Keywords
COVID-19, vaccine breakthrough; efficacy; immunodeficiency, case report
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Coronavirus (COVID-19) collection.
COVID-19, vaccine breakthrough; efficacy; immunodeficiency, case report
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is responsible for approximately three million deaths, and subsequent vast financial and social burdens due to the current global pandemic.1 The rapid and wide utilization of effective, and safe vaccines against SARS-CoV-2 infection and COVID-19 that act against viral infection, disease, transmission, and death is essential for the establishment of public health internationally.1,2 Multiple clinical trials aim to investigate the efficacy and safety of novel vaccines, although, the most crucial efficacy endpoint, which is protection against severe disease and death, is difficult to estimate.1 With emphasis on the present efficacy endpoint, the present manuscript aims to add to the existing knowledge, while presenting the case of an immunodeficient patient, who developed remarkably favorable outcomes from SAR-CoV-2 pneumonia after having been fully vaccinated against the novel coronavirus.
An 82-year-old Caucasian male presented to the emergency room of our institution with malaise, fever (max temperature 38°C), and productive cough. The patient’s vital signs were within normal range, his respiratory rate was 16/min and his mental status was normal. Clinical examination revealed reduced sounds and dry rales at lower auscultation. A laboratory workup was carried out. The complete blood count showed mild neutropenia (1450/μL, total white blood cells 3390/μL), D-Dimers 1260 mg/L and C-reactive protein 7,1 mg/L. Chest X-ray showed opacities in both lungs, especially in lower lobes consistent with the clinical findings (see Figure 1 and Figure 2). A nasopharyngeal and pharyngeal swab were sent for SARS-CoV-2 antigen and real-time polymerase chain reaction testing, respectively, the results of which were positive. Further investigation identified a B.1.1.7 variant.
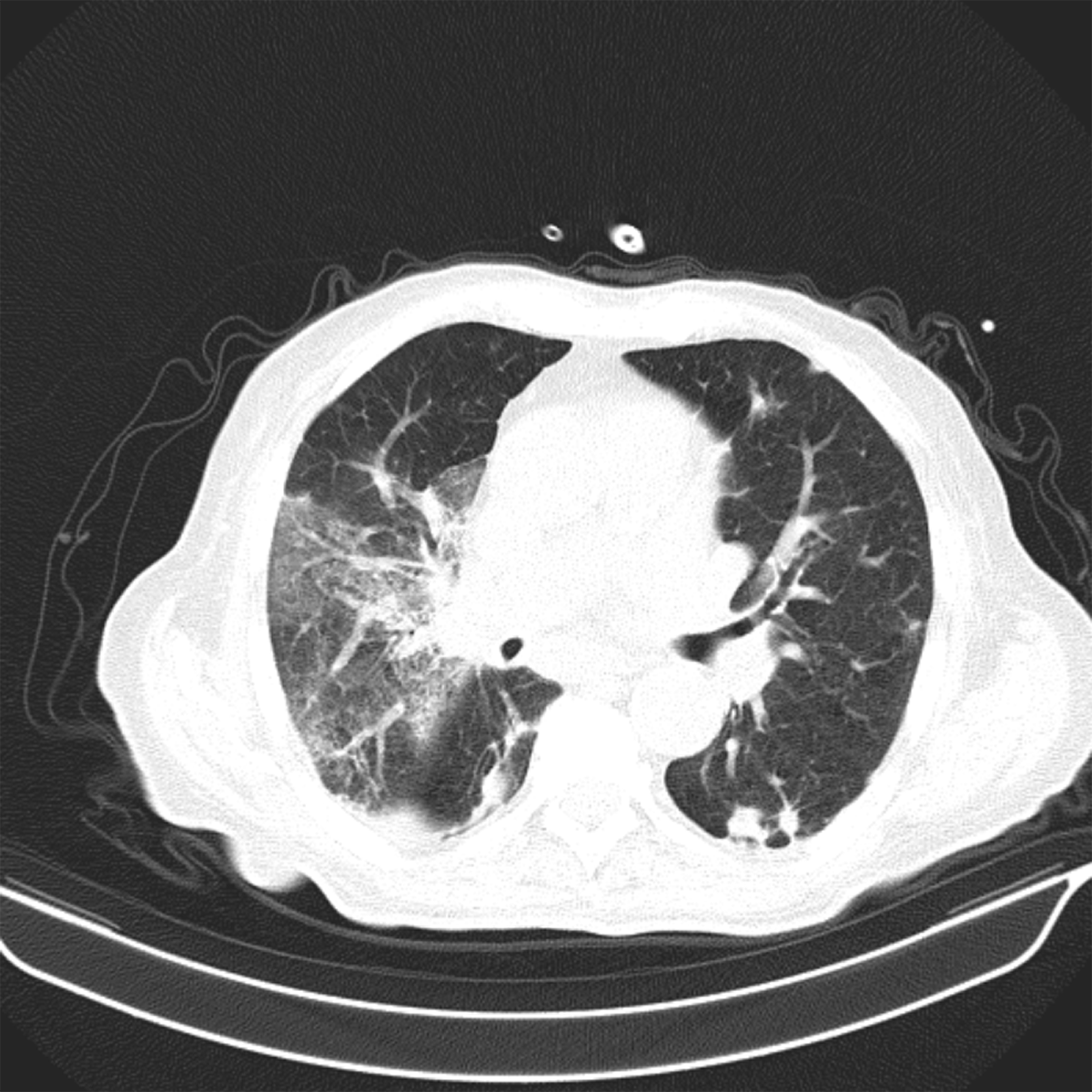

The scan reveals ground glass opacities especially with onset of fibrotic lesions and concomitant vascular enlargement. CT findings suggest >50% extent of disease.
The patient’s medical history included pancreatic cancer with lung metastasis, prostate hyperplasia, and frequent RBC transfusions. His last chemotherapy session had taken place two weeks ago and he had received the second dose of the BNT162b2 (Pfizer) vaccine against COVID-19 16 days prior. Both doses had been administered without any complications. No symptoms indicative of acute infection (fever, chills, dyspnea, tachypnea, joint pain) were mentioned between the two doses. We preceded to admission of the patient for appropriate treatment. This included remdesivir 200 mg at first day of admission then 100 mg once daily, ceftriaxone 2 gr once daily, levofloxacin 500 mg once daily, N-acetylcysteine 600 mg bid p.os, inhaled agents (ipratropium and budesonide) and prophylactic dose of low-molecular-weight heparin (LMWH) enoxaparin 4000 once daily based on patient’s weight. Supplementary oxygen was administered with nasal cannula at a flow rate of two to three liters per minute. Meanwhile a chest computed tomography (CT) was performed which was indicative of diffuse COVID-19 lesions (>50% extent). The patient was closely monitored however he did not manifest clinical deterioration or respiratory distress. No changes were made regarding the therapeutic interventions. The inflammatory markers and PaO2/FiO2 ratio remained stable and gradually ameliorated. No other complications were presented whatsoever (thromboembolism, disseminated intravascular coagulation, arrhythmia, shock, sepsis or ARDS). The patient was discharged after six days of hospitalization with no need for supplementary oxygen at home.
The global pandemic of COVID-19 seems to constitute the most challenging pandemic of the 21st century.3 A strongly efficacious vaccine is fundamental and crucial for the prevention of morbidity and severe mortality.1 Therefore, all clinically available vaccines against SARS-CoV-2 infection and COVID-19 are currently under evaluation through several multi-central clinical trials for their safety and efficacy.4
It is already documented that in comparison to unvaccinated patients, the probability of remaining asymptomatic after SARS-CoV-2 infection is lower among vaccinated persons at the period of more than 10 days after 1st dose of vaccine, and more than 0 days after 2nd dose.4
According to the study of Polack et al. (2020), between the first and the second dose, the Pfizer COVID-19 vaccine provided an efficacy of 52%, with an incidence of 39 cases of infection in the vaccinated group, in comparison to 82 cases from the placebo group. Meanwhile the integration of two doses of the BNT162b2 mRNA COVID-19 vaccine offered 95% protection against the virus in patients older than 16 years of age.2
Moreover, the study of Voysey et al. (2020) indicates that Oxford and AstraZeneca’s COVID-19 vaccine (AZD1222), presents an efficacy of 62.1% after the integration of two standard doses.5 Interestingly, for the persons that received a lower first dose combined with a standard second dose, an efficacy of 90% was observed.5
Indeed, according to the literature there have been several documented cases of COVID-19 infections after vaccination.4,6 However, all the reported cases either remained asymptomatic or presented mild symptoms.4
Interestingly our patient’s clinical course was remarkably favorable despite being immunodeficient. Herein it is implied that prior vaccination with BNT162b2 mRNA COVID-19 Vaccine had offered protection to some extent. This could be justified by the short period of hospitalization, the absence of septic and thromboembolic complications or severe respiratory distress, and finally by the fact that no high flow oxygen therapy was needed.
At this point we would like to address the issue of meticulous planning of vaccination campaigns. Given the rapid rate of COVID-19 evolution (presenting 1 × 10−3 mutations per genomic residue and year) alongside its genetic heterogeneity in every host, scientists are facing vaccination-driven virus evolution. As a result, even if massive vaccinations are implemented, there is still the risk of new infections by emerging variants. That is why authorities should take into consideration the epidemiological context of every country before vaccination campaigns are implemented.7
The current SARS-CoV-2 pandemic poses a real threat on public health due to the rapid and wide transmission of the virus. Despite the civil protection measures, the novel vaccines constitute a real game changer. To our knowledge this is the first reported vaccine breakthrough case with an extremely favorable clinical course indicating the efficacy of vaccination against COVID-19.
All data underlying the results are available as part of the article and no additional source data are required.
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patient.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
No
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Tropical medicine and infectious diseases, immunology
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious Diseases, Sepsis
Is the background of the case’s history and progression described in sufficient detail?
No
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious diseases, virology, immunology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 06 Jul 21 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)