Keywords
Major external structural birth defects, risk factors, case-control study, Kenya
Major external structural birth defects, risk factors, case-control study, Kenya
To address the reviewers' comments, we have clarified that; the hypothesized odds ratio used in sample size calculation was set at 2.0 (universally accepted), different types of birth defects were collectively used in sample size calculation rather individual defects due to the rarity of the defects coupled with the unavailability of surveillance systems for birth defects in the country, and that data collection period spanned three months from May 31st 2019 to July 31st 2019. Additionally, some of these defects are potentially fatal and could possibly introduce survivor bias in the study, thus we clarified being cognizant of this epidemiological phenomenon, however we could not minimize it considering that pathological examinations to determine the causes of death in stillbirths and miscarriages is not a routine practice in the country. Maternal residence, and paternal age were controlled for in the multivariable analyses, however maternal cigarette smoking was not controlled for due to negligible responses received from the study participants. We have also redesigned our conceptual framework, defined maternal exposure to pesticides and chronic illnesses in the context of thus study. We also improved the texts in the introduction, discussion, and conclusion sections of the manuscript.
See the authors' detailed response to the review by Rogath Kishimba
See the authors' detailed response to the review by Marcia L. Feldkamp
See the authors' detailed response to the review by Yoseph Worku
Worldwide, an estimated 7.9 million children are born every year with a birth defect of which approximately 3.3 million die before age five and around 3.2 million could be physically disabled for life1,2. More than 94% of such defects occur in developing countries where about 95% of these children do not survive beyond childhood1. Birth defects are defined as abnormalities of body structures or functions that develop during the organogenesis period (first trimester of gestation) and detectable during pregnancy, at birth, or soon after2,3. These defects may be classified as major when associated with significant adverse health effects requiring medical/surgical care; otherwise, they are described as minor1,2. Alternatively, they can be classified as external when visible at birth or soon after; or internal when advanced medical imaging techniques are required for their detection4–6. Consequently, the phrase ‘major external structural birth defects’ (MESBDs) denotes congenital physical abnormalities that are clinically obvious at birth or soon after which call for medical and/or surgical interventions1,2. The causes of these defects can be classified into three categories: (i) identifiable environmental factors (teratogens/micronutrient deficiencies); (ii) identifiable genetic factors; and (iii) complex genetic and idiopathic environmental factors, described as multifactorial inheritance1,4,7–10. One-third of these causes are attributed to identifiable environmental and genetic factors, whereas the rest are believed to be of multifactorial aetiology1,4,7–10. Additionally, an environmental endowment of women of reproductive age is thought to operate through their socioeconomic and sociodemographic characteristics leading to causes of MESBDs, described as sociodemographic-environmental factors1,4,8–10.
Organogenesis occurs in the first eight weeks of gestation; however, approximately half of pregnancies are usually unplanned/unintended, thus not recognized until the end of the second trimester1,4,11–13. Completing more years of education could improve maternal health because educated women are more likely to make informed reproductive health choices than those with low levels of education to improve birth outcomes14–17. Some of the notable maternal decisions include planned pregnancy, preconception folic acid intake in anticipation of conception, and subsequently prompt prenatal care14,16,18–23. Supplemental vitamins with folic acid are dispensed during routine antenatal care (ANC) visits, as well as health education on adequate nutrition, avoidance of environmental teratogens, and maternal infections as public health preventive strategies for MESBDs10,24. These measures could be effective only when pregnant women promptly began antenatal care within eight weeks of gestation before the intrauterine formation of MESBDs4. Folic acid is essential for normal development of the brain and spinal cord during the first 4 weeks of conception, and have been found to reduce the occurrence of neural tube defects, orofacial clefts, limb reduction defects, urinary system defects, and omphalocele; some of the most prevalent defects in the county25–27. Thus, the recommended first ANC at the 12th week of pregnancy could be a sub-optimal preventive strategy for these defects, nevertheless it improves experiences of the women during pregnancy and childbirth28. Maternal occupation as a predictor of MESBDs could be dependent on educational levels, nonetheless occupations such as farming could expose women of reproductive age to teratogenic pesticides29.
Maternal residence at conception is similarly a significant predictor of MESBDs determined by environmental etiology attributed to widespread poverty, environmental pollution, inadequate health care services, and ineffective preventive strategies; factors largely found in developing countries1,7,30. Parental age is a multifaceted risk factor whose mechanisms of actions in the intrauterine formation of MESBDs are underpinned by human biology and socio-economic endowment among women of reproductive age. From the biologic standpoint, the female gametogenesis begins before birth with the initial meiotic division (prophase stage) expected to complete shortly before ovulation, however, this is not the case always because the process may delay up to 45 years to conclude24. Thus, the oocytes take exceedingly long in the prophase stage increasing the likelihood of meiotic errors due to exposure to the environmental teratogens24. Advancing maternal age beyond 35 years is similarly a risk factor for MESBDs of genetic etiology due to chromosomal abnormalities24,31,32. Similarly, from the genetic viewpoint, genetic mutations and accumulation of chromosomal aberrations during the maturation of male germ cells have been attributed to the formation of MESBDs in utero33,34. The amount of deoxyribonucleic acid damage in sperm of men aged 36–57 is three times that of men <35 years, increasing the likelihood of these defects in aging couples34,35. From the socioeconomic perspective, parental age could be associated with MESBDs of multifactorial etiology ascribed to physiological interactions between complex genetic and idiopathic environmental attributes of women of reproductive age1,7,30,36,37.
To our knowledge, many studies on the risk factors have been published in developed countries, however, such publications are scanty in developing countries owing to the rarity of the defects, unplanned/unintended pregnancies, and difficulties in identifying these women until the end of the second trimester when the defects have already formed4. To address this gap, this study investigated maternal periconceptional exposure to environmental teratogens, sociodemographic-environmental, and multifactorial risk factors for MESBDs in Kiambu County, Kenya. The study assessed: maternal periconceptional exposure to farm-sprayed pesticides, and teratogenic therapeutic medicines proxied by maternal chronic illnesses (epilepsy and depression); multifactorial inheritance proxied by the history of siblings with birth defects, sex of the “last born” current child, nature of pregnancy, and parity; and sociodemographic-environmental factors consisting of maternal age, paternal age, residence, level of education, occupation, and adequate prenatal care proxied by gestational age at first ANC, and preconception folic acid intake. The findings of this study could provide great public health opportunities for the formulation of specific treatment strategies, preventive measures, risk-based surveillance systems, and clinical genetic services for the most prevalent MESBDs, regionally and nationally. Consequently, the objective of this study was to identify the risk factors for MESBDs among children in Kiambu County, Kenya.
A hospital-based case-control study was conducted to identify the risk factors for MESBDs. The study participants were recruited as they presented to the child welfare clinics, neonatal/pediatric units, and occupational clinics for care during the data collection period from May 31st 2019 to July 31st 2019. A case-control design was the optimal design for this study considering its suitability for the investigation of rare outcomes, as is the case with MESBDs. Even though a population-based design would have been preferable, the ease of recruiting case and control subjects within the hospital settings disproportionately favored the hospital-based design. This was an observational study, therefore was reported as per the STROBE guidelines38.
The study was conducted in 13 hospitals comprising three-county referral hospitals (Kiambu, Gatundu, and Thika), eight sub-county hospitals (Karuri, Kihara, Wangige, Nyathuna, Lari, Tigoni, Lussigetti, and Kigumo), and two faith-based hospitals (Presbyterian Church of East Africa Kikuyu Orthopedic and African Inland Church Cure International) situated within Kiambu County, Kenya. Notably, neither population-based nor hospital-based surveillance systems for MESBDs existed in the county nor the study hospitals. Nonetheless, cases detected by primary health providers during childbirth and neonatal care were recorded for the compilation of monthly hospital reports and subsequent entry into the District Health Information System (DHIS). The cases were drawn from Kiambu, Thika, Gatundu, Tigoni, Kikuyu, and Cure hospitals, which provided occupational and rehabilitative health services to children with MESBDs. The controls, on the other hand, were drawn from Kiambu, Gatundu, Thika, Karuri, Kihara, Wangige, Nyathuna, Lari-Rukuma, Tigoni, Lussigetti, and Kigumo hospitals which provided child welfare services to the under-fives. Kiambu is the second-most densely inhabited county with an estimated population of 2.4 million people out of an estimated national population of 47.5 million39. Its economic mainstay is largely agriculture, comprising tea, coffee, and dairy farming39. Of the county’s total estimated population, approximately 2.2% aged ≥5 years are living with lifelong disabilities39. A study carried out in the county between 2014 and 2018 observed defects of the musculoskeletal system as the most prevalent single system defects followed by central nervous, orofacial clefts genital, ocular, and anal organ defects25.
The study population consisted of children aged ≤5 years old seeking health services at the study hospitals during the study period spanning from May to July 2019. All children whose mothers consented to participate in the study were recruited.
Cases were defined as children aged ≤5 years born with at least one MESBD to resident women of Kiambu County and seeking health care services at the neonatal units, pediatric wards, child welfare clinics, and/or occupational therapist clinics of the study hospitals during the three-month study period. The Research Assistants (RAs) liaised with team leads of the departments listed above to identify cases of MESBDs. The team leads had been working in these departments, thus were conversant with the cases seeking services. The team leads invited the mothers of the children who met the case definition to comfortable private rooms within the departments where informed consent was sought and interviews conducted by the RAs. All cases that met this definition and whose caregivers consented to participate were prospectively recruited into the study until the required sample was attained (see Sample size determination).
Controls were defined as children aged ≤5 years born without any forms of birth defects to resident women of Kiambu County and attending routine child welfare clinics at the study hospitals during the same three-month study period. The Research Assistants liaised with team leads of the child welfare clinics to identify the children without any form of birth defects and were seeking routine immunization, and growth monitoring services. The team leads had been working in these clinics, hence were familiar with most of the under-fives seeking the services. These services are provided between 8.00 am and 5.00 pm from Monday to Friday; the team leads introduced the RAs who then briefed the potential participants on the study objectives. Because of the relatively large number of controls available, they were selected by simple randomization using sealed envelopes upon definition of the sample population and frequency-matched to the cases by the day of presentation. Informed consent was sought from the study participants who met the study eligibility criteria; those who consented to participate in the study were prospectively recruited and invited to secluded comfortable rooms within the clinics where face-to-face interviewer questionnaires were administered till the desired sample size was achieved (see sample size determination).
The sample size was estimated as per the Kelsey JL et al.40 formula specified for case-control studies as follows: -
Where: n1 is the number of cases and n2 is the number of controls; p1 is the proportion of cases whose caregivers did not begin prenatal care in the first trimester (primary exposure), p2 is the proportion of controls whose caregivers did not begin prenatal care in the first-trimester set at 57%11,12. Remarkably, Zα/2 (1.96) and Zβ (-0.84) are the values specifying the desired two-tailed confidence level (95%) and statistical power (80%), respectively. The odds ratio (OR) for the effect of the primary exposure (cases whose caregivers did not begin prenatal care in the first trimester) was hypothesized to be 2.0 (universally accepted). The ratio (r) of unexposed to exposed individuals was set at 3.0, and given the estimates, a total sample size of 408 participants was derived (102 cases, and 306 controls).
Before data collection, four nursing graduate interns were recruited and trained as RAs on sound interviewing techniques, and information derivation/validation from antenatal care (ANC) booklets. This was to ensure the data collection process spanning three months (May 31st 2019 to July 31st, 2019) was conducted in a standardized manner. The ANC booklet contains maternal profile, medical/surgical history, previous pregnancy history, clinical notes, and physical examination findings on ANC visits, among others. The maternal profile includes name, age, parity gravidity, height, weight, last menstrual period (LMP), expected date of delivery (EDD), and date of first ANC. Face-to-face structured questionnaires (see Extended data) were administered to the mothers of the study participants by RAs in comfortable secluded rooms within neonatal units and occupational therapy clinics for cases and child welfare clinics for the controls. Data were gathered retrospectively on exposures to environment-teratogens (farm-sprayed pesticides, and teratogenic medicines proxied by chronic illnesses), multifactorial inheritance (parity, nature of pregnancy, history of siblings with birth defects and sex of the “lastborn” (current) child), and sociodemographic-environmental factors (maternal age, paternal age, residence, education level, occupation, and adequate prenatal care proxied by gestational age at first ANC and preconception folic acid intake). The predictors were assessed as shown in Table 1.
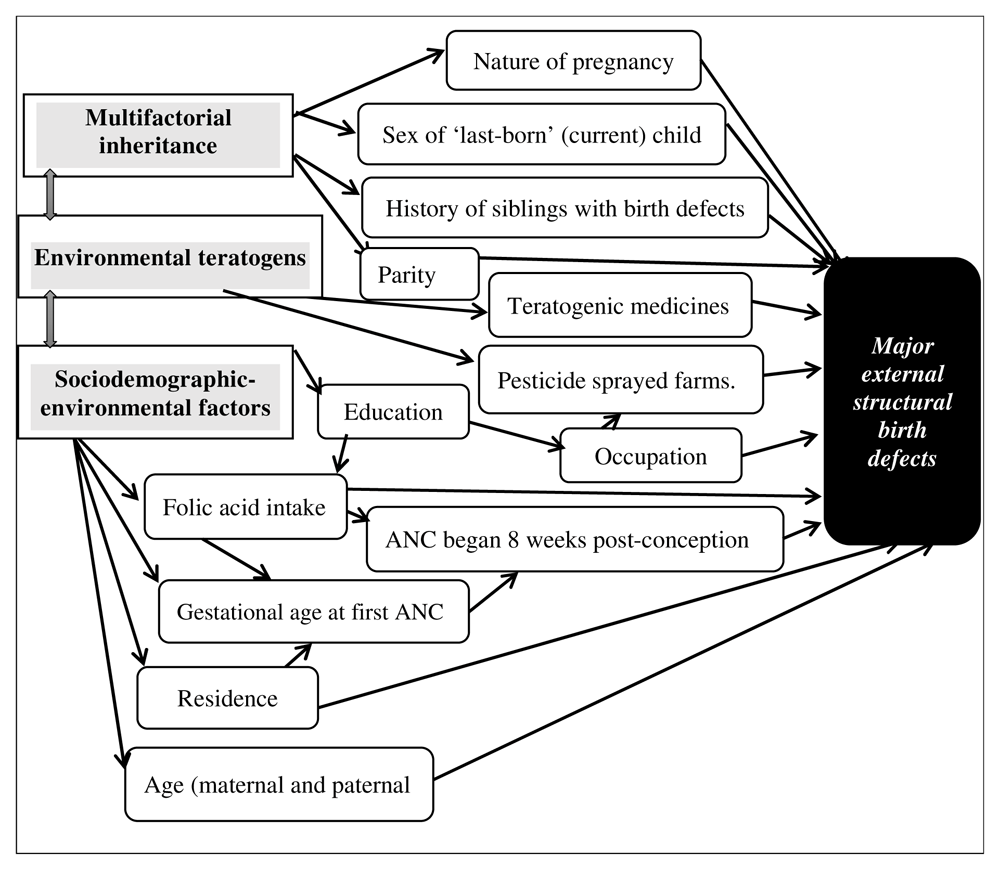
The conceptual framework was organized based on the three causal categories of MESBDs (multifactorial inheritance, environmental teratogens, and sociodemographic-environmental factors). Nonetheless, because disentangling genetic etiology (identifiable, and complex) was a scientific limitation of observational studies as is the case in our study, analysis of such factors sufficed as a multifactorial inheritance in this conceptual framework to measure maternal genetic predispositions. A conceptual framework depicting the predictor-outcome relationship is displayed in Figure 1. The flow chart of the simple-random systematic sampling strategy is shown in Figure 2.
Ethical approval for this study was obtained from the Kenyatta National Hospital [KNH]-University of Nairobi [UoN] Ethics Review Committee [Ref. No: KNH-ERC/A/44]. The purpose of the study was explained to participants and written informed consent was obtained from the mothers of the study subjects before engaging in the study.
Considering potential biases inherent in case-control studies that were likely to invalidate the study results, deliberate attempts were made to minimize their occurrence. First and foremost, the research assistants were trained on sound interviewing techniques and information derivation/validation from ANC booklets to minimize interviewer and minimize information biases, respectively. In a bid to minimize recall bias, gestational age at the first ANC was estimated from the dates of the last menstrual period and dates of the first ANC obtained from the ANC booklets.
Following data collection, filled questionnaires were manually checked daily for accuracy and completeness and subsequently entered into a Microsoft Excel spreadsheet (Microsoft Office Professional Plus 2019) by two independent data managers to reduce potential errors. The excel dataset was validated and exported to Stata software version 14.0 (Stata Corporation, Texas, USA) for further cleaning, coding, and analyses. Descriptive analyses (means, medians, standard deviations, and ranges) were used to summarize continuous variables, whereas proportions and percentages for categorical variables were generated and presented in frequency tables. Afterward, the effect of each predictor on the odds of MESBDs was assessed using univariable logistic regression models at a liberal P-value (P≤0.20)41. Gestational age at first ANC as a continuous variable was categorized into groups (<9 weeks and ≥9 weeks) for evaluation in the univariable analyses1,4,11–13. Additionally, parity as a continuous variable was grouped into two groups: =1=primiparous or >1=multiparous categories for assessment in the univariable analyses42,43. However, maternal age as a continuous variable was insignificant in the univariable analyses, thus, recategorized into two groups; <35 years, and ≥35 and reassessed for statistical significance; women aged at least 35 years have previously been reported to have an increased likelihood of giving birth to children with MESBDs44. Paternal age as a continuous variable was similarly insignificant in the univariable analyses, thus recategorized into seven groups and reassessed for statistical significance which was still insignificant. Nevertheless, paternal age was further recategorized into two groups (<35 years, and ≥35) and reassessed for statistical significance yet still insignificant; males aged at least 35 years have previously been associated with increased likelihood of defect-affected births in their female counterparts34. Variables found statistically significant in the univariable analyses were fitted to a multivariable model where a backward stepwise approach was used to eliminate variables from the model at P-value >0.05. Nature of pregnancy was however collinear in the multivariable analyses thus dropped in the final multivariable analysis. To minimize the confounding effects, elimination of non-significant predictors was only considered when their exclusion from the model did not yield more than a 30% change in the effects of the remaining variable41. Two-way interactions were fitted between the remaining variables of the final model and assessed for significance. A Hosmer-Lemeshow test was used to assess the goodness of fit of the logistic model, with a P-value of >0.05 being suggestive of a good fit.
A total of 408 study respondents (102 cases and 306 controls) were enrolled in this study. The cases consisted of cleft lip with palate 1 (0.98%), cleft palate 3 (9.94%), clubbed hand 1 (0.98%), club foot 91 (89.22%), hydrocephalus 1 (0.98%), limb defects 4 (3.92%), and persistent cloacal 1 (0.98%)45.
Sociodemographic-environmental factors: The median age of the study respondents was 26 years with a mean of 27.31 years (SD=5.73, R; 17-47) (Table 2). The median age of mothers in the case group was 28 years with a mean of 28.73 (SD=5.95, R; 19-47), whereas the median age of mothers in the control group was 26 years with a mean of 26.84 (SD=5.58, R; 17-42) (Table 2). The mean paternal age of the study respondents was 32.02 years with a standard deviation of 6.34 years, and a median age of 31 years ranging between 19 and 56 years (Table 2). Of the 408 study participants, 184 (45.10%) had attained a secondary level of education; 38 (37.25%) and 146 (47.71%) in the case and control groups, respectively (Table 2).
Environmental-teratogens: Of the 408 study respondents, 15 (3.68%) were exposed to farm-sprayed pesticides, of which four (3.92%) were in the case group and 11 (3.59%) were in the control group (Table 2).
Multifactorial inheritance: Of the 408 study respondents, 404 (98.77%) had single gestations for the current child, of which 99 (97.06%) and 304 (99.35%) were in the case and control groups, respectively (Table 2). Of the study participants, approximately 3.68% (15) of the study participants reported a history of siblings with birth defects consisting of 9 (8.82) in the case group and 6 (1.96) in the control group (Table 2). Of the 15 study participants, 12 stated the name or described the nature of the defects in their previous pregnancies/births, however, 3 participants were unable to do so. Of the 12 study respondents, 7 of the case subjects with congenital talipes equinovarus reported a history of birth defects in their previous births of which 4 subjects reported a recurrence of congenital talipes equinovarus, whereas 3 reported foot aversion, internally rotated shorthand (phocomelia), and congenital scoliosis. On the other hand, 5 control subjects reported a history of siblings with birth defects in their preceding births comprising 3 cases of congenital talipes equinovarus, 1 case of autism, and 1 case of deafness (Table 3).
Notably, the factors assessed for statistical significance in the univariable analyses and found associated with MESBDs at P≤0.20 included maternal age, residence, education, occupation, ANC visits beginning eight weeks post-conception, gestational (age) at first ANC visits, nature of pregnancy, and history of siblings with birth defects (Table 4). Subsequently, these variables were fitted to the multivariable model for the final analysis, except education being distal relative to occupation, gestational age at first ANC visits, and ANC beginning eight weeks post-conception. (Figure 1).
| Variable | Value | Odds ratio | 95% CI | P-value |
|---|---|---|---|---|
| Residence* | Others | Reference | ||
| Thika | 0.99 | 0.52-1.86 | ||
| Gatundu | 0.73 | 0.33-1.61 | <0.001 | |
| Kiambu | 0.47 | 0.22-0.98 | ||
| Ruiru | 3.07 | 1.37-6.89 | ||
| Maternal age * | <35 | Reference | ||
| ≥35 | 2.09 | 1.13-3.85 | 0.02 | |
| Paternal age | ≥35 | Reference | ||
| <35 | 1.22 | 0.73-2.03 | 0.45 | |
| Maternal education * | Tertiary | Reference | ||
| Secondary | 0.65 | 0.39-1.10 | 0.18 | |
| ≤Primary | 1.01 | 0.56-1.82 | ||
| Maternal occupation * | Farming | Reference | ||
| Employed | 1.09 | 0.43-2.77 | 003 | |
| Unemployed | 0.59 | 0.23.1.51 | ||
| Preconception folic acid intake | No | Reference | ||
| Yes | 0.94 | 0.60-1.47 | 0.78 | |
| ANC began eight weeks post gestation* | No | Reference | ||
| Yes | 1.55 | 0.90-2.66 | 0.11 | |
| Gestational age at first ANC * | <9 weeks | Reference | ||
| ≥9 weeks | 0.32 | 0.13-0.79 | 0.01 | |
| Parity | Primiparous | Reference | ||
| Multiparous | 1.14 | 0.68-1.93 | 0.62 | |
| Nature of pregnancy * | Multiple | Reference | ||
| Single | 0.22 | 0.04-1.32 | 0.10 | |
| Sex of the “lastborn” current child | Female | Reference | ||
| Male | 1.28 | 0.82-2.01 | 0.28 | |
| Siblings with a history of birth defects * | No | Reference | ||
| Yes | 4.84 | 1.68-13.95 | <0.01 | |
| Teratogenic therapeutic medicines for chronic illnesses | No medicines for chronic illnesses | Reference | ||
| Medicines for hypertension | 0.92 | 0.29-2.88 | 1.0 | |
| Medicines for other chronic illnesses | 0.85 | 0.17-4.17 | ||
| Exposure to farm-sprayed pesticides | No | Reference | ||
| Yes | 1.09 | 0.34-3.52 | 0.88 |
In the multivariable analysis, only maternal residence at conception, and history of siblings with birth defects were shown as the significant predictors MESBDs at a 5% significance level (Table 5). Compared to women who conceived while residing in other sub-counties, women who conceived when residing in Ruiru sub-county were 5.28 times likely to give birth to children with MESBDs (aOR: 5.28; 95% CI: 1.68-16.58; P<0.01); whereas women who conceived when residing in Kiambu sub-county were 27% less likely give birth to children with MESBDs (aOR: 0.27; 95% CI; 0.076-0.95; P =0.04) holding all factors constant. Additionally, compared to siblings without a history of birth defects, siblings with a history of birth defects were 7.65 times likely to be born with MESBDs (aOR: 7.65; 95% CI; 1.46-40.01; P =0.02) holding all factors constant (Table 5).
To our knowledge, this was the first case-control study conducted to identify the risk factors for MESBDs in the entire county. Our study results mimicked other findings across the world that maternal residence at conception and history of siblings with birth defects are strongly associated with the intrauterine formation of MESBDs1,30,46. Our study observed orofacial clefts comprising 1 (0.98%) cleft lip with the palate, and 3 (9.94%) cleft palates; limb reduction defects comprising 1 (0.98%) clubbed hand, and 4 (3.92%) limb defects; defects of the musculoskeletal system consisting of 91 (89.22%) clubfeet; and neural tube defects comprising 1 (0.98%) hydrocephalus and 1 (0.98%) persistent cloacal. These are some types of MESBDs associated with genetic, partially genetic, and multifactorial etiology1,30,46. The prevalence of such defects have been observed to vary by regions attributed to ethnical, and socioeconomic differences globally1,30. Siblings with a positive history of MESBDs among their preceding siblings are at most risks of being born with MESBDs, have a recurrence of similar defects among the siblings, and/or among their offspring46. This was indeed evident in this study where 4 of the case subjects with clubfoot similarly reported clubfoot in their preceding siblings, whereas 3 of the case subjects with clubfoot reported foot aversion, internally rotated shorthand (phocomelia), and congenital scoliosis each in their preceding siblings. Our study similarly made remarkable observations where case subjects with clubfoot reported concurrence of congenital pes planus, and arthrogryposis each, whereas a case subject with hydrocephalus reported concurrence of congenital pes planus, and two case subjects of limb defects reported concurrence with Down syndrome each. On the other hand, 5 control subjects reported a history of siblings with birth defects in the preceding births comprising 3 cases of clubfoot, 1 case of autism, and 1case of deafness.
Positive siblings and familial history of specific types of MESBDs have been associated with increased risks of recurrence in subsequent pregnancies24,46,47. Worldwide, the recurrence rate of NTD and Down syndrome have been approximated at 2-5% and 1%, respectively24,46,47. Thus, accurate knowledge of birth defects by families when given to the clinicians is similarly of public health significance to improve risk assessments and reproductive health planning for couples susceptible to birth defects of genetic, and multifactorial origin46. Even though our study did not show a significant statistical association between MESBDs with parental age, advanced age has been strongly associated with defects of chromosomal etiology (Down syndrome), and non-syndromic etiology (neural tube defects and orofacial clefts)1,30,34,48,. Nonetheless, our study alluded to an increased risk of chromosomal abnormalities thus suggestive of the prevalence of MESBDs of genetic origin in the county. High prevalence of Down syndrome has been observed in developing countries attributed to many older women becoming pregnant, limited family planning services, unavailability of prenatal genetic screening, diagnosis, and related services1,30. MESBDs are considered defects of public health importance, however the presence of certain defects; rare or common, minor or major, internal or external, functional or structural sometimes act as pointers to latent defects of similar significance because of the multiple genetic epidemiology, thus diagnosable later using advanced medical imaging techniques3,6,46.
Our study similarly observed maternal residence at conception as a predictor of the intrauterine formation of MESBDs. The study showed that women who got pregnant when residing in Ruiru sub-county were 5.28 times likely to give birth to children with MESBDs compared to those who got pregnant residing in other sub-counties within Kiambu County. Conversely, the study showed that women who got pregnant when residing in Kiambu sub-county were 27% less likely to give birth to children with MESBDs compared to those who got pregnant residing in other sub-counties within the county. The study showed that Kiambu sub-county was protective implying it was relatively safe for women of reproductive age to become pregnant while residing in the sub-county. Maternal residence at the time of conception as a risk factor for MESBDs could be ascribed to variations in maternal genetic, multifactorial, sociodemographic-environmental attributes. From the genetic perspective, increased frequency of single-gene defects in developing countries has been associated with increased frequency of common recessive disorders such as hemoglobin disorders, sickle cell anemia, thalassemia, oculocutaneous albinism, and cystic fibrosis because of the discerning advantage for carriers to the mortal effects of malaria, as well as recessive conditions associated with high rates of consanguineous (cousin) marriages1,30. Additionally, high prevalence of defects of chromosomal etiology in developing countries have been ascribed to women delaying childbearing beyond 35 years, limited maternal access to family planning services, and absence of clinical genetic services1,24,30,48. Sociodemographic-environmental characteristics, and physiological interactions between complex genetic disorders, and idiopathic environmental factors could also lead to the occurrence of MESBDs associated with ethnic and geographic differences1,30. Thus, the epidemiology of MESBDs in the county underscore an underlying genetic, multifactorial, sociodemographic-environmental etiology contributing to the global debate on the burden of a “silent” public health problem in developing countries1,30.
Although our study did not show an association between MESBDs with known environmental factors (teratogens and micronutrient deficiencies), pregnancies in developing countries are at increased risk of potential teratogens because of high prevalence of intrauterine infections, maternal malnutrition, low socioeconomic levels, low levels of education, deficient environmental protection policies, and insufficiently regulated access to medicines1,30. This could imply the county is performing relatively well in controlling potential environmental causes of MESBDs. The teratogens consist of; (i) congenital infections; (ii) maternal and altered metabolism; and (iii) recreational and therapeutic drugs1,30. Congenital infections comprise toxoplasmosis, other infections (syphilis, varicella-zoster, human parvovirus B19), rubella, cytomegalovirus, and herpes, denoted by an acronym “TORCH”1,30. Epilepsy and insulin-dependent diabetes are the examples of maternal illnesses and altered metabolism, whereas statins and alcohol are the examples of therapeutic and recreational drugs, respectively1,30. Our study also did not show significant associations between MESBDs with maternal occupation, gestational age at first ANC, and ANC beginning 8 weeks post-conception; factors thought to influence maternal iron-folic acid supplementation14,16,21. Folic acid is crucial for the biosynthesis, and methylation of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) which are important for cell division, differentiation, and regulation of gene expression, during rapid cell division such as embryogenesis, thus is necessary for the growth and smooth functions of human cells24,49.
Nevertheless, some limitations were inherent in this study; there was a likelihood of differential recall bias among the study respondents; cases were more likely to remember their preconception period owing to the experience of MESBDs in the last birth than the controls, thus recall bias could affect estimates of the odds ratios. The study participants with a history of siblings with birth defects either stated or described the nature of the defects however the researchers could not ascertain accuracy of the diagnoses/descriptions, while others did not know the names of the defects. Survivor bias was also an inherent limitation in this study because some defects such as neural tube defects are potentially fatal, however the study could not establish the causes of deaths among stillbirths, and miscarriages in the study hospitals because it was not a pathological standard operating procedure in the entire Kenya. Additionally, due to the extreme rarity and stochasticity of MESBDs because of the absence of public health surveillance systems, the researchers lumped all types of MESBDs in calculating the sample size, yet births defects are largely heterogenous in their etiology, thus could also lead to underestimation of the effects of the predictors on the odds of MESBDs.
These findings were indeed suggestive of genetic, multifactorial, and sociodemographic-environmental etiology of MESBDs in Kiambu County, Kenya. Thus, these findings could provide the greatest public health opportunities for health planners in the region to establish defect-specific surveillance programs, implement proven public health preventive strategies, and provide appropriate treatment interventions for the most prevalent MESBDs. Therefore, we would like to provide the following priority public health policy recommendations; establishment hospital-based surveillance systems for the most common MESBDs, and integration of clinical genetic services with routine reproductive health services, nationally. The genetic services should consist of counseling, screening, diagnosis, and associated treatments including elective termination of pregnancies for anomalies in jurisdictions with favorable legislative frameworks. Additionally, we would recommend further epidemiological, and economic evaluation studies to understand the epidemiology and economic burden of these defects in Kenya.
Havard Dataverse: Risk factors for major external structural birth defects among children in Kiambu County, Kenya: a case-control study. https://doi.org/10.7910/DVN/PYLVUW
Harvard Dataverse: Risk factors for major external structural birth defects among children in Kiambu County, Kenya: a case-control study. https://doi.org/10.7910/DVN/PYLVUW
This project contains the following extended data:
- Questionnaire_mesbds_kmbu_ke.pdf (copy of questionnaire)
- Dofile_mesbds_kmbu_ke.do (syntax used for analysis)
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
We would like to acknowledge the National Commission for Science, Technology, and Innovation (Ref. No: NACOSTI/P/19/75586/28325), Kiambu County Commissioner (Ref. No: ED.12 (A)/1/VOL.11/107), and Kiambu County Director of Health, (Ref. No: KIAMBU/HRDU/AUTHO/2019/03/06/AgotGN), for permitting us to carry out this study in the county. We would also like to acknowledge the Medical Superintendents and Directors of the thirteen hospitals for granting us permission and support during data collection. Lastly, we would like to acknowledge the efforts of all data collectors in making this exercise a reality.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Maternal and Child Health Epidemiologist
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Maternal and Child Health Epidemiologist
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiology, birth defects
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Public Health and Epidemiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 30 Apr 21 |
read | read | |
|
Version 1 01 Feb 21 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)