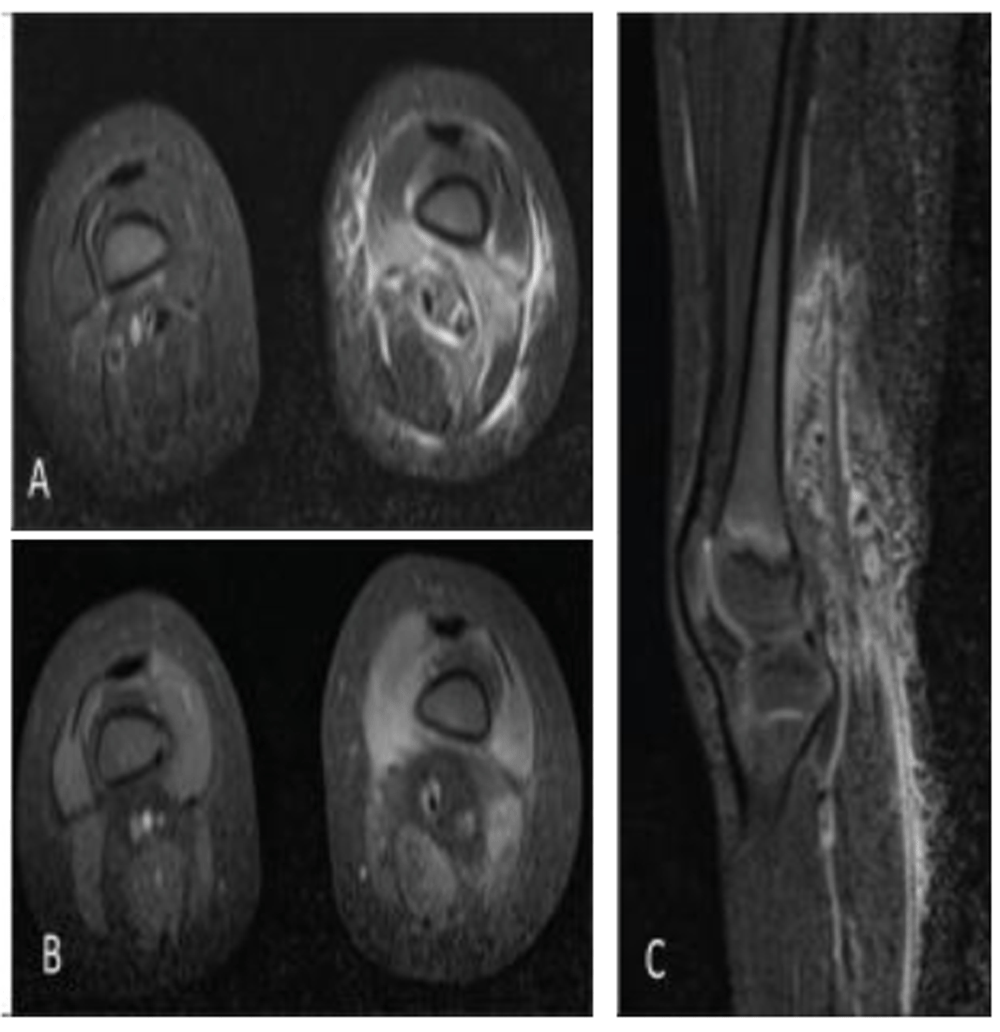
Axial (A) and sagittal (C) Short-TI Inversion Recovery (STIR) magnetic resonance imaging (MRI) images of the bilateral lower extremities demonstrate edematous signal involving the left lower extremity vastus medialis, vastus intermedius, soft tissues of the popliteal fossa, and overlying subcutaneous soft tissues. Axial fat-saturated T1-weighted postcontrast MRI of the bilateral lower extremities (B) demonstrates associated enhancement of the vastus medialis, vastus intermedius, and soft tissues of the popliteal fossa. Findings are compatible with myositis with overlying cellulitis.
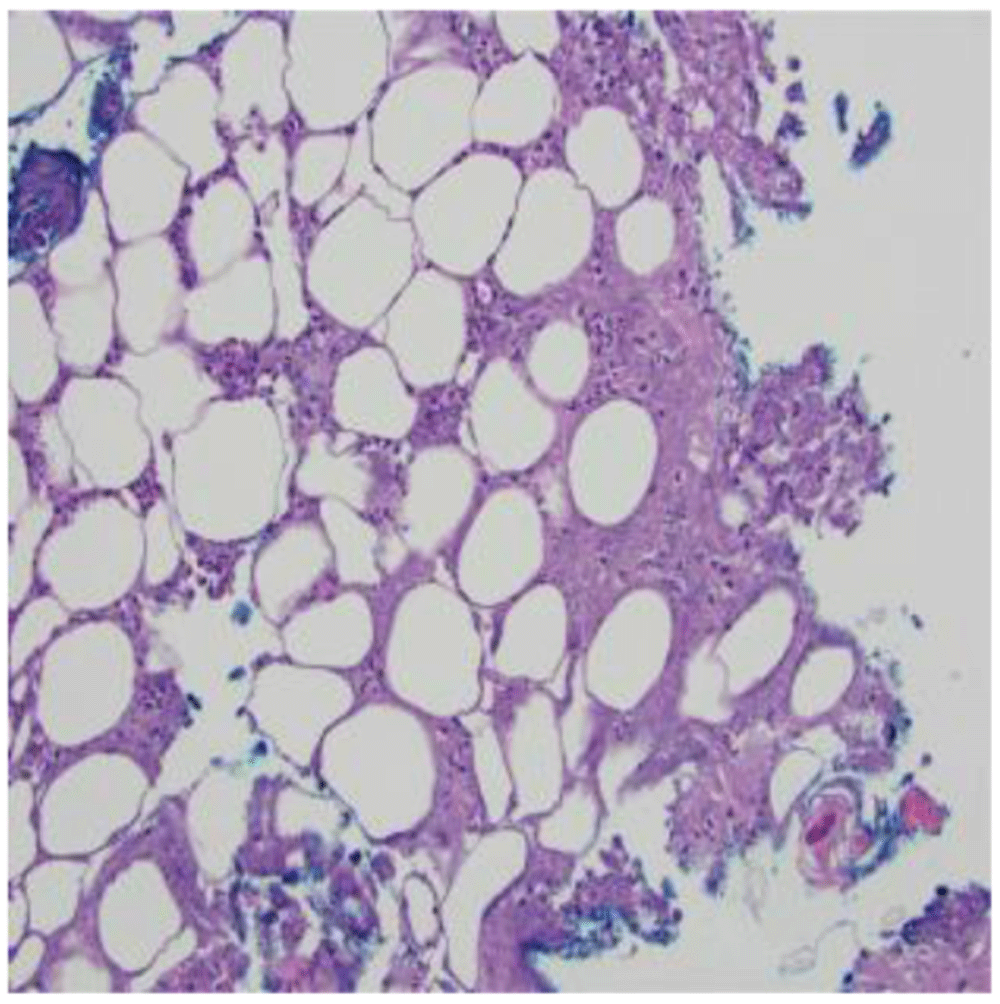
Empiric antibiotic therapy began with vancomycin (60 mg/kg/day intravenously divided every 6 hours for 3 days) and ceftriaxone (75 mg/kg/day intravenously every 24 hours for 3 days) which was then transitioned to cefazolin (100 mg/kg/day intravenously divided every 8 hours for 7 days), clindamycin (30 mg/kg/day intravenously divided every 8 hours for 7 days), amoxicillin-clavulanate (50 mg/kg/day orally divided every 12 hours for 4 days), and then to linezolid (30 mg/kg/day intravenously divided every 8 hours for 6 days) due to a lack of clinical and laboratory improvement. A lesion on her back was biopsied and demonstrated acute suppurative panniculitis and suppurative necrosis (Figure 2). Cultures were obtained with growth of P. aeruginosa and a 3-week course of cefepime (150 mg/kg/day intravenously divided every 8 hours) for SSTI was completed. Clinical and laboratory improvement occurred as demonstrated by an ESR 57 mm/hr (normal range: 0–20) and CRP <5 mg/L (normal range: 0–10). The patient was discharged while awaiting results of a workup for an inborn error of immunity.
She then returned approximately 3 weeks later with malaise, an inability to stand upright, irritability, and pain on palpation of her back as well as a refusal to ambulate. On re-admission she was afebrile, and her laboratory investigation demonstrated an ESR 89 mm/hr (normal range: 0-20), and CRP <5 mg/L (normal range: 0-10). MR imaging of her spine demonstrated enhancement of T9-T11 with an epidural abscess (Figure 3A–B). A culture was obtained by computed tomography (CT)-guided needle aspiration and empiric therapy with meropenem (120 mg/kg/day intravenously divided every 8 hours for 2 days) was begun. Growth of P. aeruginosa was documented and a 6-week course of cefepime (150 mg/kg/day intravenously divided every 8 hours) was completed. Clinical improvement occurred; however, her laboratory investigation demonstrated an ESR 80 mm/hr (normal range: 0–20) and CRP <5 mg/L (normal range: 0–10) at the completion of therapy.
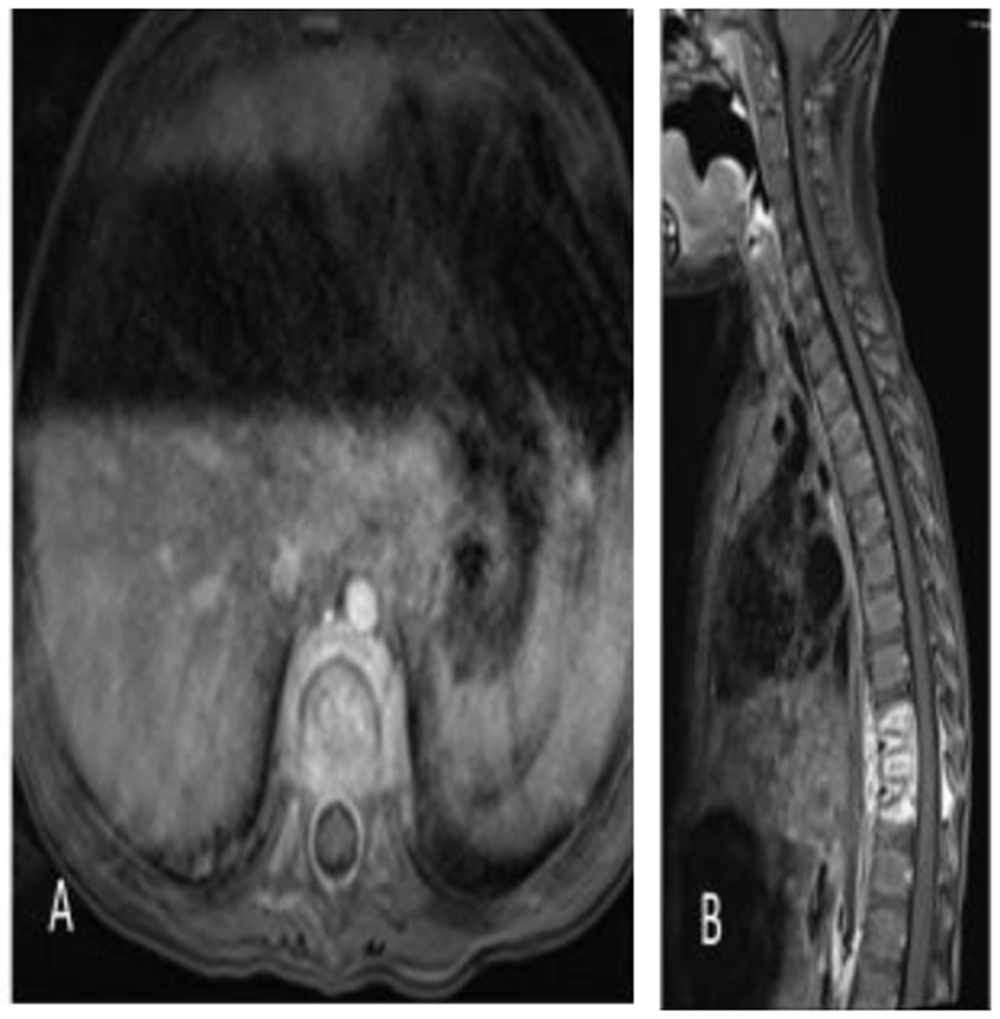
Axial (A) and sagittal (B) fat-saturated volumetric interpolated breath-hold examination (VIBE) postcontrast magnetic resonance imagery (MRI) images of the thoracic spine demonstrate multilevel thoracic osteomyelitis and discitis with associated epidural and paravertebral abscesses.
Secondary to the invasive nature of her P. aeruginosa infection, evaluation for an inborn error of immunity was completed. A normal neutrophil oxidative burst was noted. Additional laboratory assessments included a serum IgG 1331 mg/dL (normal range: 407-1009), IgA 85 mg/dL (normal range: 22-220), an elevated IgM 362 mg/dL (normal range: 43-163), and a IgE 3.8 kU/L (normal range: <97). Antibody responses documented a non-protective Haemophilus influenza type b antibody titer (0.30 mcg/L), a tetanus antibody titer which was protective (0.21 IU/mL), and pneumococcal titers which were protective (> 1.3 mcg/L) for nearly all serotypes covered by Prevnar 13. Lymphocyte immunophenotyping demonstrated a CD3+ count of 1340/ uL (normal range: 1484-5327), CD4+ count of 633 / uL (normal range: 733-3181), CD8+ count of 628/ uL (normal range: 370-2555), CD19+ count of 493/ uL (normal range: 370-2306), CD16/56+ count of 109/uL (normal range: 43-526). Decreased cytokine response to TLR agonists were documented by a commercial lab (ARUP Laboratories) using peripheral blood mononuclear cells (Figure 4). LPS-induced CD62L shedding in neutrophils is an additional potential screening test for TLR defects, but was not completed in this case4. Targeted sequencing of IRAK-4 and MyD88 was performed. A homozygous deletion of exons 8-13 was documented in IRAK-4 consistent with a diagnosis of IRAK-4 deficiency. There were no siblings; however, it is important to underscore the importance of screening other family members for the same pathogenic variants.
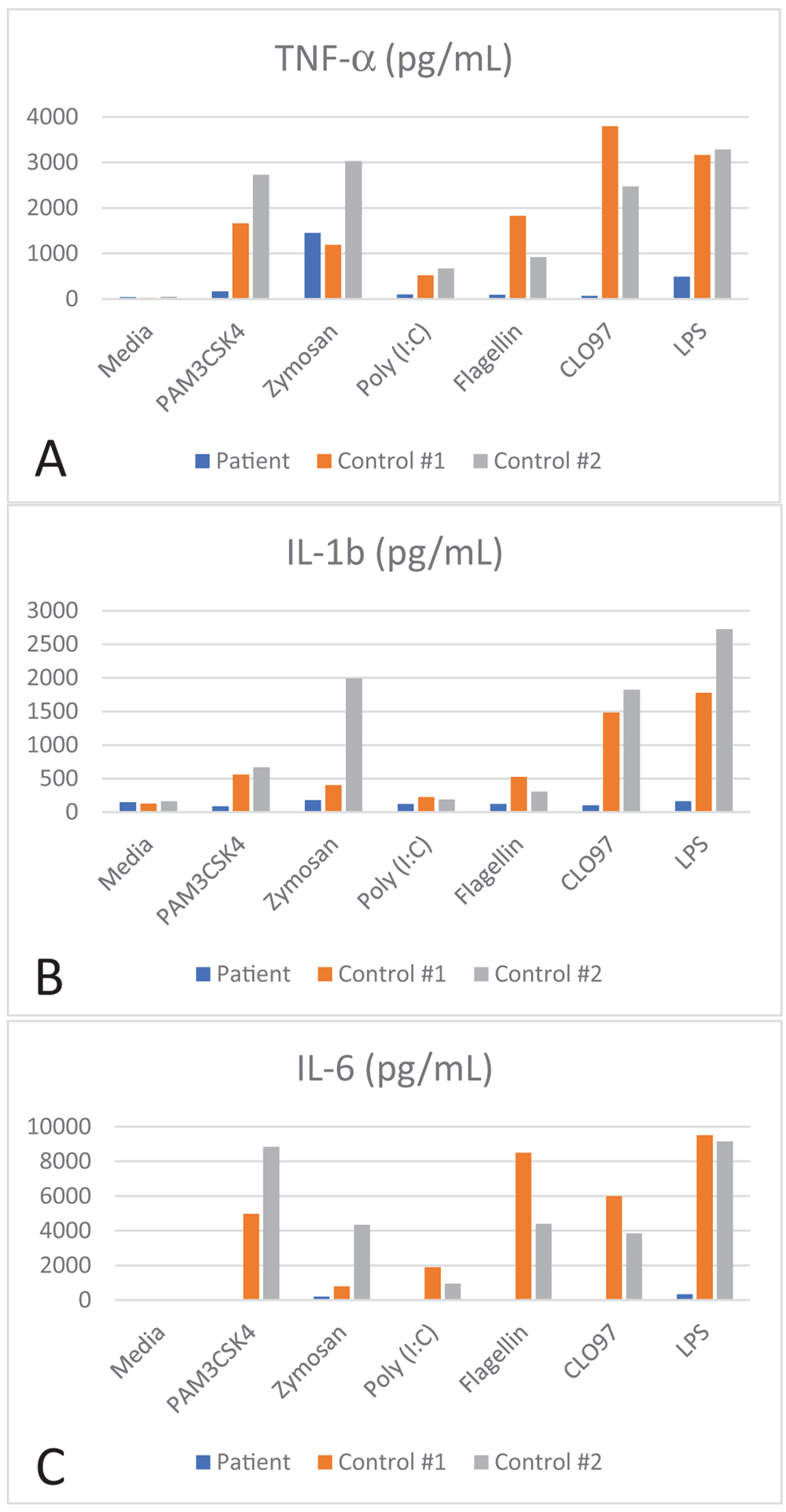
Decreased cytokine response to toll-like receptor (TLR) agonists: A) TNF alpha, B) IL-1b, and C) IL-6 was measured following peripheral blood mononuclear cell (PBMC) stimulation with PAM3CSK4 (TLR2 and TLR1), zymosan (TLR2 and TLR6), Poly (I:C) (TLR3), flagellin (TLR 5), CLO97 (TLR7 and TLR8), and LPS (TLR4).
Following the diagnosis of IRAK-4 deficiency she was start on prophylaxis with intravenous immunoglobulin (0.5 g/kg/dose intravenously every 4 weeks) as well as amoxicillin (250 mg orally each day). From her diagnosis at 2 years of age until 4 years of age she continued to experience infrequent infectious complications including a urinary tract infection (Escherichia coli), left knee swelling in association with a abscess (methicillin-susceptible Staphylococcus aureus), and a single admission for fever, cough, and post-tussive emesis. She is now 6 years of age and doing well without any recent infectious complications. She remains compliant with her prophylaxis therapy with intravenous immunoglobulin therapy and amoxicillin which she has tolerated without complications.
Detection of lipopolysaccharide and flagellin by TLRs results in the elaboration of pro-inflammatory cytokines3. TLRs possess an intracellular domain known as the Toll–IL-1R domain (TIR). Upon activation of TLRs, the recruitment of TIR-containing cytosolic adaptors such as MyD88 occurs. The adaptor MyD88 then recruits cytosolic kinases, including the IRAK complex. The IRAK complex includes two kinases including IRAK-4 and two non-catalytic subunits. This results in the activation of downstream effectors including nuclear factor κB (NF-κB) and mitogen-activated protein kinases which support the synthesis of pro-inflammatory cytokines and chemokines, such as IL-1β, -6, -8, and -12 and tumor necrosis factor alpha.
IRAK-4 deficiency is an autosomal recessive disorder which requires that affected patients have homozygous or compound heterozygous mutations in the IRAK-44–6. IRAK-4 deficient patients typically have normal basic immunological evaluations4–6. Importantly, inflammatory responses are markedly blunted as demonstrated by the severe and life-threatening invasive bacterial infection with P. aeruginosa in our patient accompanied by an absence of CRP elevation. In these patients, CRP concentrations can be strikingly misleading as IRAK-4 deficient patients demonstrate impairment in the ability to increase CRP concentrations and to mount fever responses.
Among IRAK-4 deficient patients with invasive bacterial infection, S. pneumoniae is the most frequently (~50% of episodes) implicated organism6. S. aureus and P. aeruginosa are less frequently (~20% of episodes) implicated organisms6. Patients with IRAK-4 deficiency may also experience a variety of minor non-invasive bacterial infections such as upper respiratory tract infections (otitis, sinusitis, pharyngitis) as well as SSTI (furunculosis, folliculitis cellulitis)6. Once again, the most frequently implicated organisms in these non-invasive bacterial infections among IRAK-4 deficient patients are S. pneumoniae, S. aureus, and P. aeruginosa.
Careful institution of aggressive supportive care measures is necessary for IRAK-4 deficient patients. Vaccines should include conjugated and nonconjugated vaccines for S. pneumoniae and N. meningitidis. Haemophilus influenzae type b conjugated vaccine should also be provided. Lifelong antibiotic prophylaxis with cotrimoxazole in combination with penicillin should be administered. Prophylaxis with intravenous or subcutaneous IgG should also be provided as a significant proportion of IRAK-4 deficient patients have impaired responses to glycans. Empiric parenteral antibiotic therapy against S. pneumoniae, S. aureus, and P. aeruginosa is critical whenever an infection is suspected or if the patient develops a fever. Inflammatory markers such as CRP should be considered unreliable. Importantly, patients may die from invasive bacterial infection despite prophylaxis even in the absence of fever or laboratory evidence of inflammation. The long-term prognosis of IRAK-4 deficient patients is positive as the risk of invasive infections tend to improve with age.
Although the occurrence of invasive P. aeruginosa infections in IRAK-4 deficiency is not novel, there is a vital and important educative message that bears repeating. Astute clinical judgment is necessary in the evaluation of patients with a potential inborn error of immunity. Clinical findings such as severe infections with P. aeruginosa should support the consideration of inborn errors of immunity including IRAK-4 deficiency.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication of their clinical details and/or clinical images was obtained from the parent of the patient.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
References
1. von Bernuth H, Ku CL, Rodriguez-Gallego C, Zhang S, et al.: A fast procedure for the detection of defects in Toll-like receptor signaling.Pediatrics. 2006; 118 (6): 2498-503 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Immunobiology of Immune dysregulation disorders; HSCT and gene therapy for immune defects
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Inborn Errors of Immunity/Primary Immunodeficiency Disorders
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 21 Sep 21 |
||
|
Version 1 01 Jul 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)